Numerical assessment affects aggression and competitive ability: a team-fighting strategy for the ant Formica xerophila (original) (raw)
Abstract
The relationship between numerical advantage and competitive ability is a fundamental component in contests between groups of social animals. An individual's ability to correctly assess the numerical state of its group is of vital importance. In addition to numerical dominance, the group's fighting ability also plays an important role in competitive interactions. By staging experimental fights between two Formica ant species, I show that Formica xerophila are able to assess their own group's strength prior to any competitive encounter. Ants that perceive themselves as part of a large group act more aggressively toward a competitor than ants that perceive themselves as isolated individuals. This increase in aggression improves F. xerophila's competitive ability. Furthermore, the number of individuals in a contest was found to affect competitive ability. In contests with equal number of competitors, groups of F. xerophila were more successful than individual F. xerophila. Contrary to previous predictions using Lanchester's laws of fighting, F. xerophila's ability to kill competitors increased nonlinearly with group size. This nonlinearity was due to the collective fighting strategy of an F. xerophila group isolating and engaging a single Formica integroides competitors.
Keywords: numerical assessment, social animals, competitive ability, signals, Lanchester's fighting laws
1. Introduction
In fights between groups of social animals, the winning group is likely to be the numerically superior side (Adams 1990; Traniello & Beshers 1991; Zabel et al. 1992). Therefore, social individuals' willingness to fight should increase as their group size increases. Willingness to fight, however, may also increase the probability of incurring a cost such as injury or death (Maynard Smith 1982). Thus, when social animals must fight for a resource, those individuals from the winning side that choose to fight probably will incur substantial costs. This trade-off between the benefit of winning a contest and the cost of injury or death makes the assessment of group strength potentially important for social animals. Correct numerical assessment can assist an individual in predicting the chance of victory and avoiding an unfavourable contest. For example, lions (Panthera leo) and chimpanzees (Pan troglodytes) have been observed to compare group sizes before an encounter and enter a fight only when they have a numerical advantage over a competing group (Heinsohn 1997; Wilson et al. 2001).
Aggression refers to an individual's willingness to enter a fight (Wilson 1975). Numerical assessment is only one of many factors that have been suggested to alter an individual's aggression. Other possible factors include distance between competitor's territories (Knaden & Wehner 2003), encounter rates between enemies (Langen et al. 2000; Katayama & Suzuki 2005), asymmetrical competitive abilities (Savolainen & Vepsäläinen 1988), differences in body sizes (Nowbahari et al. 1999) and owner–intruder relationships (Enquist & Leimar 1987; Adams 1990). In addition, several studies have shown that for social organisms, an individual is more likely to be aggressive as the number of aggressive group members nearby increases (Wilson 1971; Sands 1982; Zabel et al. 1992; Sakata & Katayama 2001). This process of escalated aggression has been called social facilitation (Hölldobler & Wilson 1990). Numerical assessment is subtly different from social facilitation in that the decision to be aggressive does not require either an agonistic exchange or a signal from another group member.
The relationship between numerical and individual advantage is fundamental to Lanchester's laws of combat (Lanchester 1916). According to these laws, the fighting abilities of each individual within a group and each group's numerical strength affect the mortality rates and killing rates of each side (Adams & Mesterton-Gibbons 2003). Numerical strength, however, must be considered carefully because the number of individuals present is not necessarily the same as the number of individuals that choose to fight (Kitchen et al. 2004). The likelihood of winning a fight increases as the number of aggressive individuals with excellent fighting abilities entering the battle increases. Numerical assessment can influence competitive interactions by affecting the number of aggressive individuals that enter a fight when groups of social animals compete.
No previous study to my knowledge has investigated how a social invertebrate's ability to assess its numerical state (whether isolated or part of a group) prior to a competitive interaction affects both the individual's and group's resource-holding potential versus a naturally occurring competitor. In this study, I used controlled arena experiments to determine if an individual ant can alter its level of aggression by assessing its own group's numerical state prior to an encounter with a natural competitor. If ants can assess their numerical state, and if this assessment modifies their aggression level, then ants that perceive themselves as part of a large group of nest-mates should be more aggressive toward a competitor than ants that perceive themselves as isolated individuals. To test this hypothesis, I adjusted the amount of nest-mate contact prior to a competitive encounter for one ant species while holding the level of nest-mate contact constant for the other species. I then examined the relationship between aggression, numerical state, and the consequences of aggression such as the effect on resource-holding potential, mortality, and killing ability for groups and individuals.
2. Material and methods
(a) Study site
The field site for all experiments was located in a common garden stand of Cottonwood trees (Populus spp.) (see Wimp & Whitham (2001) for details). Two congeneric ant species (Formica xerophila and Formica integroides) tend aphids (Chaitophorus populicola) on Cottonwood trees. At the beginning of the summer, scouts from both ant species investigate all the nearby trees. By midsummer, entire trees are segregated between the two ant species. Workers from nests of both ant species defend multiple trees as discrete territories, but trees that are defended by either F. xerophila or F. integroides are spatially intermingled. Formica xerophila on trees are monomorphic for body size and range in length from 4.5 to 6.5 mm, while F. integroides on trees are continuously polymorphic for body size ranging from 4.5 to 10.5 mm (C. J. Tanner 2005, unpublished data). Groups of 4–5 individual F. xerophila work together to tend and defend aphid patches. A single, large F. integroides defends multiple aphid patches, while several smaller F. integroides workers tend the aphids. On the ground, both species forage for dead arthropods individually. Preliminary studies revealed that F. integroides on the ground are approximately 40% larger than F. xerophila and chase away any F. xerophila foraging at ground baits (C. J. Tanner 2005, unpublished data). Formica xerophila retreated from every (_n_=31) encounter at a bait with F. integroides. Nevertheless, F. xerophila workers are able to successfully acquire and defend a number of trees, each with many aphid patches. The experimental fights in this study were designed to investigate the importance of F. xerophila group size on ant–ant competitive interactions.
(b) Experimental design
Ants of both species were aspirated from aphid patches from trees which were 1 m away from their respective nests. Only large F. integroides (greater than 8 mm) were collected. The ants were placed in round plastic storage containers (10 cm high, 12 cm diameter) with approximately 2 cm of soil on the bottom of each container. All ants were stored without food to control for any resource value asymmetries during contests, and all ants were conditioned in their respective storage containers for 20–40 min prior to a contest. Each storage container had only one species from one nest, and F. xerophila storage containers were further divided into sparse (five individuals per container) or dense (greater than 30 individuals per container) treatment groups. All F. integroides storage containers had dense conditions. In the 1 versus 1 contestant number trials, a single F. xerophila was taken from either the sparse or the dense treatment storage container and was dropped simultaneously with a single F. integroides into an arena identical to the storage containers except for the addition of a piece of tuna soaked in fruit juice to be used as a resource. In the 5 versus 5 contestant number trials, five individuals from either the sparse or the dense treatment storage containers were dropped simultaneously with five F. integroides into an arena with the same resource. In no trial did either species have any arena acclimatization advantage over the other species. The soil in the container bottom was mixed completely between each trial and was replaced after 20 trials. The tuna was replaced after 5 trials. This design led to four combinations of contestant number and storage treatment that consisted of: sparse storage treatment with 1 versus 1 contestant number; sparse storage treatment with 5 versus 5 contestant number; dense storage treatment with 1 versus 1 contestant number; and dense storage treatment with 5 versus 5 contestant number. I performed 20 trials of each contestant number-by-storage container density combination for a total of 80 trials.
Observations included F. xerophila aggression (lungeing toward, biting, and engaging _F. integroides_—scored as yes/no for 1 versus 1 trials, and total number of aggressors for 5 versus 5 trials), the number of encounters between F. xerophila and F. integroides before an aggressive display by F. xerophila, the number of individuals killed of each species and contest winner (a measure of resource-holding potential). Upon encountering the tuna, ants of both species would either begin dragging it away or eating it. Resource-holding potential was found by recording which species encountered and successfully held the resource for more than 30 s, which was the shortest time noted for an ant of either species to remove an item from a bait site of potential conflict in the field. Contests in which F. xerophila defended the resource were scored as ‘1’, contests in which F. integroides defended the resource were scored as ‘−1’, and draws were scored as ‘0’. Most 1 versus 1 contests lasted approximately 5–10 min, and a draw was declared if neither ant had won within 20 min. The 5 versus 5 contests were usually decided within 5 min, and a draw was declared if neither species had won within 20 min. All the individuals from the 1 versus 1 trials were collected and measured in the lab using a dissecting microscope.
The above procedure included three interspecific pairs of F. integroides and F. xerophila nests. Each interspecific nest pair tended aphids on trees near one another and did not overlap with ants on trees from other nest pairings (mean distance between nest pairs was 6 m, and mean distance between pairs of nests was 18 m). A preliminary experiment revealed that intraspecific contests between these three nests would not yield aggressive interactions in any case. Ants from each nest and for each storage container density were stored separately, and all possible combinations of interspecific ant contests from each nest were included two or three times. An initial Kruskal–Wallis test was used to investigate the effect of nest on contest winner, F. xerophila and F. integroides aggression. In no case did the nest have a significant effect in either the 1 versus 1 or 5 versus 5 trials. So, the data were pooled within each group for further analyses.
3. Results
(a) Total experiment (combination of 1 versus 1 and 5 versus 5 contests)
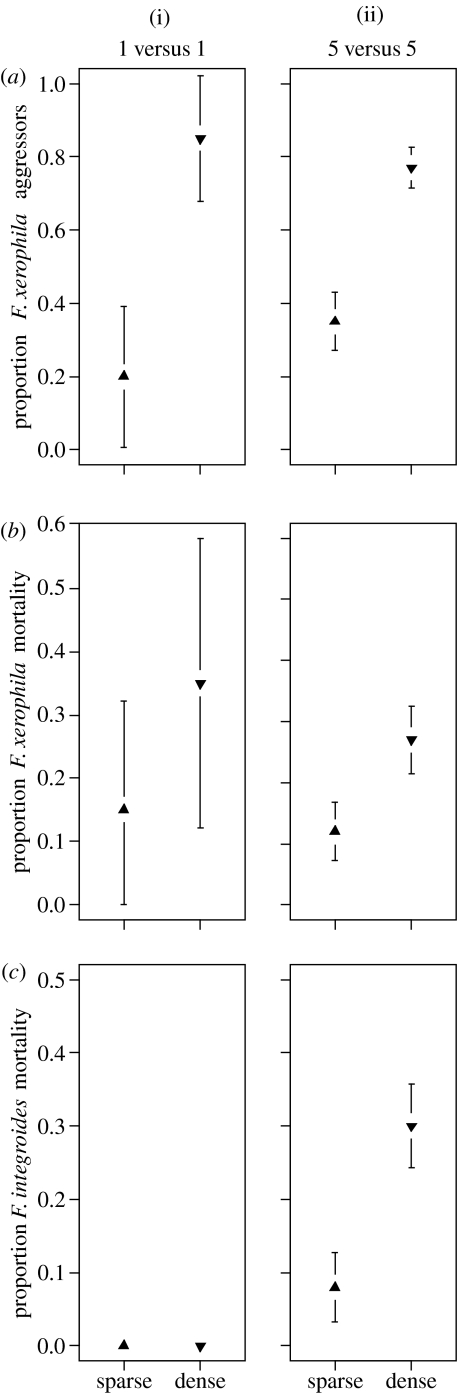
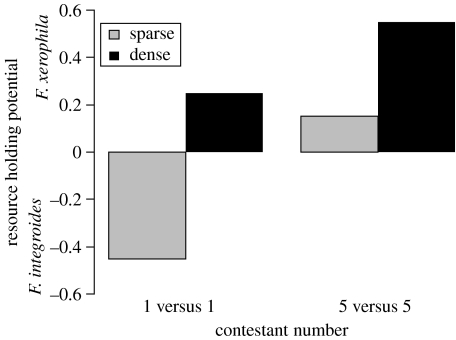
In 97% of all trials where F. xerophila acted aggressively, it did so in its first encounter with F. integroides. The remaining 3% of aggressive events occurred in the second encounter. Regardless of contestant number (1 versus 1 or 5 versus 5), storage container treatment (sparse or dense) had a significant effect on F. xerophila aggression (Cochran–Mantel–Haenszel test, _S_=39, p<0.001). Formica xerophila from dense storage containers were more aggressive than F. xerophila from sparse storage containers (figure 1). Contestant number had no effect on F. xerophila aggression after controlling for storage container treatment (_S_=21, _p_=0.697). Individual F. xerophila from dense storage containers were just as likely to be aggressive in 1 versus 1 contests as they were in 5 versus 5 contests. Storage container treatment also had a significant effect on F. xerophila mortality after controlling for contestant number (_S_=15, _p_=0.004). Formica xerophila from dense storage container treatments died more frequently than the F. xerophila from sparse storage container treatments (figure 1). Contestant number had no effect on F. xerophila mortality after controlling for storage container treatment (_S_=10, _p_=0.511). An individual F. xerophila was just as likely to die in a 1 versus 1 contest as a 5 versus 5 contest. Both contestant number (_S_=8, _p_=0.013) and storage container treatment (_S_=8, _p_=0.013) had significant effects on F. xerophila's resource-holding potential. Formica xerophila competing as a group (5 versus 5 contestant number) had a better resource-holding potential than F. xerophila competing as individuals (1 versus 1 contestant number), and F. xerophila from dense storage container treatments had a better resource-holding potential than F. xerophila from sparse storage container treatments (figure 2).
Figure 1.
(a) Mean proportion (±95% confidence intervals) of F. xerophila that act aggressively in contests for a resource in (i) the 1 versus 1 and (ii) 5 versus 5 contestant number trials. Sparse (filled up triangle) and dense (filled down triangle) refer to storage container treatment conditions. Aggressive F. xerophila were given a score of ‘1’ and F. xerophila that retreated were given a score ‘0’. (b) Mean proportion (±95% confidence intervals) of F. xerophila deaths in (i) the 1 versus 1 and (ii) 5 versus 5 contestant number trials. (c) Mean proportion (±95% confidence intervals) of F. integroides deaths in (i) the 1 versus 1 and (ii) 5 versus 5 contestant number trials.
Figure 2.
Score of contest outcomes (measure of resource-holding potential) for F. xerophila versus F. integroides. A value of +1 signifies F. xerophila victory, and a score of −1 signifies F. integroides victory. Each bar represents a different group of 20 contests. Grey bars represent sparse storage treatment conditions for F. xerophila. Black bars represent dense F. xerophila storage treatment conditions. 1 versus 1 and 5 versus 5 refer to contestant number.
(b) 1 versus 1 contestant number trials
Formica xerophila from dense storage container treatments were more aggressive than those from sparse storage container treatments (generalized linear model, family: Binomial, _Z_=−3.718, p<0.001). Formica xerophila body size, F. integroides body size and the interaction between body sizes were all not significant in explaining F. xerophila aggression (_p_=0.151, 0.521 and 0.955, respectively). The proportion of aggressive F. xerophila that died (10 out of 21) was greater than that of the non-aggressive F. xerophila (0 out of 19) (one-sided Fisher's exact test, p<0.001). Aggressive behaviour was also significant in explaining F. xerophila's resource-holding potential (ordinal logistic regression, Wald _Z_=3.94, p<0.001). More aggressive behaviour from F. xerophila led to an improved resource-holding potential. Of the 21 aggressive F. xerophila (17 from dense and 4 from sparse storage treatments), F. integroides retreated six times. Aggressive F. xerophila won six trials, and five of these six times occurred after F. integroides retreated. Of the 15 fights between aggressive F. xerophila and F. integroides, 10 ended in F. xerophila deaths.
(c) 5 versus 5 contestant number trials
The number of aggressive F. xerophila per trial from dense storage container treatments was greater than that of the sparse storage container treatments (generalized linear model, _t_=−9.086, p<0.001). An increase in the number of aggressive F. xerophila in each trial significantly increased F. xerophila's resource-holding potential (ordinal logistic regression, Wald _Z_=2.72, _p_=0.007). An increase in the number of aggressive F. xerophila in each trial also significantly increased the number of F. xerophila deaths (generalized linear model, _t_=3.94, p<0.001). The number of F. xerophila lost in each trial had no significant effect on resource-holding potential (ordinal logistic regression, Wald _Z_=−0.58, _p_=0.560). The number of F. integroides lost, however, did significantly affect F. xerophila's resource-holding potential (ordinal logistic regression, Wald _Z_=2.95, _p_=0.003). A greater number of aggressive F. xerophila led to an increase in F. integroides mortality, and an increase in F. integroides mortality improved F. xerophila's resource-holding potential (figure 1).
4. Discussion
These results suggest that, in a way similar to chimpanzees (Wilson et al. 2001) and lions (Heinsohn 1997), individual F. xerophila are capable of assessing their own group's numerical state prior to a competitive encounter, and are more likely to be aggressive when they recognize themselves as part of a larger group. Likewise, F. xerophila that perceive themselves to be isolated are more timid and avoid costly confrontations (figure 1). This process, presumably due to a physiological change (Wilson 1975), occurred before any interspecific interaction or conspecific behavioural display took place. The number of ants in the contest did not affect the aggression levels. Only storage container density, a condition prior to any encounter, affected an individual's decision to enter a fight (i.e. it was not social facilitation).
The experimental fights in this study lasted longer and ended in more fatalities than observed encounters in the field. When these two species meet on aphid patches, the occupant species takes the role of aggressor and the intruder usually retreats by either running away or falling from the tree. Retreat was not as feasible in these experimental fights when compared to encounters in the wild. Therefore, the escalated fighting and subsequent fatalities probably suggest what would happen if the intruder could not retreat.
Aggressive F. xerophila from dense storage containers were more successful in gaining a resource (figure 2) and died more often (figure 1) than their conspecifics from sparse storage containers. Formica xerophila from sparse containers usually retreated from F. integroides. Formica xerophila from dense containers, however, usually attacked F. integroides. If F. integroides retreated, then F. xerophila usually went on to gain the resource. If F. integroides attacked back, however, a fight ensued, which often ended in F. xerophila fatality. These results are consistent with Searcy & Nowicki (2005), who suggested that aggressive displays that are most successful in causing a competitor to retreat are usually the same displays that cause a competitor to attack.
Signals such as aggression can express information about intent or ability to a competitor (Ciani 2000; Searcy & Nowicki 2005). Formica xerophila aggression probably conveyed significant information to F. integroides regarding F. xerophila's expected numerical state. This is an interesting case to examine potential signals because F. xerophila's intent might depend on its ability. Formica integroides encountering an aggressive F. xerophila could have recognized this behaviour as a signal that more F. xerophila were nearby. Groups of F. xerophila have an advantage over F. integroides, and it would be useful information for F. integroides to know how many F. xerophila it would be facing if a fight occurred. Manipulation of the degree of aggression, even in 1 versus 1 contests, changed the ant–ant dynamics and competitive outcome. Therefore, F. xerophila aggression appears to convey meaningful information to F. integroides. This prospective signalling mechanism provides a possible explanation for the lack of escalated fighting at resource patches in the field. The correlation between intent and ability probably presents a cost to F. xerophila in displaying an aggressive signal. If individual F. xerophila regularly display aggressive behaviour, then F. integroides should not connect F. xerophila aggression with group size and should attack aggressive F. xerophila. Most individual fights between F. xerophila and F. integroides end in F. xerophila fatalities. This cost is likely to be important in maintaining honest signalling from F. xerophila.
In addition to aggression, contestant number was important for F. xerophila's resource-holding potential (figure 2). Formica xerophila were more successful at defending a resource in 5 versus 5 contests than in 1 versus 1 contests. Contestant number, however, had no effect on F. xerophila mortality (figure 1). The effect of contestant number on resource-holding potential was due to the individual behaviours of the F. xerophila workers. Individual F. xerophila workers displayed the same level of aggressive behaviour in 1 versus 1 and 5 versus 5 trials, but in the 5 versus 5 contests, groups of aggressive F. xerophila fought with single F. integroides worker. As many as four, but usually two or three, aggressive F. xerophila would simultaneously engage a single F. integroides. Fighting in groups improved F. xerophila's competitive ability without increasing its risk of death. For this reason, F. xerophila have an advantage by attacking as a group rather than as an individual. Within the 5 versus 5 contests, the number of F. xerophila that would engage in a fight affected the outcome and was related to individual aggression levels (and hence storage container treatment). Therefore, similar to Kitchen et al. (2004), I found that the number of aggressive social individuals present (or functional group size), and not the absolute number, determined the group's fighting ability. Many aggressive F. xerophila workers resulted in several dynamic divide-and-conquer skirmishes. This type of behaviour is consistent with the divide-and-conquer strategy suggested by Franks & Partridge (1993) for numerically dominant competitors. Although neither species had a global numerical advantage, by attacking in groups F. xerophila locally outnumbered F. integroides at each engagement. There are several species of ants with substantial individual fighting abilities that produce propaganda substances to deter their more numerous enemies from attacking in groups (Franks & Partridge 1993). In the experiments that I conducted, however, there was no evidence that F. integroides fought with any kind of group strategy, or were capable of stopping F. xerophila from using a group strategy, even though F. xerophila was not actually numerically dominant.
The effect of group size on fighting ability (measured in rates of kills and losses) is not always clear (Franks & Partridge 1993; Adams & Mesterton-Gibbons 2003; Plowes & Adams 2005). Franks & Partridge (1993) suggested that a larger fighting force ensures fewer casualties, although Plowes & Adams (2005) did not find this to be the case for Solenopsis invicta. Although the study presented here did not explicitly test for the effects of numerical dominance (both sides had equal numbers in all trials), I found that group size affected F. xerophila killing rate and not mortality rate (figure 1). Attacking as a group created localized numerical dominance, and in doing so provided a mechanism for a nonlinear positive relationship between F. xerophila group size and killing rate. Furthermore, F. xerophila mortality did not affect F. xerophila's resource-holding potential, but F. integroides mortality did. Aggressive F. xerophila were more likely to both gain the resource and die. So, in this case, the winning group did not necessarily suffer fewer casualties. A decline in F. integroides numbers led to improved F. xerophila's resource-holding potential because fewer F. integroides presented less necessity to fight, and more opportunity to discover/maintain the resource. This nonlinearity between group number and killing ability is not conveyed in typical Lanchester models of fighting and should be considered more closely in future theoretical and empirical analyses. In the same way that an increase in functional group size due to aggression has a nonlinear positive effect, a decrease in functional group size due to mortality could have a nonlinear negative effect on resource-holding potential, which would present an additional cost to worker loss not yet considered.
Prior to these experiments, F. integroides was thought to be the behaviourally dominant ant species in this community (Wimp & Whitham 2001). It is clear from these results that when F. xerophila act aggressively and operate in groups, they are more successful than F. integroides. In trees, F. xerophila are found in groups and successfully defend aphids. On the ground, however, where the ants forage for protein, F. xerophila move as individuals and lose competitive encounters with F. integroides. It is not clear why F. xerophila do not travel as aggressive groups in a manner similar to Argentine ants (Linepithema humile) (Holway & Suarez 1999). The difference in the distribution of resources between trees and ground probably has an effect on the competitive dynamics between the two ant species (Vahl et al. 2005). This suggestion, however, requires further investigation.
Regardless of any other factor affecting aggression, these results have shown that numerical assessment can dictate an individual ant's aggression and affect its competitive ability in complex ways. The level of aggression for individuals and the numerical relationship between sides can work in partnership to affect the group's functional size. Therefore, numerical assessment should be considered more closely in empirical and theoretical studies involving various types of interference competition.
Acknowledgements
I thank Y. Uemura for his help in the field. I am also grateful to F. Adler, K. Houck, E. Adams and P. Nonacs for their valuable discussions and comments on the earlier drafts of this paper. Two anonymous reviewers added their helpful comments. R. N. Snelling identified both Formica species.
References
- Adams E.S. Boundary disputes in the territorial ant Azteca trigona: effects of asymmetries in colony size. Anim. Behav. 1990;39:321–328. doi:10.1016/S0003-3472(05)80877-2 [Google Scholar]
- Adams E.S, Mesterton-Gibbons M. Lanchester's attrition models and fights among social animals. Behav. Ecol. 2003;14:719–723. doi:10.1093/beheco/arg061 [Google Scholar]
- Ciani A.S. When to get mad: adaptive significance of rage in animals. Psychopathology. 2000;33:191–197. doi: 10.1159/000029142. doi:10.1159/000029142 [DOI] [PubMed] [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: the effect of variation in resource value. J. Theor. Biol. 1987;127:187–205. [Google Scholar]
- Franks N.R, Partridge L.W. Lanchester battles and the evolution of combat in ants. Anim. Behav. 1993;45:197–199. doi:10.1006/anbe.1993.1021 [Google Scholar]
- Heinsohn R. Group territoriality in two populations of African lions. Anim. Behav. 1997;53:1143–1147. doi: 10.1006/anbe.1996.0316. doi:10.1006/anbe.1996.0316 [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Holway D.A, Suarez A.V. Animal behavior: an essential component of invasion biology. Trends Ecol. Evol. 1999;14:328–330. doi: 10.1016/s0169-5347(99)01636-5. doi:10.1016/S0169-5347(99)01741-3 [DOI] [PubMed] [Google Scholar]
- Katayama N, Suzuki N. The importance of the encounter rate between ants and herbivores and of ant aggressiveness against herbivores in herbivore exclusion by ants on Vicia angustifolia (Leguminosae) with extrafloral nectaries. Appl. Entomol. Zool. 2005;40:69–76. doi:10.1303/aez.2005.69 [Google Scholar]
- Kitchen D.M, Horwich R.H, James R.A. Subordinate male black howler monkey (Alouta pigra) responses to loud calls: experimental evidence for the effects of intra-group male relationships and age. Behaviour. 2004;141:703–723. doi:10.1163/1568539042245196 [Google Scholar]
- Knaden M, Wehner R. Nest defense and conspecific enemy recognition in the desert ant Cataglyphis fortis. J. Insect Behav. 2003;16:717–729. doi:10.1023/B:JOIR.0000007706.38674.73 [Google Scholar]
- Lanchester F.W. Aircraft in warfare. Appleton; New York, NY: 1916. [Google Scholar]
- Langen T.A, Tripet F, Nonacs P. The red and the black: habituation and the dear-enemy phenomenon in two desert Pheidole ants. Behav. Ecol. Sociobiol. 2000;48:285–292. doi:10.1007/s002650000223 [Google Scholar]
- Maynard Smith J. Evolution and the theory of games. Cambridge University Press; Cambridge, UK: 1982. [Google Scholar]
- Nowbahari E, Fénéron R, Malberbe M. Effect of body size on aggression in the ant, Cataglyphis niger (Hymenoptera; Formicidae) Aggressive Behav. 1999;25:369–379. doi:10.1002/(SICI)1098-2337(1999)25:5<369::AID-AB5>3.0.CO;2-C [Google Scholar]
- Plowes N.J.R, Adams E.S. An empirical test of Lanchester's square law: mortality during battles of the fire ant Solenopsis invicta. Proc. R. Soc. B. 2005;272:1809–1814. doi: 10.1098/rspb.2005.3162. doi:10.1098/rspb.2005.3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Katayama N. Ant defence system: a mechanism organizing individual responses into efficient collective behavior. Ecol. Res. 2001;16:395–403. doi:10.1046/j.1440-1703.2001.00404.x [Google Scholar]
- Sands W.A. Agonistic behavior of African soldierless Apicotermitinae (Isoptera: Termiditae) Sociobiology. 1982;7:61–73. [Google Scholar]
- Savolainen R, Vepsäläinen K. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos. 1988;51:135–155. [Google Scholar]
- Searcy W.A, Nowicki S. Princeton University Press; Princeton, NJ: 2005. The evolution of animal communication. [Google Scholar]
- Traniello J.F.A, Beshers S.N. Maximization of foraging efficiency and resource defense by group retrieval in the ant Formica schaufussi. Behav. Ecol. 1991;29:283–289. doi:10.1007/BF00163986 [Google Scholar]
- Vahl W.K, Lok T, van der Meer J, Piersma T, Weissing F.J. Spatial clumping of food and social dominance affect interference competition among ruddy trunstones. Behav. Ecol. 2005;16:834–844. doi:10.1093/beheco/ari067 [Google Scholar]
- Wilson E.O. The insect societies. Belknap Press; Cambridge, MA: 1971. [Google Scholar]
- Wilson E.O. Sociobiology. Harvard University Press; Cambridge, MA: 1975. [Google Scholar]
- Wilson M.L, Hauser M.D, Wrangham R.W. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav. 2001;61:1203–1216. doi:10.1006/anbe.2000.1706 [Google Scholar]
- Wimp G.M, Whitham T.G. Biodiversity consequences of predation and host plant hybridization on an aphid–ant mutualism. Ecology. 2001;82:440–452. doi:10.2307/2679871 [Google Scholar]
- Zabel C.J, Glickman S.E, Frank L.G, Woodmansee K.B, Keppel G. Coalition formation in a colony of prepubertal spotted hyenas. In: Harcourt A.H, De Wall F.B.M, editors. Coalitions and alliances in humans and other animals. Oxford University Press; Oxford, UK: 1992. pp. 113–135. [Google Scholar]