Self-recognition in an Asian elephant (original) (raw)
Abstract
Considered an indicator of self-awareness, mirror self-recognition (MSR) has long seemed limited to humans and apes. In both phylogeny and human ontogeny, MSR is thought to correlate with higher forms of empathy and altruistic behavior. Apart from humans and apes, dolphins and elephants are also known for such capacities. After the recent discovery of MSR in dolphins (Tursiops truncatus), elephants thus were the next logical candidate species. We exposed three Asian elephants (Elephas maximus) to a large mirror to investigate their responses. Animals that possess MSR typically progress through four stages of behavior when facing a mirror: (i) social responses, (ii) physical inspection (e.g., looking behind the mirror), (iii) repetitive mirror-testing behavior, and (iv) realization of seeing themselves. Visible marks and invisible sham-marks were applied to the elephants' heads to test whether they would pass the litmus “mark test” for MSR in which an individual spontaneously uses a mirror to touch an otherwise imperceptible mark on its own body. Here, we report a successful MSR elephant study and report striking parallels in the progression of responses to mirrors among apes, dolphins, and elephants. These parallels suggest convergent cognitive evolution most likely related to complex sociality and cooperation.
Keywords: cognition, mirror self-recognition, theory of mind, intelligence, empathy
Mirror self-recognition (MSR) is exceedingly rare in the animal kingdom (1). Attempts to demonstrate MSR outside of the Hominoidea (i.e., humans and apes) have thus far failed (2), with the notable exception of one report on dolphins (Tursiops truncatus) (3). Animals that demonstrate MSR typically go through four stages: (i) social response, (ii) physical mirror inspection (e.g., looking behind the mirror), (iii) repetitive mirror-testing behavior (i.e., the beginning of mirror understanding), and (iv) self-directed behavior (i.e., recognition of the mirror image as self) (3, 4). The final stage is verified if a subject passes the “mark test” by spontaneously using the mirror to touch an otherwise imperceptible mark on its own body (1). Application of the mark is recommended only if the preceding criteria have been met (5). Animals without MSR tend to remain at stages 1 and 2. Even if the degree to which the mirror image is confused with a stranger is debatable for some non-MSR species (6), these animals likely lack understanding of who is in the mirror.
Gallup was the first to hypothesize about a phylogenetic connection between MSR and empathy (7), a connection supported by evidence for consolation behavior in apes but not monkeys (8, 9). A possible ontogenetic connection between MSR and empathy is reflected in the coemergence of MSR and “sympathetic concern” during child development (10, 11). Dolphins and elephants represent interesting additions to MSR tests because, like the hominoids (8, 12), they are highly empathic animals known for so-called “targeted helping” [i.e., helping that takes the specific needs of others into account (8)] aimed at both conspecifics and humans. As in dolphins (13, 14), there are numerous reports of elephants physically supporting or trying to lift up injured or incapacitated conspecifics (15–17). In view of the aforementioned hypothetical connection with empathy, the elephant's known social complexity (15, 16, 18) and its relatively large and complex brain (19, 20), we introduced three adult female Asian elephants (Elephas maximus) at the Bronx Zoo in New York City to a jumbo-size mirror (244 × 244 cm) in a variant of the classical mark test (1) using both visual and “sham” marks (3).
Elephants have the advantage that they can touch most of their own body with their trunks, thus permitting an unequivocal mark test. A previous failed attempt to demonstrate MSR in two Asian elephants presented the animals with a relatively small mirror that was kept at a distance, well out of trunk-reach (21). Assuming that physical exploration of the mirror surface should be part of the learning process (5) and that mirror size matters, we built an almost 2.5-m-tall elephant-resistant mirror to allow close-up inspection of the reflective surface (Fig. 1). Here we demonstrate that all three subjects reached the aforementioned third and fourth stages of MSR progression and that one subject passed the mark test.
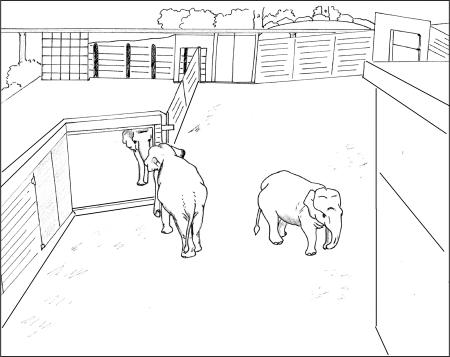
Fig. 1.
Elephant yard with open mirror. A drawing of a typical open-mirror session (drawing by F. Plotnik from a still taken from the rooftop camera). One elephant stands at the mirror, while another stands off to the side. The elephant yard in which the mirror was installed is not visible to the public.
Results and Discussion
Baseline, Controls, and Initial Mirror Exposure.
There were five experimental phases: baseline (T1), covered mirror (T2), open mirror (T3), covered-mirror sham (T4), and the mark test (T5). Happy, Maxine, and Patty all spent far more time close to the mirror during 3 days of open vs. covered mirror (i.e., T3 vs. T2; Happy, 19.4 vs. 0.2%; Maxine, 37.9 vs. 1.3%; and Patty, 49.7 vs. 3.3%), indicating that time spent at the mirror was due to its reflective qualities rather than to the novelty of the apparatus. During T3, all three subjects showed investigative behavior of the mirror surface and frame including touching and probable sniffing. For Maxine and Patty, trunk-over-wall exploration (i.e., the swinging of the trunk over and behind the wall on which the mirror was mounted) declined from the first through the fourth day of mirror exposure (Maxine, 10 to 0 times; Patty, 13 to 4 times). Happy never put her trunk over the mirror wall. Maxine and Patty also attempted to physically climb the mirror wall to look over and behind it (see Movie 1, which is published as supporting information on the PNAS web site), and both, on separate occasions, seemed to try to get their trunks underneath and behind the mirror by kneeling down in front of it. Their behavior was highly unusual (i.e., animal-care staff had rarely observed similar attempts by the elephants to look over or underneath enclosure walls). Remarkably, all three subjects showed a total absence of social interaction with their mirror image, such as species-typical visual, vocal, or agonistic displays (15, 16, 18, 22).
All three elephants displayed behavior consistent with mirror-testing and self-directed behavior during T3 (open mirror) and T5 (mark test), such as bringing food to and eating right in front of the mirror (a rare location for such activity), repetitive, nonstereotypic trunk and body movements (both vertically and horizontally) in front of the mirror, and rhythmic head movements in and out of mirror view; such behavior was not observed in the absence of the mirror (see Movie 2, which is published as supporting information on the PNAS web site, for an example). On more than one occasion, the elephants stuck their trunks into their mouths in front of the mirror or slowly and methodically moved their trunks from the top of the mirror surface downward. In one instance, Maxine put her trunk tip-first into her mouth at the mirror, as if inspecting the interior of her oral cavity, and in another instance, she used her trunk to pull her ear slowly forward toward the mirror. Because these behaviors were never observed in T1 or T2 (the initial, “no mirror” control conditions), or at any other time, they indicate the elephants' tendency to use the mirror as a tool to investigate their own bodies. Apes are known for very similar self-investigation in front of the mirror, such as picking with their fingers at their teeth (1, 23), which is considered a precondition for the mark test (5). Similar to the time frame observed in chimpanzees (1), Happy reached this criterion for the mark test within 3 days, and Maxine and Patty reached this criterion within 4 days.
The Mark Test.
On the first day of the mark test (T5), a visible mark (Fig. 2) was applied to the right side of each elephant's head, and an invisible sham-mark was applied to the left side of the head. The sham-mark controlled for both olfactory and tactile cues (i.e., texture), leaving only a visual component to differentiate between mark and sham-mark (see Supporting Text, which is published as supporting information on the PNAS web site, for the chemical composition of the marking substances). Lone sham-marks had been used previously in T4 to test this control while avoiding habituation by the other elephant to the visual component of the mark. The elephants never touched the sham-mark under this previous condition, suggesting the predicted absence of odor or tactile cues. A controlled-mark condition similar to T4, but using the visual mark instead of the sham-mark in a covered-mirror condition, would have been an ideal addition to our testing procedure but could not be implemented because of the presence of the elephant's partner. Husbandry concerns prevented us from isolating each elephant during testing; hence any visual mark might have attracted the partner's attention and risked the loss of mark salience by the time actual mark tests were conducted in front of the open mirror.
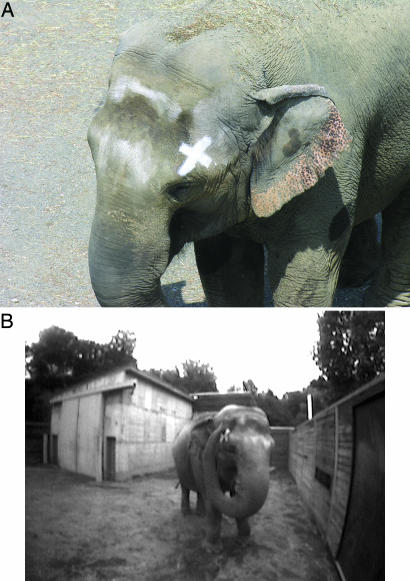
Fig. 2.
Mark and mark-touching. (A) Happy with a visual X-shaped mark on her head, (B) Happy at the mirror touching the mark with the tip of her trunk. This still image was captured from a video camera embedded in the mirror. The locations of the mark and the sham-mark were counterbalanced on the left and right side of the elephant's head on consecutive mark days. Also see Movies 1–3.
One elephant, Happy, passed the mark test on the first day of marking. Caretakers did not notice her touching either the mark or sham-mark before being released into the elephant yard. After being released into the yard, she walked straight to the mirror where she spent 10 seconds, then walked away. Seven minutes later she returned to the mirror, and over the course of the next minute she moved in and out of view of the mirror a couple of times, until she moved away again. In the following 90 seconds, out of view of the mirror, she repeatedly touched the visible mark but not the sham-mark. She then returned to the mirror, and while standing directly in front of it, repeatedly touched and further investigated the visible mark with her trunk (see Movie 3, which is published as supporting information on the PNAS web site).
Combining all experimental phases (T1–T5), Happy touched her own head with her trunk a total of 47 times. Comparing the frequency distribution of head touches across three aggregate conditions [i.e., (i) the first mark test, (ii) the open-mirror tests before marking, and (iii) all nonmirror conditions combined] with the expected frequency distribution based on Happy's observation time under those three conditions, a significant difference was found (χ2 = 130.83, df = 2, P < 0.001). On the mark day itself, Happy showed dramatically increased head touching early in the session, most of which (i.e., 12 of 14 times; Fig. 3; measured as rate per minute) occurred during or within 90 seconds after proximity to the mirror. All 12 touches during or right after mirror exposure came in contact with or close to (within 20 cm) the visible mark on the right side of Happy's head. Head touches never came in contact with or close to the sham-mark on the left side of Happy's head (binomial test 12 vs. 0, z = 3.18, P = 0.0008). Happy's right-side vs. left-side bias on the first mark day differed significantly from that for head touches on nonmark days (Fisher's exact test, P = 0.025). In other words, Happy's touching of the right side of her head (particularly the mark itself) on the first mark day deviated from her general head touching during all previous conditions in both its higher frequency and its bias toward the side with the visible mark. In addition, although female African elephants (Loxodonta africana) often touch their heads because of temporal gland secretion (15), female Asian elephants do not; their temporal gland is bilateral, vestigial, and nonsecretory (24), thus eliminating concerns about their propensity to touch this area.
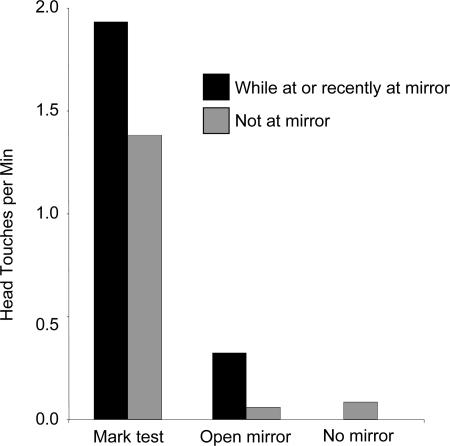
Fig. 3.
Rate of head touching by Happy across four conditions. For mark tests and open-mirror tests, the black bars show the rate per minute of self-touching by Happy while at the mirror or within 90 seconds of having stepped away from the mirror. Gray bars show the rate of touching before mirror exposure or >90 seconds after having left the mirror location. For comparison, the self-touching rate during covered mirror and baseline is provided (“no mirror”), which is the combined data for the mirror-closed (T2) and mirror-absent (T1) conditions.
Passing and Failing the Mark Test.
In contrast to Happy, Maxine and Patty failed to show increased self-touching of either the mark or sham-mark. Maxine and Patty were marked twice; Happy was marked three times. On the second day of marking, Happy never approached the mirror nor did she touch either the mark or sham-mark. She was re-marked on a third day during which time she stationed herself at the mirror but touched neither the mark nor the sham-mark. We repeated the marking procedure on all three elephants after 2 months; none of them touched either the mark or sham-mark during this second phase of marking, although all of them continued to show self-directed behavior at the mirror.
The fact that one elephant passed the mark test but two did not is not inconsistent with data recorded for other species because even in the most extensively tested species with MSR, the chimpanzee (Pan troglodytes), fewer than half of the individuals may pass the test according to some studies (23, 25). Similarly, absence of responses to the mark after multiple exposures resembles the reaction of MSR-capable apes, which generally lose interest in the mark within minutes of mirror exposure, apparently realizing that the mark is inconsequential (26). For this reason, multiple mark tests on a single individual are considered to compromise mark salience and are therefore uninterpretable (5).
Happy, Maxine, and Patty all continued to show self-directed behavior at the mirror, indicating that they may have only lost interest in the mark but not in their own reflection. Although we encourage further testing of Asian elephants, because of the overexposure of our three elephants to the marking materials and the descriptions of elephants' extraordinary memory (15, 16), we would not expect these three elephants to pass during further rounds of testing.
According to the manufacturer, the ingredients of the mark and sham-mark material (both face paints) are identical except for the pigmentation components (see Supporting Text); the titanium dioxide, which is used to make the mark paint “white,” is odorless according to its Material Safety Data Sheet (MSDS). The zinc sulfide, which is used to make the sham-mark paint luminescent, may have a slight odor, but we would expect that if the zinc sulfide odor in the sham-mark paint was detectable and differentiated from the mark by the elephants, they would have been attracted to the sham-mark rather than the visual mark. However, as our data show, no such attraction was evident. Therefore, we conclude that the odor and tactile components of the mark and the sham-mark are either equal or negligible and that any differential touching of the mark should be due to its visual component.
The later negative outcomes in all three subjects seem to confirm the absence of tactile and odor clues of the marks. One would further suspect a lack of “concern” about bodily appearance and cleanliness in elephants compared with primates. Whereas primates often groom specific spots on their bodies (27, 28), elephants rarely autogroom with their trunks (29, 30). Rather, they “substrate groom,” which includes dust-throwing and mud-bathing (30, 31). This manner of “grooming” actually adds debris to the body. It may very well be that because of an elephant's large body-surface area (29) and the mud and sand it often carries around on its body, attention to detail is not a priority. A small paint mark may be trivial to them.
The behavior of the elephants was strikingly similar to that of other animals who have demonstrated MSR. Although none of the elephants aimed social behavior at the mirror, they all, like the apes and dolphins, exhibited exploratory and mirror-testing behavior before more explicitly self-directed activities. Further studies on elephants of different ages, gender, and personal history will be needed to confirm and further elucidate the capacity for MSR in these animals. Our study suggests that mirror size and access to the mirror surface should be considered in replication attempts.
The mark-touching by one elephant is compelling evidence that this species has the capacity to recognize itself in a mirror. Finding strong parallels among apes, dolphins, and elephants in both the progression of behavioral stages and actual responses to a mirror provides compelling evidence for convergent cognitive evolution. Perhaps MSR indexes an increased self–other distinction that also underlies the social complexity and altruistic tendencies shared among these large-brained animals.
Materials and Methods
Subjects.
Subjects were adult female Asian elephants housed and observed in pairs (Happy/Samuel R. and Maxine/Patty) at the Bronx Zoo, located in New York City. The youngest elephant (Samuel R.) was not included in the study. Maxine (35 years of age), Patty (35 years of age), and possibly Happy (34 years of age) had infrequent but previous exposure to a small mirror that was leaned against a tree beyond their reach as part of their enrichment program. They were not exposed to any mirrors in the year before the present study; however, like all animals at the zoo, they had experience with reflective surfaces, such as pools of water.
Procedures.
Maxine and Patty were shifted from their holding area to the outdoor elephant yard for 1 h (≈0915–1015 h) each morning for observation and then shifted into a zoo exhibit no later than 1030 h. From 1115 to 1215 h, Happy and Samuel R. were also given access to the same elephant yard for observation, but in contrast to Maxine and Patty, they were not restricted to the yard. For husbandry reasons, they retained access to the holding area.
There were five experimental phases, during which each pair of elephants was videotaped from a roof above the exhibit yard: baseline (T1; 1 h per day for 4 days), covered mirror (T2; 1 h per day for 3 days), open mirror (T3; 1 h per day for 4 days), covered-mirror sham (T4; 1 h for 1 day), and the mark test (T5; 1 h per day, see Results and Discussion). A low-frequency sensitive microphone was installed behind the wall adjacent to the mirror to record elephant vocalizations (these data were not analyzed for this study). The mirror was built and installed over a period of 2 weeks but remained covered during T2 (covered mirror). For T3 (open mirror), three camera angles were used, one of which, as described previously, was operated from the roof above the elephant yard [using a Sony (Tokyo, Japan) PDF-150 digital video (miniDV) camera]. A second camera (Optura Xi miniDV camera; Canon, Lake Success, NY) with a side view of the mirror location was installed on a tripod located outside of the elephant yard at ground level. The third camera was a 3-mm-diameter charge-coupled device Elmo (Plainview, NY) lipstick color camera embedded in the mirror and interfaced with an external miniDV camera behind the mirror wall. Although the elephants were always videotaped for a period of ≈1 h per day, because the elephants had access to areas of the yard and building that were not visible, actual observation time varied depending on how much time each subject spent in camera view.
On the first day of mirror exposure for each pair, the holding area door was slightly opened for a period of 10 min to allow the subjects time to habituate to the mirror. The door was then fully opened to allow the elephants entrance into the yard. The elephants were observed for a previously undetermined number of days (but for the same length of daily observation time) until they reached behavioral criteria. Before the mark test, we conducted T4 (i.e., 1 day of sham-marking in a covered-mirror condition). The elephant was sham-marked in the center of her forehead and observed for 1 h. We then conducted 2 more days of covered-mirror observation (T2) immediately followed by 1 day of open-mirror observation (T3) before proceeding to the first mark test (T5). On marking days, one elephant per pair was marked and sham-marked by an elephant keeper in the holding area. Using three fingers, the X-shaped mark and same-shaped sham-mark (≈12 cm diagonal length and ≈4 cm wide) were applied to opposite sides of the elephant's head in the area diagonally above the eye and in front of the ear and counterbalanced on subsequent days of marking. Any touching of the mark and sham by the elephant before being released into the yard (a period that never exceeded 5 minutes) was noted by the keepers. After the elephants were released into the yard, all procedures were identical to those of the open-mirror condition (T3). The mark test was repeated on each of the three subjects after a 2-month break with a differently shaped mark following the aforementioned procedure. No two elephants in the same pair were ever marked on the same day, and all paint was removed after each mark test.
Mirror.
Two 0.6-cm-thick pieces of 122 × 244-cm acrylic (Plaskolite, Columbus, OH) mirror were glued to plywood to produce a full 244 × 244-cm mirror with a negligible yet fully braced seam down the middle. The mirror was then framed with steel support and bolted to the yard wall ≈30 cm off the ground. The mirror cover (i.e., a metal door painted with flat, nonreflective brown paint) was then installed with a reinforced hinge and back-supported with steel on the adjacent wall. The door was locked in either the open or the closed position depending on the experimental procedure.
Mirror Location.
The elephants were considered “at the mirror” if they were within 4 m of the mirror apparatus as judged by their position relative to a visible grating in the wall to the left of the mirror.
Coding Procedure.
While coding sessions where all three cameras were used (T3–T5), two tape decks and monitors were used to code behaviors while at the mirror. All sessions (T1–T5) were coded by using as many of the three tapes as was necessary to verify accuracy. In general, the roof camera tapes were coded, with the mirror camera and the side-view camera tapes used to verify the elephants' behaviors when facing the mirror (and thus when their backs were to the roof camera).
Data were coded by using a behavioral ethogram developed during baseline (T1) observations. All data were then organized and analyzed by using Excel PivotTables (Microsoft, Redmond, WA). All data were coded by J.M.P.; however, 100% agreement was reached on the frequency and location of all mark touches during T5 (the mark test) by J.M.P. and D.R., who coded Happy's first mark session independently.
Supplementary Material
Supporting Information
Acknowledgments
We thank Gordon Gallup, Jr., and Joyce Poole for their helpful comments on the study and manuscript. We are grateful to J. Mahoney, P. Thomas, P. Kalk, K. Theis, G. Stark, G. Gordian, G. Fergason, W. Canino, C. Vitale, and M. Medina of the Bronx Zoo Mammal Department for their assistance in conducting the study. We also thank R. Lattis and J. Breheny for supporting this project; the Bronx Zoo Machine and Carpentry Shops for construction of the mirror apparatus; T. Veltre, L. Groskin, J. Deveney, and D. Mulewski for audio/visual support; D. Moore, K. Payne, A. Murray, H. Lyn, and M. Maust for their assistance in this study; and J. McDowell and N. Bliwise for statistical advice. Finally, we thank Palmer Paint Products, Inc. for providing advice on the marking material. This project was conducted at the Wildlife Conservation Society (WCS)'s Bronx Zoo and was supported by WCS's Living Institutions Animal Enrichment Program, the Living Links Center at the Yerkes National Primate Research Center, and the Department of Psychology at Emory University.
Abbreviation
MSR
mirror self-recognition.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gallup GG., Jr Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Gallup GG., Jr . In: Animal Models of Human Emotion and Cognition. Haug M, Whalen RE, editors. Washington, DC: American Psychological Association; 1999. pp. 175–194. [Google Scholar]
- 3.Reiss D, Marino L. Proc Natl Acad Sci USA. 2001;98:5937–5942. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan JP, Gallup GG, Jr, Falk D. The Face in the Mirror: The Search for the Origins of Consciousness. New York: HarperCollins; 2003. [Google Scholar]
- 5.Gallup GG., Jr . In: Self-Awareness in Animals and Humans: Developmental Perspectives. Parker ST, Mitchell RW, Boccia ML, editors. Cambridge, UK: Cambridge Univ Press; 1994. pp. 35–50. [Google Scholar]
- 6.de Waal FBM, Dindo M, Freeman CA, Hall M. Proc Natl Acad Sci USA. 2005;102:11140–11147. doi: 10.1073/pnas.0503935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallup GG., Jr Am J Primatol. 1982;2:237–248. doi: 10.1002/ajp.1350020302. [DOI] [PubMed] [Google Scholar]
- 8.de Waal FBM. In: Feelings & Emotions: The Amsterdam Symposium. Manstead T, Frijda N, Fischer A, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 379–399. [Google Scholar]
- 9.de Waal FBM, Aureli F. In: Reaching Into Thought: The Minds of the Great Apes. Russon AE, Bard KA, Parker ST, editors. Cambridge, UK: Cambridge Univ Press; 1996. pp. 80–110. [Google Scholar]
- 10.Bischof-Köhler D. Schweiz Z Psychol. 1988;47:147–159. [Google Scholar]
- 11.Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M. Dev Psychol. 1992;28:126–136. [Google Scholar]
- 12.de Waal FBM. Good Natured: The Origins of Right and Wrong in Humans and Other Animals. Cambridge, MA: Harvard Univ Press; 1996. [Google Scholar]
- 13.Caldwell MC, Caldwell DK. In: Whales, Dolphins, and Porpoises. Norris KS, editor. Berkeley, CA: Univ of California Press; 1966. pp. 755–789. [Google Scholar]
- 14.Connor RC, Norris KS. Am Nat. 1982;119:358–372. [Google Scholar]
- 15.Moss C. Elephant Memories: Thirteen Years in the Life of an Elephant Family. New York: Fawcett Columbine; 1988. [Google Scholar]
- 16.Poole J. Coming of Age With Elephants: A Memoir. New York: Hyperion Books; 1996. [Google Scholar]
- 17.Hamilton-Douglas I, Bhalla S, Wittemyer G, Vollrath F. App Anim Behav Sci. 2006;100:87–102. [Google Scholar]
- 18.Payne K. In: Animal Social Complexity: Intelligence, Culture, and Individualiz Societies. de Waal FBM, Tyack PL, editors. Cambridge, MA: Harvard Univ Press; 2003. pp. 57–85. [Google Scholar]
- 19.Shoshani J. TREE. 1998;13:480–487. doi: 10.1016/s0169-5347(98)01491-8. [DOI] [PubMed] [Google Scholar]
- 20.Shoshani J, Kupsky WJ, Marchant GH. Brain Res Bull. 2006;70:124–157. doi: 10.1016/j.brainresbull.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Povinelli DJ. J Comp Psychol. 1989;103:122–131. [Google Scholar]
- 22.Olson D, editor. Elephant Husbandry Resource Guide. Lawrence, KS: Allen Press; 2004. [Google Scholar]
- 23.Povinelli DJ, Rulf AB, Landau KR, Bierschwale DT. J Comp Psychol. 1993;107:347–372. doi: 10.1037/0735-7036.107.4.347. [DOI] [PubMed] [Google Scholar]
- 24.Brown RE. In: Social Odours in Mammals. Brown RE, Macdonald DW, editors. Vol 1. New York: Oxford Univ Press; 1985. pp. 235–244. [Google Scholar]
- 25.Swartz KB, Evans S. Primates. 1991;32:483–496. [Google Scholar]
- 26.Povinelli DJ, Gallup GG, Jr, Eddy TJ, Bierschwale DT, Engstrom MC, Perilloux HK, Toxopeus IB. Anim Behav. 1997;53:1083–1088. [Google Scholar]
- 27.van Lawick-Goodall J. Anim Behav Monogr. 1968;1:161–311. [Google Scholar]
- 28.de Waal FBM. Chimpanzee Politics: Power and Sex Among Apes. New York: Harper & Row; 1982. [Google Scholar]
- 29.Mooring MS, Benjamin JE, Harte CR, Herzog NB. Anim Behav. 2000;60:35–45. doi: 10.1006/anbe.2000.1461. [DOI] [PubMed] [Google Scholar]
- 30.Leuthold W. African Ungulates: A Comparative Review of Their Ethology and Behavioral Ecology. New York: Springer-Verlag; 1977. [Google Scholar]
- 31.Estes R. The Behavior Guide to African Mammals: Including Hoofed Mammals, Carnivores, Primates. Berkeley, CA: Univ of California Press; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information