The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life (original) (raw)
Abstract
Infants undergoing open heart surgery often have all or part of their thymus removed. The activity of the immune system has not been investigated thoroughly in these children, and only shortly after the operation. Therefore, it was decided to investigate the activity of the immune system in more detail in children several years after their operation. Peripheral blood samples from 19 children who had undergone open heart surgery during their first months of life was collected (study group) and from 19 age- and gender-matched children (control group). The activity of the immune system was evaluated by measuring the number of different cell types in peripheral blood, the phenotype of lymphocytes and the response of T cells following in vitro stimulation by mitogen, tetanus toxoid and measles antigen. The study group had significantly lower counts of total lymphocytes, which was reflected in a lower number of T cells but not B cells. Furthermore, the study group had significantly lower proportion of T cells (CD3+) and helper T cells (CD4+), but not cytotoxic T cells (CD8+). The level of neutrophils in peripheral blood was significantly higher in the study group. This may indicate enhanced innate immunity when the acquired immunity is defective. The results indicate a shift to extrathymic T cell maturation, which is less efficient for CD4+ helper cells than for CD8+ cytotoxic cells.
Keywords: congenital/, surgery, heart defects, immune functions, infant, thymectomy, thymus gland
INTRODUCTION
T cells derive from bone marrow stem cells and migrate at a very early stage to the thymus. This central lymphoid organ in the upper anterior thorax, just above the heart, provides the specialized microenvironment in which receptor gene rearrangement and T cell maturation occur [1].
The thymus is almost fully developed at birth. It increases in weight over the first 6 months of life and the rate of T cell production by the thymus is greatest before puberty. After puberty, the thymus begins to shrink and the production of new T cells reduces to a lower rate in adults [1,2]. There is a progressive replacement of the perivascular spaces with fat, but despite that the remaining cortical and medullary tissue in the ageing thymus is histologically normal [2,3].
The importance of the thymus in immunity was first discovered through experiments on mice. It was found that thymectomy in neonatal mice resulted in partial immunodeficiency, principally affecting cell-mediated immune responses [4]. Rodents are less mature than humans at birth but the thymus is known to be highly active in the first months of life in humans [1]. Neonatal thymectomy might therefore be expected to have adverse effects on immune function in children. It is, nevertheless, common to carry out thymectomy, total or partial, in the preliminary stages of open heart surgery for the correction of congenital cardiac malformations in order to facilitate the cannulation of the great vessels.
There is very little information on the impact of neonatal thymectomy in humans. According to a 25-year-old study, thymectomy in children over 6 months of age is not believed to have clinical consequences [5]. Two more recently published reports specifically addressing this issue have documented a decreased number of T cells, CD4+ T cells (helper T cells), CD8+ T cells (cytotoxic T cells) and diminished proliferative response to phytohaemagglutinin and concanavalin A [6,7]. In these two studies children were tested for immune functions from 3 months to 3 years after they underwent thymectomy at the time of cardiopulmonary bypass in the first months of life.
To investigate further the effect of thymectomy on the immune functions in children we have studied a group of children around 10 years after undergoing thymectomy as part of open heart surgery in the first few months of life. Tests performed to evaluate the immune system were haematological analysis, cell culture to evaluate functional activity and differentiation of T lymphocytes, serological measurements of immunoglobulins and autoantibodies, and flow cytometric analysis of lymphocyte immunophenotype. The phenotype CD8+ CD62L+ CD103+ that has been described recently as characteristic of recent thymic emigrants [8] was specifically analysed. The purpose of the study was to determine the long-term effects of partial or total neonatal thymectomy in humans, in particular on T cells, autoimmune diseases and infections.
PATIENTS AND METHODS
Subjects
Nineteen children aged 5–16 years (average age 10·1 years) who underwent partial or total thymectomy during an operation for congenital heart disease in the first months of life (average age 2·6 months) were enrolled into the study. Eleven children had ventricular septal defect, six had transposition of the great vessels, one child had pulmonary venous connection and one had pulmonary stenosis. One healthy control subject was matched for age and gender to each study patient. The average age of the control group was 10·2 years. Written consent was obtained from all parents and the study was conducted with permission from the Icelandic National Bioethics Committee and the Privacy and Data Protection Authority.
All the children in the study group were selected on the criteria of having a heart defect which was not going to influence their health after it had been corrected by surgery. Furthermore, there were no records of any blood transfusions or further early childhood illness after surgical treatment of the heart defect in the study group.
Data and sample collection
Information on original diagnosis, the surgical operation, number of post-operative infections and hospital stay of each subject were retrieved from hospital records. All subjects answered a standardized questionnaire with their parents, including questions about history of infections (otitis, bronchitis, pneumonia, meningitis and candidiasis), asthma, eczema, allergy, psoriasis, arthritis, vaccinations and antibiotic use for the last year. A peripheral venous blood sample was collected from all subjects.
Tests of immune system function
Studies performed to evaluate the immune system are summarized in Table 1.
Table 1.
Tests of immune function
| Full blood count and white cell differential | Lymphocyte surface markers CD3, CD4, CD8, CD16, CD19, CD45RA, CD45RO, CD56, CD62L, CD103 and TCR γ/δ | Lymphocyte stimulation (proliferation and IFN-γ release) by mitogen, tetanus toxoid and measles virus | Serum immunoglobulin concentration | Serum autoantibodies, i.e. rheumatoid factor (RF) and antinuclear antibodies (ANA) |
|---|
Haematological studies
Full blood count and white cell differential count was performed at the Department of Haematology, Landspitali University Hospital using standard procedures (Coulter Counter analysis).
Flow cytometry
The following phenotypes were studied: CD3+ (all T cells), CD3+ CD4+ (helper T cells), CD3+ CD8+ (cytotoxic T cells), CD3+ CD45RA+ (naive T cells), CD3+ CD45RO+ (T memory cells), CD19+ (all B cells), CD16+ CD56+ [natural killer (NK) cells], CD3+ TCRγδ+ (γδ T cells) and CD8+ CD62L+ CD103+. The phenotype CD8+ CD62L+ CD103+ has been described recently as characteristic of recent thymic emigrants [8]. Three-colour flow cytometric analysis was performed using a FACStarPlus flow cytometer (Becton Dickinson, San Jose, CA, USA) using FITC, PE and PE CyChrome 5 (PC5) as the fluorescent parameters. Lymphocyte immunophenotyping was based on staining with conjugated mouse antihuman monoclonal antibodies (MoAbs) to the following lymphocyte surface antigens in combinations of three: IgG1/IgG2a/IgG1, CD3/CD103/CD19, CD3/CD45RA/CD45RO, CD3/CD4/CD8, CD3/CD19/TCR γδ, CD3/CD16/CD56, CD62L/CD103/CD8. Saturating amounts of MoAbs (according to the manufacturer's protocol) and 50 µl EDTA anticoagulated whole blood was added directly to each tube. The mixture was incubated on ice for 30 min. Red blood cells were lysed with 1 ml lysis buffer (Becton Dickinson), samples centrifuged for 5 min and washed with 1 ml of phosphate buffered saline (PBS) wash. The samples were recentrifuged for 5 min and fixed with 400 µl of 0·4% paraformaldehyde. For each sample, forward and side-angle light scatter profiles were used to acquire data for 10 000 events representing viable lymphocytes. Data were saved and analysed with CellQuest software (Becton Dickinson). Results were expressed as percent positive cells compared with cells stained with isotype control antibodies only.
Cell culture
To evaluate functional activity and differentiation of T lymphocytes, the proliferation and cytokine production [interferon (IFN)-γ] was measured. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation on Histopaque solution (Sigma, Stockholm, Sweden). The cells were seeded at 2 × 105 cells per well in RPMI-1640 (Gibco, Invitrogen, Paisley, UK) supplemented with 10% fetal calf serum (FCS, Gibco) and penicillin–streptomycin mixture (Gibco) in 96-well plates (Nunc, Invitrogen) and incubated at 37°C in a humidified incubator containing 5% CO2. The cells were cultured in triplicate wells for 7 days in the presence of the mitogen phytohaemagglutinin (PHA; L4144, Sigma) at 15 µg/ml, or three antigens, tetanus toxoid (TT; Fort Dodge, USA, at 1 : 400–1 : 10800); measles virus (MV; Department of Virology, Landspitali University Hospital, at 1 : 1400–1 : 37800) and bovine serum albumin (BSA; ICN, OH, USA, at 200–7·3 µg/ml). Cells cultured in medium alone served as negative control. Icelandic children are all vaccinated against tetanus and measles in the first and second years of life and should therefore mount an immune response against the TT and MV antigens. BSA should, on the other hand, not be immunogenic.
Cytokine production (IFN-γ).
Culture supernatant was collected after 96 h and stored at −70°C until tested for IFN-γ levels by enzyme-linked immunosorbent assay (ELISA; Duoset, R&D Systems, Oxford, UK).
Proliferation of lymphocytes. The cells were pulsed with 0·5 µCi of [6–3H]-thymidine (TRK61, Amersham, Little Chalfont, UK) on day 7 and harvested on day 8 using Filtermate harvester (Packard, USA). The amount of radioactivity was measured in a scintillation counter (TopCount, Packard) with results expressed as counts per minute (cpm). Stimulation index (SI) was calculated (experiment/medium alone control) and the mean for both groups calculated.
Immunoglobulins and autoantibodies
Autoantibodies (RF and ANA) and the concentration of IgM, IgG, IgA, IgE and IgG subclasses was measured at the Department of Immunology, Landspitali University Hospital, using their standard methods (nephalometry for immunoglobulins, ELISA for RF and IgG subclasses and immunofluorescence for ANA).
Statistical analysis
The significance of differences between means was determined by non-paired Student's _t_-test as the data were normally distributed. Wilcoxon rank sum test was applied when analysing distribution of the results; a χ2 test was used to compare clinical data. P < 0·05 was considered significant.
RESULTS
Haematological parameters
The results of routine haematological tests are shown in Table 2. All values were within the normal range but the study group showed different values from the control group for three parameters. The study group had lower counts for lymphocytes (P = 0·0001) but higher counts for neutrophils (P = 0·01), giving a ratio of neutrophils to lymphocytes of 2·00 compared with 0·96 for the controls. Platelet counts were lower in the study group compared with the control group (P = 0·01).
Table 2.
Comparison of blood status between study and control groups
| Cell type | Study group 109/l (n = 19) | Control group 109/l (n = 19) | _P_-value |
|---|---|---|---|
| Red blood cells | 4·68 ± 0·38 | 4·77 ± 0·31 | 0·47 |
| White blood cells | 6·14 ± 1·98 | 5·93 ± 1·28 | 0·69 |
| Hemoglobin | 135 ± 9·74 | 136 ± 8·96 | 0·75 |
| Platelets | 273 ± 59·1 | 322 ± 58·4 | 0·01 |
| Lymphocytes | 1·79 ± 0·53 | 2·68 ± 0·73 | 0·0001 |
| Neutrophils | 3·60 ± 1·37 | 2·58 ± 0·93 | 0·01 |
| Monocytes | 0·53 ± 0·19 | 0·46 ± 0·13 | 0·20 |
| Eosinophils | 0·25 ± 0·16 | 0·18 ± 0·13 | 0·15 |
| Basophils | 0·02 ± 0·04 | 0·03 ± 0·05 | 0·44 |
When the data were analysed for those children with known thymectomy (total or partial) the results were the same as for the whole study group (data not shown).
Immunophenotype
Tables 3 and 4 show the results of the flow cytometric analysis of surface antigens, expressed either as percentage positive or as total number, respectively. The study group had significantly lower numbers of lymphocytes expressing CD3 and either CD4 (P < 0·001), CD8 (P < 0·001), CD45RO (P = 0·01), CD45RA (P = 0·001), CD103 (P = 0·01) or TCRγδ (P = 0·004). Furthermore, the study group had a significantly lower proportion of lymphocytes with the surface antigens CD3 (P = 0·02) and CD3 and CD4 (P = 0·05), whereas the proportion of CD8+ T cells was not reduced (P = 0·26).
Table 3.
Percentage of lymphocytes by immunophenotype
| CD antigen | No. in each group | Study group % | Control group % | _P_-value |
|---|---|---|---|---|
| CD3+ | 19 | 59·26 ± 9·85 | 66·74 ± 9·49 | 0·02 |
| CD3+ CD4+ | 19 | 30·14 ± 10·26 | 36·55 ± 8·87 | 0·05 |
| CD3+ CD8+ | 19 | 26·70 ± 8·66 | 29·47 ± 6·05 | 0·26 |
| CD19+ | 18 | 15·27 ± 10·35 | 11·68 ± 5·20 | 0·12 |
| CD3+ CD45RO+ | 19 | 29·86 ± 9·47 | 28·87 ± 10·79 | 0·76 |
| CD3+ CD45RA+ | 19 | 42·39 ± 13·85 | 51·13 ± 13·59 | 0·06 |
| CD16+ CD56+ | 10 | 12·75 ± 5·10 | 9·17 ± 3·14 | 0·09 |
| CD8+ CD62L+ CD103+ | 10 | 4·37 ± 10·26 | 1·26 ± 0·41 | 0·37 |
| Small CD8+ CD62L+ CD103+ | 10 | 20·20 ± 12·88 | 12·89 ± 9·66 | 0·19 |
| CD3+ CD103+ | 9 | 1·03 ± 0·39 | 1·34 ± 0·53 | 0·18 |
| CD3+ TCRγδ+ | 19 | 2·75 ± 1·51 | 3·65 ± 2·46 | 0·18 |
Table 4.
Total number of lymphocytes by immunophenotype
| CD antigen | No. in each group | Study group 109/l | Control group 109/l | _P_-value |
|---|---|---|---|---|
| CD3+ | 19 | 1·08 ± 0·45 | 1·80 ± 0·60 | <0·001 |
| CD3+ CD4+ | 19 | 0·54 ± 0·26 | 1·0 ± 0·45 | <0·001 |
| CD3+ CD8+ | 19 | 0·49 ± 0·25 | 0·77 ± 0·19 | <0·001 |
| CD19+ | 18 | 0·28 ± 0·17 | 0·33 ± 0·20 | 0·43 |
| CD3+ CD45RO+ | 19 | 0·53 ± 0·24 | 0·74 ± 0·24 | 0·01 |
| CD3+ CD45RA+ | 19 | 0·78 ± 0·43 | 1·42 ± 0·65 | 0·001 |
| CD16+ CD56+ | 10 | 0·27 ± 0·17 | 0·27 ± 0·10 | 0·98 |
| CD8+ CD62L+ CD103+ | 10 | 0·06 ± 0·12 | 0·03 ± 0·01 | 0·53 |
| Small CD8+ CD62L+ CD103+ | 10 | 0·36 ± 0·17 | 0·35 ± 0·25 | 0·93 |
| CD3+ CD103+ | 9 | 0·53 ± 0·24 | 0·03 ± 0·01 | 0·01 |
| CD3+ TCRγδ+ | 19 | 0·05 ± 0·03 | 0·10 ± 0·06 | 0·004 |
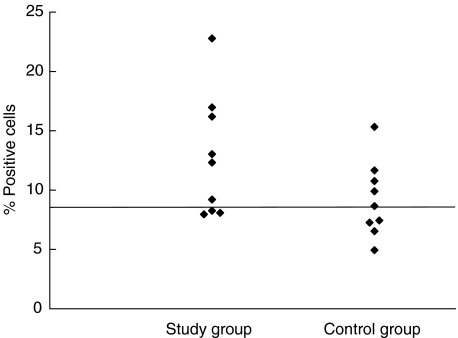
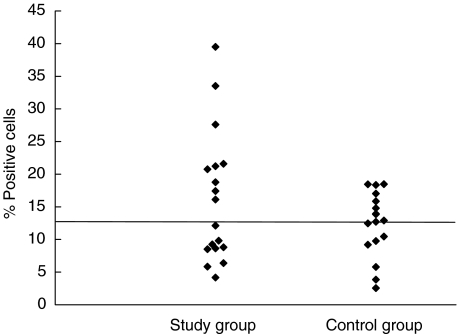
The number of lymphocytes expressing CD19 or CD16 and CD56 was not different between the study group and the control group (P = 0·43 and P = 0·98, respectively). However, the study group had a higher proportion of lymphocytes expressing these surface antigens (CD19, P = 0·116, and CD16 and CD56, P = 0·091), but the difference between the two groups was not significant. Figures 1 and 2 show the distribution of the results for these two cell populations. For both cell types there appeared to be a difference in the distribution of values around the median value of the control group, as seen in Figs 1 and 2. The difference was, however, not significant using Wilcoxon's rank sum test (P = 0·09 and P = 0·43 for NK and B cells, respectively). It has to be noted that assessment of NK cells was performed only on 10 blood samples from each group.
Fig. 1.
The distribution of the proportion of CD16+ CD56+ lymphocytes. The line shows the median value of the control group. P = 0·093 (Wilcoxon's rank sum test). Median for control group: 8·66.
Fig. 2.
The distribution of the proportion of CD19+ lymphocytes. The line shows the median value of the control group. P = 0·43 (Wilcoxon's rank sum test). Median for control group: 12·805.
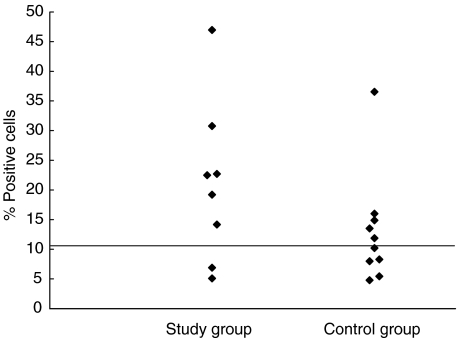
Figure 3 shows the distribution of small CD8+ cells, which express both CD62L and CD103 (the alleged small recent thymic emigrants). Again, the difference between the two groups did not reach statistical significance (P = 0·43; Wilcoxon's rank sum test).
Fig. 3.
The distribution of the proportion of small CD8+ cells in peripheral blood, which express both CD62L and CD103. The line shows the median value of the control group. P = 0·43 (Wilcoxon's rank sum test). Median for control group: 10·21.
When the data were analysed for those children with known thymectomy (total or partial) the results were the same as for the whole study group (data not shown).
T cell function
There was no difference in T cell functions between the study group and the control group when cells were stimulated with TT and MV and the effect measured by cell proliferation and IFN-γ production. All subjects showed significant proliferative response to tetanus toxoid (mean SI 2·8 for study group and 2·2 for controls) as well as PHA (mean SI 8·6 for study group and 7·4 for controls), but neither group responded significantly to measles antigen (mean SI 1·8 for test group and 1·2 for controls) or BSA (mean SI 2·0 for study group and 1·7 for controls). No significant IFN-γ production was induced by any of the stimuli except PHA (mean 1833 pg/ml for study group and 1752 pg/ml for control group), but it may be noted that measurable release was seen with BSA in the study group (mean 310 pg/ml for study group and 68 pg/ml for control group).
Autoantibodies
None of the study and control subjects had a measurable ANA or raised levels of RF.
Immunoglobulins
The concentration of IgA and IgG1 was significantly lower in the study group compared with the control group (P = 0·05 and P = 0·02, respectively). Lower levels were also seen for total IgG and IgG2 but the difference between the two groups was not significant (P = 0·28 and P = 0·21, respectively, Table 5).
Table 5.
Concentrations of immunoglobulin classes and IgG subclasses
| No. in each group | Study group g/l | Control group g/l | _P_-value | |
|---|---|---|---|---|
| IgM | 19 | 0·93 ± 0·41 | 1·04 ± 0·37 | 0·38 |
| IgA | 19 | 1·16 ± 0·60 | 1·55 ± 0·58 | 0·05 |
| IgE | 10 | 20·87 ± 23·80 | 33·09 ± 48·14 | 0·48 |
| IgG | 19 | 8·69 ± 1·81 | 9·38 ± 2·06 | 0·28 |
| IgG1 | 10 | 5·86 ± 1·30 | 7·39 ± 1·41 | 0·02 |
| IgG2 | 10 | 1·74 ± 0·81 | 2·19 ± 0·71 | 0·21 |
| IgG3 | 10 | 0·42 ± 0·14 | 0·43 ± 0·16 | 0·87 |
| IgG4 | 10 | 0·62 ± 0·60 | 0·86 ± 0·83 | 0·46 |
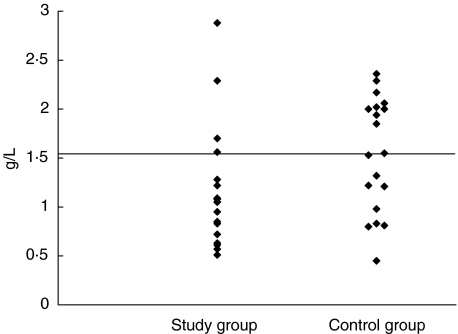
Figure 4 shows distribution of IgA values in the two groups. The difference in the distribution was significant according to the Wilcoxon rank sum test (P = 0·046).
Fig. 4.
The distribution of IgA levels. The line shows the median value of the control group. P = 0·046 (Wilcoxon's rank sum test). Median for control group: 1·55.
Clinical data
Total removal of the thymus was confirmed by the surgical report for five study patients and seven study patients had undergone partial thymectomy. In three cases no surgical report was available and in four cases the thymus was not mentioned in the description. One study patient had not undergone thymectomy. Because thymectomy was performed only for ease of surgical access to the heart and great vessels, and was not the reason for the operation, it was not possible to assess the completeness of the procedure.
None of the study patients had required hospital admission because of infection. No other differences were observed between the groups. The incidence of candidiasis in the study group was eight and six for the control group (P = 0·50), and the figures for pneumonia were six in the study group and two for the control group (P = 0·11). All participants had a normal vaccination history.
DISCUSSION
The purpose of this study was to determine the long-term effects of partial or total neonatal thymectomy in humans, by testing for laboratory and clinical parameters of T lymphocyte function in children around 10 years after they had undergone open-heart surgery in the first few months of life. The main differences found between the two groups were lower total counts of lymphocytes but higher counts for neutrophils in the study group, giving a ratio of neutrophils to lymphocytes of 2·00 compared with 0·96 in the control group. The lower number of lymphocytes was accounted for by a significantly lower number of T cells but B cell numbers were normal. The reduction of T cells was reflected in lower numbers of both CD4 and CD8 T cells, of γδT cells, and T cells of both naive and memory phenotype. In addition, significantly lower proportions of lymphocytes with the surface antigens CD3 (all T cells) and CD3 and CD4 (helper T cells) were found in the study group, whereas the proportion of CD8+ cells (cytotoxic T cells) was the same in both groups. Unexpectedly, platelet counts were lower in the study group compared with the control group. The reduction of CD3+ T cells did not reach the critically low level of approximately 20%, which has been associated with a significant increase in clinical infections [6]. In addition, the study group showed a trend towards a higher proportion of lymphocytes expressing the surface antigens CD16 and CD56 (natural killer cells).
Further analysis of the T cell population showed that half of the study group subjects had a relatively high proportion of cells showing the phenotype recently attributed to small recent thymic emigrants, that is small CD8+ cells which express both CD62L and CD103 [8]. Significantly lower values for IgG1 and IgA among the study group suggest some impairment of Th2 function [9], whereas Th1 activity appeared to be unaffected as reflected in normal IFN-γ production. It is of interest that stimulation with BSA, which was used as negative antigen control, resulted in measurable IFN-γ production in samples from the study group. In normal individuals T regulatory cells would be expected to participate in preventing such a response [10]. These T regulatory cells have been studied extensively in mice and found to express the surface antigens CD4 and CD25 and develop in the thymus [11–13]. Depletion of these T regulatory cells results in the mice developing various autoimmune diseases. Our study did not reveal any significant clinical consequences of neonatal thymectomy or any indications of autoimmunity.
Surprisingly few studies on the impact of neonatal thymectomy in humans have been reported [6, 7, 14]. These reports have documented a decreased number of T cells and CD4+ T cells compared with control groups. One study revealed a lower number of CD8+ T cells and diminished response to phytohaemagglutinin [7]. Despite this evidence of decreased immune function, no important clinical consequences of thymectomy in early life, such as infections or autoimmune diseases, were noted in these reports but none of them had follow-up beyond 3 years post-surgery.
The present study agrees with the findings of previous studies by showing a significant decrease in the number of all T cells and T cell subsets (CD4+, CD8+ and TCRγδ+), but not in B cells, in the study group. Furthermore, the study reveals a lower proportion of CD4+ T cells in the study group but no decrease in the proportion of CD8+ T cells. However, a reduction in proliferative responses to mitogens was not confirmed. The older studies did not contain more detailed analyses of T cells, which in this study revealed a decrease in the proportion of naïve T cells (CD45RA+) as well as, perhaps paradoxically, increased proportions of the alleged recent thymic emigrants in some of the patients.
All the children in this study were operated on in the same hospital (Harley Street Clinic, London, UK) and only two surgeons performed the operations. The biggest limitation of this study is the lack of ability to measure retained thymus on the basis of the surgical reports. Wells and associates [6] studied prospectively a group of neonates who underwent thymectomy at the time of heart surgery and they had difficulties in measuring retained thymus. Even though they removed the entire encapsulated thymus themselves, they could not determine how much thymic tissue remained.
Partial or total thymectomy thus had a small but measurable effect on the number of circulating T cells, with both CD4+ and CD8+ cells affected, although the proportion of CD4+ cells was more affected than the proportion of CD8+ cells. The presence of mature T cells in the apparent absence of the thymus could be explained in three ways. First, the thymus may not have been completely removed during the operation, as discussed above, leaving sufficient residual thymic tissue. Secondly, the current T cell population could be derived from the expansion of cells released from the thymus before it was removed. This would imply continuous progeny from post-thymic cells for up to 12 years. The third possibility is that the role normally served by the thymus has been taken over by extrathymic tissue. In a recently published study, Guy-Grand et al. [15] have demonstrated that this role, as regards production of gut intraepithelial lymphocytes, is fulfilled by mesenteric lymph nodes. Extrathymic T cell maturation was shown to differ from the thymic counterpart in being less efficient and having a bias towards γδ T cells. The data from our study are indeed consistent with a slower rate of production, particularly for CD4+ T cells, which is supported by the low proportion of naive T cells. The study by Guy-Grand et al. also shows that the extrathymic pathway is normally suppressed by thymus-derived T cells but can be reactivated by thymic depletion. The relatively high proportion of cells with the phenotype CD8+ CD62L+ CD103+ in our study in some of the patients seems puzzling. In the light of the preponderance of CD8+ cells it might be speculated that these cells come from the gut epithelium, CD103 being an epithelial retention receptor [16].
The study group showed some indication of compensation for the reduction in T cell number and function by increased activity of innate, non-specific immune mechanisms, i.e. neutrophils and NK cells (CD16+ CD56+). Ramos and associates [14] have suggested that the human thymus is partially involved in the control of the release of circulating T cells and may negatively modulate some NK subsets, as well as NK activity, during the first year of life but not later.
In conclusion, this study has demonstrated measurable changes in some T cell parameters consistent with decreased rate of T cell production, probably of extrathymic origin, in children who underwent heart surgery and partial or total thymectomy as neonates. This study, which has a longer follow-up period than previous studies, indicates that these children are nevertheless healthy for the first 10 years of life. Overall T cell function and differentiation in these children is not very different from the control group and there is the same number of infectious illnesses in these two groups. It remains to be seen whether the slight but significant differences in CD4+ T cell numbers and function could have clinical consequences later in life, especially under circumstances where there is greater stress on T cells, possibly resulting in higher incidence of cancer or autoimmunity.
Acknowledgments
The financial assistance of the Students Innovation Fund, Reykjavik, Iceland and Memorial Research Fund of the Children's Hospital Iceland is gratefully acknowledged. We thank the children and their parents for their willingness to participate in this study. Finally we are most grateful to the nursing staff of the Children Hospital Iceland, at Landspitali University Hospital for their assistance.
REFERENCES
- 1.Janeway C, Travers P, Walport M, Shlomchik M. ImmunobiologyThe immune system in health and disease. 5. NY: Garland Publishing; 2001. [Google Scholar]
- 2.Jamieson BD, Douek DC, Killian S, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–75. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 3.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Miller JF. Immunological functions of the thymus. Lancet. 1961;2:748–9. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein A, Pelet B, Schweizer V. Immunological decay in thymectomized infants. Helv Paediatr Acta. 1976;30:425–33. [PubMed] [Google Scholar]
- 6.Wells WJ, Parkman R, Smogorzewska E, et al. Neonatal thymectomy: does it affect immune function? J Thorac Cardiovasc Surg. 1998;115:1041–6. doi: 10.1016/S0022-5223(98)70403-9. [DOI] [PubMed] [Google Scholar]
- 7.Brearley S, Gentle TA, Baynham MI, et al. Immunodeficiency following neonatal thymectomy in man. Clin Exp Immunol. 1987;70:322–7. [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland RD, Douek DC, Koup RA, et al. Identification of a human recent thymic emigrant penotype. Proc Natl Acad Sci USA. 2000;97:4214–20. doi: 10.1073/pnas.070061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Vancott JL, Okahashi N, et al. The role of Th1 and Th2 cells for mucosal IgA responses. Ann NY Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- 10.Read S, Powrie F. CD4 (+) regulatory T cells. Curr Opin Immunol. 2001;13:644–9. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM, McHugh RS, Piccirillo CA. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:8–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 12.Shevach EM. Certified professionals: CD4 (+) CD25 (+) suppressor T cells. J Exp Med. 2001;193:F41–6. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon BS, Mason D. The third function of the thymus. Immunol Today. 2000;21:95–9. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- 14.Ramos SB, Garcia AB, Viana SR, et al. Phenotypic and functional evaluation of natural killer cells in thymectomized children. Clin Immunol Immunopathol. 1996;81:277–81. doi: 10.1006/clin.1996.0189. [DOI] [PubMed] [Google Scholar]
- 15.Guy Grand D, Azogui O, Celli S, et al. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med. 2003;197:333–41. doi: 10.1084/jem.20021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agace WW, Higgins JMG, Sadasivan B, et al. T-lymphocyte–epithelial-cell interactions: integrin αE (CD103) β7, LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–8. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]