Phosphodiesterase inhibitors (original) (raw)
Abstract
Phosphodiesterases are a diverse family of enzymes that hydrolyse cyclic nucleotides and thus play a key role in regulating intracellular levels of the second messengers cAMP and cGMP, and hence cell function. Theophylline and papaverine have historically been used therapeutically and are known to be weak inhibitors of PDE, but to what extent this contributed toward their clinical efficacy was poorly defined. However, the discovery of 11 isoenzyme families and our increased understanding of their function at the cell and molecular level provides an impetus for the development of isoenzyme selective inhibitors for the treatment of various diseases. This review focuses on the development of PDE3 inhibitors for congestive heart failure, PDE4 inhibitors for inflammatory airways disease and most successfully, PDE5 inhibitors for erectile dysfunction
Keywords: Phosphodiesterase, PDE4, PDE5, asthma, erectile dysfunction, inflammation
Introduction
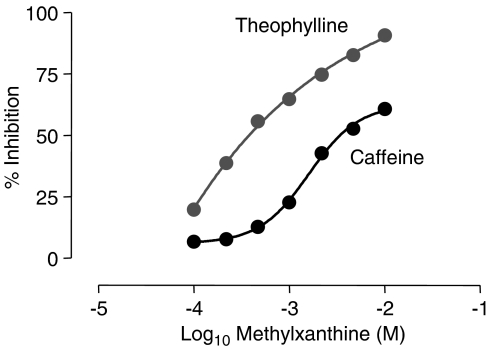
The phosphodiesterase (PDE) story begins with the work of Henry Hyde Salter in 1886. An asthmatic he noted that when he drank a strong cup of coffee on an empty stomach, his breathing eased, an effect attributed to the bronchodilator properties of caffeine. Although the mechanism of action at the time was unknown, it has since been shown that caffeine was acting as a non-selective, albeit weak, PDE inhibitor. Subsequently, analogues of caffeine including theophylline were successfully introduced as treatments for airway disease. The seminal work by Earl Sutherland and Ted Rall published in 1958, first identified the heat-stable nucleotide, cyclic adenosine monophosphate (cAMP) in liver extracts as a second messenger and suggested that it mediated many of the cellular effects of neurotransmitters and hormones. The discovery of cAMP was followed 5 years later by the identification of a second intracellular second messenger, cyclic guanosine monophosphate (cGMP), in rat urine (Ashman et al., 1963). In this same study, PDE was identified as the enzyme capable of inactivating cAMP, and it was shown that this enzyme could be activated by magnesium ions and importantly could be inhibited by caffeine providing a plausible mechanism of action for the diverse activities of this drug (see Figure 1; Sutherland, 1958).
Figure 1.
Inhibition of cAMP-PDE activity by methylxanthines. cAMP was incubated with purified cAMP-PDE at 30°C for 30 min in the presence of increasing concentrations of methylxanthines. Data redrawn from Butcher & Sutherland (1962).
From a very early period, it was hypothesised that there were a number of different isoforms of PDE distinguished primarily by their substrate specificity and sensitivity to calcium-calmodulin (CaM) and these isoenzymes were numbered according to their elucidation order. They were first differentiated in the early 1970s in rat and bovine tissue (Beavo et al., 1970). Initially, three enzymes were identified and known as CaM-PDE, cAMP-PDE and cGMP-PDE, which were further characterised by the use of selective inhibitors for these enzymes (Hidaka & Endo, 1984; Nicholson et al., 1991). With the advent of the molecular age, the number of PDE isoforms identified increased and so in 1995, the nomenclature for the PDE family was standardised (Beavo, 1995). Today 11 isoenzyme groups, encompassing over 50 isoforms, have been identified including the recently characterised PDE4A11 (Wallace et al., 2005) (see Table 1).
Table 1. The PDE superfamily.
| PDE isoenzyme | No. of isoforms | Substrate | Km (μM) cAMP | Km (μM) GMP | Tissue expression | Specific inhibitors |
|---|---|---|---|---|---|---|
| 1 | 8 | Ca2+/calmodulin-stimulated | 1–30 | 3 | Heart, brain, lung, smooth muscle | KS-505a |
| 2 | cGMP-stimulated | 50 | 50 | Adrenal gland, heart, lung, liver, platelets | EHNA (MEP-1) | |
| 3 | 4 | cGMP-inhibited, cAMP-selective | 0.2 | 0.3 | Heart, lung, liver, platelets, adipose tissue, inflammatory cells | Cilostamide Enoxamone Milrinone Siguazodan |
| 4 | 20 | cAMP-specific | 4 | Sertoli cells, kidney, brain, liver, lung, inflammatory cells | Rolipram, Roflumilast Cilomilast | |
| 5 | 3 | cGMP-specific | 150 | 1 | Lung, platelets, vascular smooth muscle | Sildenafil, Zaprinast |
| 6 | cGMP-specific | 60 | Photoreceptor | Dipyridamole | ||
| 7 | 3 | cAMP-specific, high-affinity | 0.2 | Skeletal muscle, heart, kidney, brain, pancreas, T lymphocytes | BRL-50481 | |
| 8 | cAMP-selective, | 0.06 | Testes, eye, liver, skeletal muscle, heart, kidney, ovary, brain, T lymphocytes | none | ||
| 9 | 4 | cGMP-specific, | 0.17 | Kidney, liver, lung, brain | BAY 73-6691 | |
| 10 | 2 | cGMP-sensitive, cAMP-selective | 0.05 | 3.0 | Testes, brain | none |
| 11 | 4 | cGMP-sensitive, dual specificity | 0.7 | 0.6 | Skeletal muscle, prostate, kidney, liver, pituitary and salivary glands, testes | none |
PDE activity is found in every cell in the body, although there is distinct cellular and subcellular distribution of the 11 isoenzymes, which has provided many possibilities for increasingly selective therapeutic targets (reviewed by Lugnier, 2005). In identifying isoenzyme selective targets for specific diseases, a substantial amount of work was undertaken by pharmacologists working in the U.K., particularly in characterising tissue expression, subcellular distribution and modulation of tissue function by isoenzyme selective inhibitors. This includes work by Nicholson and Shahid at Organon studying PDE expression in cardiac tissues and the airways (de Boer et al., 1992; Torphy et al., 1993; Shahid & Nicholson, 1995) and Miles Houslay and co-workers in Glasgow who have contributed significantly to our understanding of PDE4 and its various subtypes (Houslay, 2001).
The development of PDE3 inhibitors to treat congestive heart failure
PDE3 has high affinity for cAMP but can also hydrolyse cGMP. However, it hydrolyses cAMP at 10 times the rate it hydrolyses cGMP and therefore cGMP effectively acts as a competitive inhibitor for cAMP and consequently for PDE3 (Lugnier, 2005). As a result of its high expression in both the vasculature and the airways, PDE3 was identified as a potential therapeutic target in cardiovascular disease and asthma, and indeed, PDE3 inhibitors have subsequently been shown to relax vascular and airway smooth muscle, inhibit platelet aggregation (reviewed by Barnes et al., 1988) and induce lipolysis (Manganiello et al., 1995). However, the unequivocal effect of PDE3 inhibitors as positive inotropic agents provided a strong rationale for developing such drugs for the treatment of chronic heart disease (Nicholson et al., 1991). A number of PDE3 selective inhibitors, including milrinone, were developed to treat patients with heart failure. However, chronic treatment with milrinone was associated with an increased risk of mortality and has consequently somewhat jaundiced the view of PDE3 as a drug target (Packer et al., 1991). Nonetheless, milrinone is still used in the acute treatment of heart failure, and cilostazol, another PDE3 inhibitor, is used in the treatment of intermittent claudication.
Development of PDE4 inhibitors for the treatment of inflammatory airways disease
PDE4, formerly known as cAMP-PDE, is a cAMP-specific PDE and is the predominant isoenzyme in the majority of inflammatory cells, with the exception of platelets, implicated in inflammatory airways disease. It is expressed in the airways smooth muscle, brain and cardiovascular tissues (Muller et al., 1996) and is the largest PDE subfamily with over 35 different isoforms identified thus far. The molecular structure, compartmentalisation and function have been extensively investigated (Houslay, 2001) and as such, PDE4 is the most widely characterised PDE isoenzyme. In the early 1970s, rolipram, a cAMP-PDE inhibitor, was developed as a potential drug to treat depression as it was demonstrated that elevation of cAMP could enhance noradrenergic neurotransmission in the central nervous system. Although rolipram proved to be an effective antidepressant, side effects of nausea and gastrointestinal disturbance terminated its clinical development (Scott et al., 1991). In addition, the worldwide success of serotonin selective reuptake inhibitors in treating depression usurped PDE4 inhibitors as a potential therapy in this field. Nonetheless, there is a current resurgence underway in this area (Renau, 2004) and in addition, to the development of PDE4 inhibitors to treat other CNS indications such as memory enhancement (Inflazyme pharmaceuticals, 2005).
The success of theophylline in treating asthmatic patients (see also Barnes, this issue), the finding that raising intracellular levels of cAMP within inflammatory cells inhibited their function and the wide distribution of PDE4 in inflammatory cells and the lung led to the exploration of isoenzyme selective PDE4 inhibitors as potential treatments for airway disease (Torphy & Undem, 1991). Indeed, the first generation PDE4 inhibitors were shown to be effective at inhibiting a wide range of inflammatory cell function in vitro including eosinophil (Dent et al., 1991), lymphocyte (Giembycz et al., 1996), basophil (Weston et al., 1997) and neutrophil activation (Nielson et al., 1990). Furthermore, they were highly effective at suppressing inflammation in animal models of respiratory disease (Torphy & Undem, 1991). The ability of PDE4 inhibitors to also induce relaxation of isolated human bronchus (Cortijo et al., 1993) gave rise to the hope that PDE4 inhibitors could perhaps possess both anti-inflammatory and bronchodilator activity.
A number of pharmaceutical companies went on to develop potent second generation PDE4 selective inhibitors, and scientists at Celltech in Slough U.K., developed CDP840 (Hughes et al., 1996), which in 1997, became the first orally active PDE4 inhibitor to demonstrate a beneficial effect in patients with asthma at doses producing no reported serious adverse effects. CDP840 had no direct bronchodilator activity nor did it inhibit acute bronchoconstriction in response to antigen challenge. This acute response is referred to as the early asthmatic response (EAR) and is known to be a consequence of mast cell degranulation and release of mediators including histamine as a result of antigen binding to high affinity IgE cell surface receptors. However, CDP840 did significantly suppress the late asthmatic response (LAR) by 30% at doses that did not elicit significant gastrointestinal side effects (Harbinson et al., 1997). This was a very significant observation, as the LAR is viewed by clinicians to represent the inflammatory component of airway disease and showed that PDE4 inhibitors could indeed be anti-inflammatory drugs and that it was possible to obtain this effect without the side effects that dogged earlier drugs of this class.
Cilomilast developed by Ted Torphy and co-workers at SmithKline Beecham (now GSK) and roflumilast developed by Dr C Schudt and co-workers at Byk Gulden (now Altana) are orally active selective PDE4 inhibitors in late clinical development (Brown, 2005; Rabe et al., 2005). In COPD patients, cilomilast significantly improved FEV1 and quality of life scores, as well as reducing exacerbation rates, although it has still to be approved by the FDA. However, gastrointestinal disturbances such as emesis and nausea are still evident with this drug such that it is likely that the doses currently used in man are at the bottom end of the dose response curve and therefore the optimal effects of this class of drug are not being achieved with cilomilast. Roflumilast (250 and 500 _μ_g, p.o.) significantly suppressed LAR by 27 and 43%, respectively, in patients with asthma. It also reduced the EAR (25 and 28%, respectively), although by a smaller margin than the LAR (Van Schalkwyk et al., 2005). Significantly, studies have demonstrated the efficacy of roflumilast in patients with both asthma and COPD, where roflumilast improved lung function and reduced exacerbation rates, and thus it remains a promising new therapy to treat this disease (Rabe et al., 2005). One recent study has shown that roflumilast is equivalent to taking inhaled beclomethasone diproprionate in the treatment of mild to moderate asthma, suggesting that such drugs may prove to be a viable alternative therapy to inhaled glucocorticosteroids (reviewed by Lipworth, 2005). Roflumilast is undergoing further clinical evaluation in patients with both asthma and COPD, although it is still a drug that has significant gastrointestinal side effects, particularly at high doses, which may prove problematic.
Thus, there still remains a challenge to design even better PDE4 inhibitors with an improved therapeutic index and a number of different strategies are being pursued to achieve this. The work of Souness & Rao (1997) working at Rhone Poulenc Rorer, latterly Aventis, in Dagenham suggested that PDE4 existed in two distinct conformations, one present predominantly in the CNS and parietal glands, which binds rolipram with high affinity termed HPDE4, and one, which binds rolipram with low affinity that is mainly present in inflammatory cells termed LPDE4. Accordingly, binding to the HPDE4 was predicted to be related to the adverse side effects associated with rolipram. However, cilomilast has reduced potency against HPDE4 but nonetheless is still emetic in patients treated with this drug. Another approach followed the recognition that there were four genetically distinct PDE4 subtypes; termed PDE4A-D. Conti and co-workers were the first to produce PDE4 knock-out mice deficient in either PDE4B or PDE4D and studies with these mice suggested that PDE4D was associated with emesis (Robichaud et al., 2002), and in the development of airways hyperresponsiveness in response to cholinergic stimulation (Hansen et al., 2000). Further studies with PDE4B knock-out mice showed that this PDE4 subtype was essential for LPS-induced generation of the cytokine TNF-α and thus, a PDE4B selective inhibitor could potentially be an effective anti-inflammatory agent without inducing emesis (Jin & Conti, 2002). Another potential avenue that could be exploited to improve drug selectivity and reduce side effects would be the targeting of specific PDE4 isoenzymes that are only expressed under inflammatory situations (Chan et al., 2003). To date there is no evidence for altered PDE4 expression and function in inflammatory cells from asthmatic subjects (Landells et al., 2001; Jones et al., 2005) although increased expression of PDE4A4 has been documented in macrophages from subjects with COPD (Barber et al., 2004). Whether selective targeting of this enzyme will lead to a better drug than roflumilast remains to be seen. In addition, the expression of PDE7 in inflammatory cells has been acknowledged and while inhibition of this enzyme alone does not suppress inflammatory cell function, however, combined use of PDE4 with PDE7 inhibitors provides a greater inhibition than PDE4 alone. Therefore, a hybrid PDE4/7 inhibitor may provide more effective anti-inflammatory activity and reduce side effects. It is of interest, therefore, that novel PDE4 inhibitors are now being developed, which claim to lack significant gastrointestinal side effects, including HT0712 (Inflazyme pharmaceuticals, 2005) and GRC 3886 (Glenmark Pharmaceuticals, 2005).
PDE5 inhibitors for the treatment of erectile dysfunction
A variety of treatments have been historically used to treat erectile dysfunction that influence the cyclic nucleotide signalling pathway in vascular smooth muscle including PGE1, papaverine and pentoxifylline. It was recognised that papaverine and pentoxifylline mediated vasorelaxation by a number of mechanisms including non-selective PDE inhibition (Allenby et al., 1991) and these drugs can be considered as forerunners to the clinically successful PDE5 inhibitors used today for the treatment of erectile dysfunction, although at the time, PDE5 inhibition as a mechanism to account for the actions of these particular drugs was not established.
PDE5, a cGMP-specific PDE was first identified in rat platelets in 1978 and was originally known as cGMP-PDE (Hamet & Coquil, 1978). Early on it was shown that cGMP-PDE could be specifically inhibited by zaprinast, and this was widely used to explore the functional role of what we now know as the PDE5 isoenzyme (reviewed by Murray, 1993). Zaprinast was designated M&B22948 by May and Baker in Dagenham and designed as a mast cell stabilising drug for treating allergic diseases, and in this capacity M&B22948 became the first orally active isoenzyme selective PDE5 inhibitor to be given in man. It was originally administered to patients with exercise-induced asthma and was shown to have moderate bronchodilator effects (Rudd et al., 1983). However, PDE5 inhibitors had no inhibitory effect on inflammatory cells other than mast cells, but they were able to induce vascular smooth muscle relaxation and therefore PDE5 was considered a possible therapeutic target in cardiovascular disease (Murray, 1993). Indeed, it was demonstrated that an elevation in cGMP mediated by zaprinast was associated with vascular smooth muscle relaxation of isolated rat aorta (Lugnier et al., 1983; Rapoport & Murad, 1983); observations which led to the initiation of research programmes by a number of pharmaceutical companies to develop PDE5 inhibitors for a range of diseases. Pfizer in Sandwich, Kent, in particular, took the PDE5 inhibitor sildenafil into the clinic as a treatment for angina pectoris.
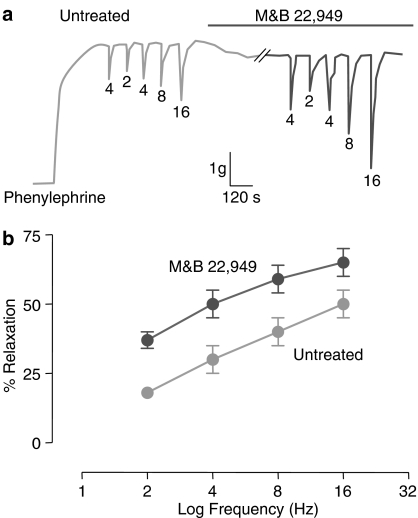
Other laboratories were undertaking studies to enhance understanding of the vascular and neurological control of the corpus cavernosum to better determine the basis of erections. In 1990, it was reported that electrical field stimulation (EFS) induced relaxation of rabbit corpus cavernosum smooth muscle cells. This response was also correlated with the formation of nitric oxide (NO) and a rise of intracellular levels of cGMP within smooth muscle cells, suggesting that enhanced NO production could potentially mediate penile erection (Ignarro et al., 1990). This was followed by the seminal work of Rajfer et al. (1992) demonstrating that the NO pathway was also triggered by EFS stimulation in human corpus cavernosum to bring about relaxation, a mechanism which was reduced in tissue obtained from patients with impotence. As part of a very thorough investigation of this NO pathway regulating the relaxation of the corpus cavernosum, it was demonstrated that the PDE5 inhibitor zaprinast could enhance NO-induced relaxation of isolated corpus cavernosum (Rajfer et al., 1992). These findings suggested that PDE5 inhibitors may also be useful for treating impotence (reviewed by Murray, 1993) (see Figure 2).
Figure 2.
The PDE5 inhibitor M&B 22,949 (Zaprinast) augmented relaxation of human corpus cavernosum to electrical field stimulation (Hz). Data showing relaxation of human corpus cavernosum in an isolated preparation (a) or the mean±s.e.m. for 11 subjects (b); to different stimulation frequencies in untreated and M&B 22,949 (1–3 mM)-treated tissue. Data re-drawn from Raijfer et al. (1992).
The data from the Phase 1 clinical trials with sildenafil in angina patients proved disappointing. However, while sildenafil provided no significant therapeutic improvement over the existing nitrate therapy, it was noted that one of the commonly reported side effects in this study was penile erection. This observation led to the important decision by Pfizer to change the focus of the sildenafil research programme to investigate their drug as a potential treatment of erectile dysfunction. In late 1993, the first clinical trial examining the efficacy of sildenafil for the treatment of erectile dysfunction was undertaken and it confirmed the potential of the drug to treat patients with this condition (Boolell et al., 1996). Over the next 4 ½ years, over 5000 patients received sildenafil in clinical trials and in March 1998, the FDA approved it for the treatment of erectile dysfunction. Since then, an estimated 177 million prescriptions in over 120 countries have been written and sildenafil has had a revolutionary impact on the understanding and treatment of this common condition. In vitro studies with isolated human corpus cavernosum, similar to the earlier studies by Rajfer et al. (1992), were undertaken and these showed that sildenafil is approximately 240 times more potent than zaprinast at inhibiting PDE5 (Ballard et al., 1998). Nonetheless, sildenafil is sometimes associated with visual disturbances due to activity against PDE6, an enzyme found in the retina, and also has a relatively short half-life. Therefore, while sildenafil has been very successful, it has some limitations and this has led to the development of newer PDE5 inhibitors. Two more PDE5 inhibitors, vardenafil and tadalafil, are now approved for use as treatments for erectile dysfunction. Vardenafil is more potent than sildenafil and tadalafil, and has a half-life of approximately 17 h, which allows more natural engagement of sexual activity. Furthermore, tadalafil is far less active against the PDE6 isoenzyme (selectivity ratio vs PDE5: 780) than either sildenafil (6.8) or vardenafil (2.9) and consequently, the incidence of visual side effects associated with PDE6 inhibition in the photoreceptor cells is greatly reduced (<0.1% tadalafil compared with 3% sildenafil) (Maggi et al., 2000).
Conclusion
Non-selective PDE inhibitors including theophylline and papaverine have been used therapeutically for over 70 years for a range of diseases. However, it is only in the last 10 years, that potent PDE selective drugs have begun to make an impact in the treatment of disease, and the worldwide success of sildenafil in treating erectile dysfunction is evidence of the effect such drugs can have. Selective PDE inhibitors are being investigated in a wide range of diseases (summarised in Table 2) including the use of PDE2 inhibitors in sepsis; PDE5 inhibitors to treat sexual dysfunction in females, cardiovascular disease and pulmonary hypertension; and PDE4 inhibitors to treat asthma, COPD, allergic rhinitis, psoriasis, multiple sclerosis, depression, Alzheimer's disease and schizophrenia. As we increase our understanding of the physiological roles of the individual PDE isoforms, in parallel with the development of even more selective inhibitors of these enzymes, it is highly likely that better therapeutically active drugs will emerge.
Table 2. Disease targets for isoenzyme selective PDE inhibitors.
| PDE family | Drug targets |
|---|---|
| 2 | Sepsis, Acute Respiratory Distress Syndrome (ARDS) |
| 3 | Airways disease, fertility |
| 4 | Allergic Rhinitis, Psoriasis, Multiple Sclerosis, Depression, Alzheimer's Disease, Schizophrenia, Memory loss, Cancer, Dermatitis |
| 5 | Pulmonary hypertension, female sexual dysfunction, cardiovascular disease, premature ejaculation, stroke, leukaemia, renal failure |
| 7 | Inflammation |
Glossary
CaM
calcium-calmodulin
cAMP
cyclic adenosine monophosphate
cGMP
cyclic guanosine monophosphate
EAR
early asthmatic response
LAR
late asthmatic response
LPS
lipopolysaccharide
NO
nitric oxide
PDE
phosphodiesterase
PGE
prostaglandin
TNF
tumour necrosis factor
References
- ALLENBY K.S., BURRIS J.F., MROCZEK W.J. Pentoxifylline in the treatment of vascular impotence – case reports. Angiology. 1991;42:418–420. doi: 10.1177/000331979104200511. [DOI] [PubMed] [Google Scholar]
- ASHMAN D.F., LIPTON R., MELICOW M.M., PRICE T.D. Isolation of adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate from rat urine. Biochem. Biophys. Res. Commun. 1963;11:330–334. doi: 10.1016/0006-291x(63)90566-7. [DOI] [PubMed] [Google Scholar]
- BALLARD S.A., GINGELL C.J., TANG K., TURNER L.A., PRICE M.E., NAYLOR A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- BARBER R., BAILLIE G.S., BERGMANN R., SHEPHERD M.C., SEPPER R., HOUSLAY M.D., HEEKE G.V. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and non smokers. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., CHUNG K.F., PAGE C.P. Inflammatory mediators and asthma. Pharmacol. Rev. 1988;40:49–84. [PubMed] [Google Scholar]
- BEAVO J.A. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A., HARDMAN J.G., SUTHERLAND E.W. Hydrolysis of cyclic guanosine and adenosine 3′,5′-monophosphates by rat and bovine tissues. J. Biol. Chem. 1970;245:5649–5655. [PubMed] [Google Scholar]
- BOOLELL M., ALLEN M.J., BALLARD S.A., GEPI-ATTEE S., MUIRHEAD G.J., NAYLOR A.M., OSTERLOH I.H., GINGELL C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996;8:47–52. [PubMed] [Google Scholar]
- BROWN W.M. Cilomilast GlaxoSmithKline. Curr. Opin. Inves. Drug. 2005;6:545–558. [PubMed] [Google Scholar]
- BUTCHER R.W., SUTHERLAND E.W. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J. Biol. Chem. 1962;237:1244–1250. [PubMed] [Google Scholar]
- CHAN S.C., REIFSNYDER D., BEAVO J.A., HANIFIN J.M. Immunochemical characterisation of the distinct monocyte cyclic AMP-phosphodiesterase from patients with atopic dermatitis. J. Allergy Clin. Immunol. 1993;91:1179–1188. doi: 10.1016/0091-6749(93)90321-6. [DOI] [PubMed] [Google Scholar]
- CORTIJO J., BOU J., BELETA J., CARDELUS I., LLENAS J., MORCILLO E., GRISTWOOD R.W. Investigation into the role of phosphodiesterase IV in bronchorelaxation, including studies with human bronchus. Br. J. Pharmacol. 1993;108:562–568. doi: 10.1111/j.1476-5381.1993.tb12841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOER J., PHILPOTT A.J., VAN AMSTERDAM R.G., SHAHID M., ZAAGSMA J., NICHOLSON C.D. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br. J. Pharmacol. 1992;106:1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENT G., GIEMBYCZ M.A., RABE K.F., BARNES P.J. Inhibition of eosinophil cyclic nucleotide PDE activity and opsonised zymosan-stimulated respiratory burst by ‘type IV'-selective PDE inhibitors. Br. J. Pharmacol. 1991;103:1339–1346. doi: 10.1111/j.1476-5381.1991.tb09790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., CORRIGAN C.J., SEYBOLD J., NEWTON R., BARNES P.J. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br. J. Pharmacol. 1996;118:1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenmark Pharmaceuticals 2005. GRC3886. Report. .http://www.glenmarkpharma.com/research/clinical.html.
- GUAY D., HAMEL P., BLOUIN M., BRIDEAU C., CHAN C.C., CHAURET N., DUCHARME Y., HUANG Z., GIRARD M., JONES T.R., LALIBERTE F., MASSON P., MCAULIFFE M., PIECHUTA H., SILVA J., YOUNG R.N., GIRARD Y. Discovery of L-791,943: a potent, selective, non emetic and orally active phosphodiesterase-4 inhibitor. Bioorg. Med. Chem. Lett. 2002;12:1457–1461. doi: 10.1016/s0960-894x(02)00190-7. [DOI] [PubMed] [Google Scholar]
- HAMET P., COQUIL J.F. Cyclic GMP binding and cyclic GMP phosphodiesterase in rat platelets. J. Cyclic. Nucleotide. Res. 1978;4:281–290. [PubMed] [Google Scholar]
- HANSEN G., JIN S., UMETSU D.T., CONTI M. Absence of muscarinic cholinergic airway responses in mice deficient in the cyclic nucleotide phosphodiesterase PDE4D. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6751–6756. doi: 10.1073/pnas.97.12.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBINSON P.L., MACLEOD D., HAWKSWORTH R., O'TOOLE S., SULLIVAN P.J., HEATH P., KILFEATHER S., PAGE C.P., COSTELLO J., HOLGATE S.T., LEE T.H. The effect of a novel orally active selective PDE4 isoenzyme inhibitor (CDP840) on allergen-induced responses in asthmatic subjects. Eur. Respir. J. 1997;10:1008–1014. doi: 10.1183/09031936.97.10051008. [DOI] [PubMed] [Google Scholar]
- HIDAKA H., ENDO T. Selective inhibitors of three forms of cyclic nucleotide phosphodiesterase--basic and potential clinical applications. Adv. Cyclic. Nucleotide. Protein Phosphorylation. Res. 1984;16:245–259. [PubMed] [Google Scholar]
- HOUSLAY M.D. PDE4 cAMP-specific phosphodiesterases. Prog. Nucleic. Acid Res. Mol. Biol. 2001;69:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- HUGHES B., HOWAT D., LISLE H., HOLBROOK M., JAMES T., GOZZARD N., BLEASE K., HUGHES P., KINGABY R., WARRELLOW G., ALEXANDER R., HEAD J., BOYD E., EATON M., PERRY M., WALES M., SMITH B., OWENS R., CATTERALL C., LUMB S., RUSSELL A., ALLEN R., MERRIMAN M., BLOXHAM D., HIGGS G. The inhibition of antigen-induced eosinophilia and bronchoconstriction by CDP840, a novel stereo-selective inhibitor of phosphodiesterase type 4. Br. J. Pharmacol. 1996;118:1183–1191. doi: 10.1111/j.1476-5381.1996.tb15522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGNARRO L.J., BUSH P.A., BUGA G.M., WOOD K.S., FUKUTO J.M., RAJFER J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Inflazyme pharmaceuticals 2005. HT0712, a selective PDE4 inhibitor. .www.inflazyme.com/corporate_profile.
- JIN S.L., CONTI M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES N.A., LEPORT M, HOLAND T, VOS T, MORGAN M, FINK M, PRUNIAUX M.-P, BERTHELIER C, O'CONNOR B.J, BERTRAND C, PAGE C.P.2005Phosphodiesterase (PDE) 7 in inflammatory cells from patients with asthma and COPD Pulm. Pharmacol. Therapin press). [DOI] [PubMed]
- LANDELLS L.J., SZILAGY C.M., JONES N.A, BANNER K.H., ALLEN J.M., DOHERTY A., O'CONNOR B.J., SPINA D., PAGE C.P. Identification and quantification of phosphodiesterase 4 subtypes in CD4 and CD8 lymphocytes from healthy and asthmatic subjects. Br. J. Pharmacol. 2001;133:722–729. doi: 10.1038/sj.bjp.0704120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPWORTH B.J. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- LUGNIER C.2005Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents Pharmacol. TherEpub ahead of print. [DOI] [PubMed]
- LUGNIER C., STIERLE A., BERETZ A., SCHOEFFTER P., LEBEC A., WERMUTH C.G., CAZENAVE J.P., STOCLET J.C. Tissue and substrate specificity of inhibition by alkoxy-aryl-lactams of platelet and arterial smooth muscle cyclic nucleotide phosphodiesterases relationship to pharmacological activity. Biochem. Biophys. Res. Commun. 1983;113:954–959. doi: 10.1016/0006-291x(83)91091-4. [DOI] [PubMed] [Google Scholar]
- MAGGI M., FILIPPI S., LEDDA F., MAGINI A., FORTI G. Erectile dysfunction: from biochemical pharmacology to advances in medical therapy. Eur. J. Endocrinol. 2000;143:143–154. doi: 10.1530/eje.0.1430143. [DOI] [PubMed] [Google Scholar]
- MANGANIELLO V.C., TAIRA M., DEGERMAN E., BELFRAGE P. Type III cGMP-inhibited cyclic nucleotide phosphodiesterases (PDE3 gene family) Cell Signal. 1995;7:445–455. doi: 10.1016/0898-6568(95)00017-j. [DOI] [PubMed] [Google Scholar]
- MULLER T., ENGELS P., FOZARD J.R. Subtypes of the type 4 cAMP phosphodiesterases: structure, regulation and selective inhibition. Trends Pharmacol. Sci. 1996;17:294–298. doi: 10.1016/0165-6147(96)10035-3. [DOI] [PubMed] [Google Scholar]
- MURRAY K.J. Phosphodiesterase VA inhibitors. Drug News & Perspectives. 1993;6:150–156. [Google Scholar]
- NICHOLSON C.D., CHALLISS R.A., SHAHID M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol. Sci. 1991;12:19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- NIELSON C.P., VESTAL R.E., STURM R.J., HEASLIP R. Effects of selective phosphodiesterase inhibitors on the polymorphonuclear leukocyte respiratory burst. J. Allergy Clin. Immunol. 1990;86:801–808. doi: 10.1016/s0091-6749(05)80186-1. [DOI] [PubMed] [Google Scholar]
- PACKER M., CARVER J.R., RODEHEFFER R.J., IVANHOE R.J., DIBIANCO R., ZELDIS S.M., HENDRIX G.H., BOMMER W.J., ELKAYAM U., KUKIN M.L. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- RABE K.F., BATEMAN E.D., O'DONNELL D., WITTE S., BREDENBROKER D., BETHKE T.D. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- RAJFER J., ARONSON W.J., BUSH P.A., DOREY F.J., IGNARRO L.J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N. Engl. J. Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- RAPOPORT R.M., MURAD F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J. Cyclic. Nucleotide. Protein Phosphor. Res. 1983;9:281–296. [PubMed] [Google Scholar]
- RENAU T.E. The potential of phosphodiesterase 4 inhibitors for the treatment of depression: opportunities and challenges. Curr. Opin. Investig. Drugs. 2004;5:34–39. [PubMed] [Google Scholar]
- ROBICHAUD A., STAMATIOU P.B., JIN S.L., LACHANCE N., MACDONALD D., LALIBERTE F., LIU S., HUANG Z., CONTI M., CHAN C.C. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor- mediated anesthesia, a behavioral correlate of emesis. J. Clin. Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDD R.M., GELLERT A.R., STUDDY P.R., GEDDES D.M. Inhibition of exercise-induced asthma by an orally absorbed mast cell stabilizer (M & B 22,948) Br. J. Dis. Chest. 1983;77:78–86. [PubMed] [Google Scholar]
- SCOTT A.I., PERINI A.F., SHERING P.A., WHALLEY L.J. In-patient major depression: is rolipram as effective as amitriptyline. Eur. J. Clin. Pharmacol. 1991;40:127–129. doi: 10.1007/BF00280065. [DOI] [PubMed] [Google Scholar]
- SHAHID M., NICHOLSON C.D. The analysis and assay of cyclic nucleotide phosphodiesterase isoenzyme activity. Methods Mol. Biol. 1995;41:129–150. doi: 10.1385/0-89603-298-1:129. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., RAO S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9:227–236. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E.W. Fractionation and characterisation of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- TORPHY T.J., UNDEM B.J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991;46:512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORPHY T.J., UNDEM B.J., CIESLINSKI L.B., LUTTMANN M.A., REEVES M.L., HAY D.W. Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscle. J. Pharmacol. Exp. Ther. 1993;265:1213–1223. [PubMed] [Google Scholar]
- VAN SCHALKWYK E., STRYDOM K., WILLIAMS Z., VENTER L., LEICHTL S., SCHMID-WIRLITSCH C., BREDENBROKER D., BARDIN P.G. Roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, attenuates allergen-induced asthmatic reactions. J. Allergy Clin. Immunol. 2005;116:292–298. doi: 10.1016/j.jaci.2005.04.023. [DOI] [PubMed] [Google Scholar]
- WALLACE D.A., JOHNSTON L.A., HUSTON E., MACMASTER D., HOUSLAY T.M., CHEUNG Y.F., CAMPBELL L., MILLEN J.E., SMITH R.A., GALL I., KNOWLES R.G., SULLIVAN M., HOUSLAY M.D. Identification and characterization of PDE4A11, a novel, widely expressed long isoform encoded by the human PDE4A cAMP phosphodiesterase gene. Mol. Pharmacol. 2005;67:1920–1934. doi: 10.1124/mol.104.009423. [DOI] [PubMed] [Google Scholar]
- WESTON M.C., ANDERSON N., PEACHELL P.T. Effects of phosphodiesterase inhibitors on human lung mast cell and basophil function. Br. J. Pharmacol. 1997;121:287–295. doi: 10.1038/sj.bjp.0701115. [DOI] [PMC free article] [PubMed] [Google Scholar]