Molecular Cytogenetic Analyses of Immunoglobulin Loci in Nodular Lymphocyte Predominant Hodgkin’s Lymphoma Reveal a Recurrent IGH-BCL6 Juxtaposition (original) (raw)
Abstract
Chromosomal translocations juxtaposing different oncogenes to the immunoglobulin (IG) loci are the hallmark of various B-cell lymphomas. Because the tumor cells in nodular lymphocyte predominant Hodgkin’s lymphoma (NLPHL) are also derived from B cells, we examined whether NLPHL harbors chromosomal translocations that affect IG loci. Fluorescence in situ hybridization was applied to 24 NLPHL cases using probes flanking the IGH, IGK, and IGL loci as well as the BCL6 gene. Fourteen of these cases were additionally analyzed by combined immunofluorescence and fluorescence in situ hybridization. Chromosomal breakpoints in the IGH locus were detected in five NLPHL. All these cases also contained a BCL6 breakpoint. Triple-color interphase cytogenetics demonstrated the presence of an IGH-BCL6 juxtaposition, indicating a t(3;14)(q27;q32) in all five cases. There was no evidence for breakpoints affecting the IGK or IGL loci. Our results show that translocations juxtaposing the BCL6 oncogene next to the IGH locus are recurrent in NLPHL.
Nodular lymphocyte predominant Hodgkin’s disease (NLPHL) is a rare type of B-cell lymphoma characterized by a nodular or a nodular and diffuse tumor proliferate.1 Large neoplastic cells, the lymphocytic and histiocytic (L&H) cells, lie within in a large mesh network of follicular dendritic cells that are filled with nonneoplastic lymphocytes. They usually account for only ∼1% of the neoplastic infiltrate and express B-cell markers.2,3,4 Analysis of rearranged immunoglobulin (IG) genes showed a derivation from germinal center B cells selected for expression of a functional antigen receptor.5,6,7,8 The cytogenetic background of NLPHL is poorly characterized.9 Clonal karyotypes of only approximately a dozen NLPHL cases studied by conventional cytogenetics have been published. Most of these karyotypes were hyperploid with multiple unresolved chromosomal changes. Comparative genomic hybridization from microdissected L&H cells of NLPHL revealed recurrent genomic imbalances.10
In early B-cell differentiation the immunoglobulin genes of B-cell precursors are rearranged. By class switch recombination, germinal center B cells change the isotype of the expressed B-cell receptor. During the process of somatic hypermutation, nucleotide substitutions, deletions, and duplications are inducted into rearranged V-genes. Each of these three processes involves double-strand DNA breaks that enable chromosomal translocations to take place.11 Chromosomal translocations juxtaposing oncogenes into the IG heavy-chain (IGH) locus at band 14q32 or less frequently the light-chain lambda (IGL) and kappa (IGK) loci, located at 22q11 and 2p12, respectively, are the hallmark of several mature B-cell lymphomas. These translocations result in deregulated expression of the oncogenes coming under the control of IG enhancers.11,12 One of these oncogenes, BCL6, encoding a transcriptional repressor, is located at 3q27. The BCL6 gene is frequently translocated to IG but also non-IG partners in germinal center derived B-cell lymphomas. Just recently, an interphase cytogenetic study has reported translocations with breakpoints in the BCL6 locus in 11 of 23 NLPHLs.13
Considering the B-cell origin of the L&H cells of NLPHL and the high incidence of translocations in IG loci in B cell, it is reasonable to assume that translocations in IG loci might also occur in NLPHL. Thus, we investigated a series of 24 NLPHLs by fluorescence in situ hybridization (FISH) for translocations involving the IGH, IGL, and IGK loci. To corroborate the findings by Wlodarska and colleagues13 in an independent series we also studied all cases for breakpoints involving the BCL6 gene. To verify the FISH results we further analyzed 14 of the cases from which sufficient material was available by combined fluorescence immunophenotyping (FICTION technique) for CD20 and FISH for IGH and BCL6 breakpoints.
Materials and Methods
Patient Material
Frozen tumor tissue samples from 24 patients with a NLPHL diagnosis were originally submitted for diagnostic purposes.
Histology and Immunohistochemistry
Morphology of the tumors was analyzed using histological sections stained with Giemsa. For immunohistology, CD15 and CD20 antibodies (DakoCytomation, Hamburg, Germany) were applied and detected using the UltraVision detection system (Lab Vision Corp., Fremont, CA). In all cases, the neoplastic L&H cells proved to be CD15-negative and CD20-positive.
FISH and FICTION
Cell nuclei were extracted out of 40-μm-thick frozen sections by mechanical separation in ice-cold phospho-buffered saline with a nylon net with 10-μm aperture diameters. Nuclei were gently dehydrated through an ethanol series of increasing grade (79%, 85%, and 96%). After collecting them on cytospins nuclei were pretreated for 5 minutes at 37°C with 0.0005% pepsin, fixed for 2 minutes in 1% paraformaldehyde, and again dehydrated through an ethanol series.
The dual-color break-apart assays LSI IGH and LSI BCL6 were obtained from Vysis (Downers Grove, IL). FISH was performed following the manufacturer’s instructions. FISH analyses for the detection of chromosomal breakpoints in the IGL and IGK loci were performed using recently established dual-color flanking probes.14 Cases harboring signal constellations indicative for chromosomal breakpoints in both the IGH locus and BCL6 gene were further studied with a triple-color FISH assay to detect the presence of an IGH/BCL6 juxtaposition. This assay was composed of the LSI IGH dual-color probe in combination with a previously published BCL6 probe set15 labeled with diethyl aminomethyl coumarin.16 The performance of this three-color probe was tested in negative controls and t(3;14)(q27;q32)-positive non-Hodgkin lymphomas.
Combined immunophenotyping and interphase cytogenetics (FICTION technique) was performed on frozen sections from 14 cases included in the FISH analyses in which suitable material was still available. Briefly, 5-μm-thick frozen sections were thawed and dried for 30 minutes at room temperature, fixed in acetone for 10 minutes, and air-dried. Slides were incubated for 30 minutes at room temperature with a primary monoclonal antibody anti-CD20 (DakoCytomation, Hamburg, Germany) diluted (1:50) in PBS-nonfat milk buffer. Detection of the primary antibody was performed with a rabbit anti-mouse antibody conjugated with Alexa-594 (1:100; Molecular Probes, Leiden, The Netherlands) and incubated for 30 minutes at room temperature. After immunophenotyping, slides were fixed in Carnoy’s fixative (ethanol:acetic acid, 3:1) for 10 minutes and in paraformaldehyde solution (1%) for 1 minute. Then, slides were dehydrated through increasing ethanol concentrations (70%, 85%, and absolute) and air-dried. LSI IGH or LSI BCL6 (1.5 μl) were applied to the cell-containing area of the slide and covered with a round 10-mm coverslip. Both probe and target DNA were simultaneously denatured at 70°C for 7 minutes and incubated overnight at 37°C. Posthybridization washes were performed according the rapid wash protocol provided by Vysis. 4,6-Diamidino-2-phenylindole was used as DNA counterstaining. A minimum of 10 CD20+ large cells were scored whenever possible (Table 2). Slides were analyzed using an Axioskop-2 fluorescence microscope (Zeiss, Göttingen, Germany) equipped with appropriate filter sets (AHF, Tübingen, Germany) and documented using the ISIS imaging system (MetaSystems, Altlussheim, Germany).
Table 2.
Detailed Results of the FICTION Analyses for the Detection of Breakpoints in the IGH and BCL6 Loci
| Case | Large CD20+ cells evaluated | |||
|---|---|---|---|---|
| IGH | BCL6 | |||
| Signal pattern | No. of cells scored | Signal pattern | No. of cells scored | |
| NP1 | 1F,1R,1G* | 17 | 1F,1R,1G* | 15 |
| 1F | 2 | |||
| 2F | 3 | |||
| NP2 | 2F | 10 | 2F | 10 |
| NP3 | 2F | 5 | 2F | 5 |
| 4F | 1 | 3F | 1 | |
| 5F | 1 | |||
| NP4 | 2F | 10 | NE | |
| NP5 | NE | NE | ||
| NP6 | 1–2F,1–2R,1–2G | 10 | 1–2F,1–2R,1–2G | 10 |
| NP7 | 2F | 7 | 2F | 6 |
| 3F | 1 | 3F | 3 | |
| 4F | 2 | 4F | 1 | |
| NP8 | 2F | 10 | 2F | 10 |
| NP9 | 2F | 10 | 2F | 10 |
| NP10 | 2F | 10 | 2F | 10 |
| NP11 | 1–2F1–2R1–2G | 10 | 1–2F1–2R1–2G | 10 |
| NP19 | 0–1F,0–1R,0–1G* | 10 | 1–2F,0–1R,0–1G | 10 |
| NP20 | 0–1F,1–2R,1–3G | 10 | 0F,1–2R,1–3G | 10 |
| NP21 | 2F | 10 | 2F | 10 |
Results
All FISH experiments with extracted nuclei were blindly evaluated using recently defined criteria17 by two independent investigators (C.R. and J.I.M.-S.). Larger nuclear size and the frequent hyperploid genomic status were used as criteria to identify putative L&H cells. At least 10 cells meeting these criteria were evaluated per case. Fifty small diploid cells were scored as internal negative controls. The diagnostic cut-offs for the IGH, IGL, and IGK probes were calculated as mean of false-positive results in negative controls plus three times the SD and were 9.2%, 13.3%, and 9.6%, respectively. These cut-offs are slightly higher as those previously published,14,17 most likely due to the different nature, preservation, and preparation of the samples studied in the present series. For the BCL6 probe, the cut-off was virtually zero because no split signals were detected in any small diploid cells.
Of the 24 NLPHL samples analyzed by FISH with the probe flanking the IGH locus 3 cases (13%) showed a significant number of putative L&H cells with a signal pattern indicating a chromosomal breakpoint in the IGH locus. As the number of putative L&H cells with signal patterns indicative for translocations affecting the IGL and IGK loci did not exceed the cut-off level in any of the 24 cases studied we did not detect any breakpoints affecting IG light chains. A significant number of large atypical cells with signal patterns indicating the presence of a breakpoint in the BCL6 locus were detected by FISH in four (17%) patients (Table 1).
Table 1.
Summary of the FISH and FICTION Analyses for the Detection of Breakpoints in the IGH, IGL, IGK, and BCL6 Loci
| IGH | IGK | IGL | BCL6 | ||
|---|---|---|---|---|---|
| Total (n) | 24 | 24 | 24 | 24 | |
| FISH results | Cases with breakpoint (n) | 3 | 0 | 0 | 4 |
| Percentage of cases with breakpoint | 13% | 0% | 0% | 17% | |
| Total cases (n) | 13 | nd | nd | 12 | |
| FICTION results | Cases with breakpoint (n) | 5* | nd | nd | 5* |
| Percentage of cases with breakpoint | 38% | nd | nd | 42% |
To prove the FISH results and to demonstrate that the chromosomal changes detected by FISH were present in the L&H cells, FICTION analyses were attempted in all 14 cases in which sufficient cryopreserved material was still available. Putative L&H cells were identified by virtue of their larger cell and nuclear size, and expression of the CD20 antigen. In the FICTION analyses, the frequent hyperploid nature of L&H cells was not considered because truncation of the nucleus by cryosection can lead to artificial signal loss. Of 13 evaluable cases, presence of an IGH breakpoint was confined to the CD20+ large cell population in the 3 NLPHL cases in which such a breakpoint was also identified by FISH. Two additional cases with CD20+ L&H cells containing breakpoints in the IGH locus were detected by FICTION (Figure 1A, Table 1, cases NP1 and NP19, Table 2). FICTION analyses with CD20 and BCL6, which were evaluable in 12 cases, revealed signal constellations indicating breaks in the BCL6 locus in the CD20+ L&H cells of 5 cases, including the 4 cases detected by FISH and 1 additional NLPHL without evidence for a BCL6 break by FISH (Table 1, case NP1; Table 2).
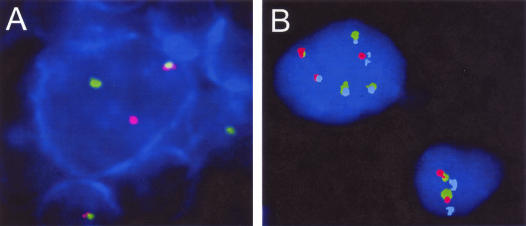
Figure 1.
A: Combined immunophenotyping and FISH for the detection of CD20 and breakpoints in the IGH locus in case NP19. The CD20-positive large cell (blue) displays a split of the signals flanking the IGH locus indicating the presence of a translocation. The CD20 antigen detected with Alexa-594 (red fluorescence) is displayed in blue. B: Triple-color FISH assay for the detection of the t(3;14)(q27;q32) translocation juxtaposing the BCL6 and IGH loci (false color display) in case NP20. This probe set was made of the LSI IGH flanking probe (IGH telomeric in green and IGH centromeric in red) combined with a _BCL6-_spanning probe labeled in DEAC (pale blue signals). The small nucleus on the right displays the regular signal constellation: two red/green co-localizations indicating intact IGH gene and two pale blue signals pointing to intact BCL6 alleles. The large nucleus on the left shows multiple split of green and red signals indicating a breakpoint in the IGH locus. Disrupted red and green signals co-localize with pale blue BCL6 signals pointing to a t(3;14)(q27;q32) translocation involving the BCL6 and IGH loci as well as the presence of several derivative chromosomes. This signal pattern was found recurrently in this case.
All five NLPHL with signal patterns indicating IGH breaks also displayed BCL6 signal patterns indicative of a breakpoint. The simultaneous occurrence of breakpoints in the IGH and BCL6 loci suggested the presence of a t(3;14)(q27;q32). To investigate the presence of this translocation, these cases were studied by FISH (three cases) or FICTION (two cases) simultaneously applying differentially labeled probes for IGH and BCL6. All five cases showed a signal pattern indicative for a t(3;14)(q27;q32) translocation in all putative L&H cells evaluated (Figure 1B, Table 2).
Discussion
In this study we analyzed the IG heavy- and light-chain loci of 24 NLPHL cases for chromosomal translocations in an open approach using FISH and FICTION techniques. The comparison of the results obtained by FISH and FICTION in this study clearly shows that FICTION seems to be the more sensitive and therefore the more qualified technique for interphase cytogenetic analyses of NLPHL. The FICTION analysis can be restricted to the CD20+ atypical large-cell population, which might be missed by FISH due to its low frequency. Moreover, it cannot be ruled out that the mechanical separation of cell nuclei through a nylon net damages large nuclei, especially nuclei of L&H cells, more frequent than those of small cells so that the samples finally collected on cytospins for FISH analyses may be depleted of L&H cell nuclei. However, although the L&H cells are considered to be frequently hyperploid, as also shown in this study by FISH, the majority of the CD20+ atypical large cells suggestive for L&H cells in 9 of the 13 cases evaluated by FICTION displayed two co-localized signals for IGH and BCL6. This discrepancy is most likely due to sectioning artifacts in the thin cryosections. Alternatively, it could also indicate that hyperploidy is not a consistent phenomenon in NLPHL.
So far, recurrent chromosomal aberrations affecting 14q32 have not been described in NLPHL by classical cytogenetics. This might be due to the scarcity of cytogenetic data, the presence of many unresolved changes and the subtelomeric location of the IGH locus which can lead to cryptic translocations.18 In this study, translocations affecting the IGH locus are reported for the first time, which were present in 38% of the NPLHLs analyzed by FICTION. BCL6 was identified as the partner gene in all cases with IGH breaks studied herein suggesting the presence of a translocation t(3;14)(q27;q32). The proportion of cases with BCL6 breakpoints in this series of NLPHL is similar to that of the recently published independent collective of 23 patients samples.13 The incidence of translocation t(3;14)(q27;q32) found in NLPHL seems similar to the frequency observed in diffuse large B-cell lymphoma19,20 and progression from NLPHL to diffuse large B-cell lymphoma has been described.21,22
The variant translocations involving IGL and IGK represent ∼5 to 10% of all IG translocations in B-cell lymphomas.23,24,25 We did not detect any breakpoints affecting IG light-chain loci in the present series, which might be simply due of the small sample size or the fact that FISH is less sensitive than FICTION to detect chromosomal breakpoints if the tumor cell content is low. In this context, it is noteworthy that a translocation t(3;22)(q27;q11) affecting BCL6 and IGL has been previously detected in two NLPHL,10,26,27 suggesting recurrent deregulation of BCL6 in NLPHL also by juxtaposition next to IG light-chain loci. Moreover, a recent publication has reported the presence of heterogeneous translocation partners of BCL6 affecting non-IG loci in chromosome bands 9p13, 7p12, and 4q32.27 In contrast to the obvious heterogeneity of BCL6 partners in NLPHL, IGH was the only translocation partner of BCL6 in the present series.
Summarizing the data presented herein and those from the independent series by Wlodarska and colleagues,13 it can be assumed that BCL6 activation by juxtaposition predominantly to IG loci is the first recurrent chromosomal change in NLPHL, which is shared with other germinal center-derived B-cell neoplasms. Future studies are required to elucidate whether the presence of BCL6 translocations defines a subgroup of NLPHLs with a distinct biological behavior as recently described in follicular lymphoma.28
Acknowledgments
We thank Andreas Bräuninger and Ralf Küppers for critical reading of the manuscript; and Sabine Albrecht, Dorit Schuster, and Claudia Becher for their excellent technical assistance.
Footnotes
Supported by the Interdisziplinäres Zentrum für Klinische Krebsforschung (IZKF, Kiel) and the Deutsche Krebshilfe.
C.R. and J.I.M.S. contributed equally to this study.
References
- Hansmann ML, Stein H, Harris NL, Jaffe ES. Philadelphia: Lippincott Williams & Wilkins,; Pathology of Lymphocyte Predominant Hodgkin’s Disease. 1999 [Google Scholar]
- Pinkus GS, Said JW. Hodgkin’s disease, lymphocyte predominance type, nodular–further evidence for a B cell derivation. L and H variants of Reed-Sternberg cells express L26, a pan B cell marker. Am J Pathol. 1988;133:211–217. [PMC free article] [PubMed] [Google Scholar]
- Mason DY, Banks PM, Chan J, Cleary ML, Delsol G, de Wolf Peeters C, Falini B, Gatter K, Grogan TM, Harris NL. Nodular lymphocyte predominance Hodgkin’s disease. A distinct clinicopathological entity. Am J Surg Pathol. 1994;18:526–530. doi: 10.1097/00000478-199405000-00014. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Küppers R, Rajewsky K, Bräuninger A, Hansmann ML. L&H cells in lymphocyte-predominant Hodgkin’s disease. N Engl J Med. 1998;338:763–765. doi: 10.1056/NEJM199803123381113. [DOI] [PubMed] [Google Scholar]
- Ohno T, Stribley JA, Wu G, Hinrichs SH, Weisenburger DD, Chan WC. Clonality in nodular lymphocyte-predominant Hodgkin’s disease. N Engl J Med. 1997;337:459–465. doi: 10.1056/NEJM199708143370704. [DOI] [PubMed] [Google Scholar]
- Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Falini B, Delsol G, Isaacson PG, Pileri S, Stein H. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med. 1997;337:453–458. doi: 10.1056/NEJM199708143370703. [DOI] [PubMed] [Google Scholar]
- Bräuninger A, Küppers R, Strickler JG, Wacker HH, Rajewsky K, Hansmann ML. Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc Natl Acad Sci USA. 1997;94:9337–9342. doi: 10.1073/pnas.94.17.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansmann ML, Godde-Salz E, Hui PK, Müller-Hermelink HK, Lennert K. Cytogenetic findings in nodular paragranuloma (Hodgkin’s disease with lymphocytic predominance; nodular) and in progressively transformed germinal centers. Cancer Genet Cytogenet. 1986;21:319–325. doi: 10.1016/0165-4608(86)90212-8. [DOI] [PubMed] [Google Scholar]
- Franke S, Wlodarska I, Maes B, Vandenberghe P, Delabie J, Hagemeijer A, De Wolf-Peeters C. Lymphocyte predominance Hodgkin disease is characterized by recurrent genomic imbalances. Blood. 2001;97:1845–1853. doi: 10.1182/blood.v97.6.1845. [DOI] [PubMed] [Google Scholar]
- Küppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R, Gaidano G. Lymphomas. DeVita VT Jr, Hellmann S, Rosenberg SA, editors. Philadelphia: Lippincott Williams and Wilkins,; 2001:2215–2235. [Google Scholar]
- Wlodarska I, Nooyen P, Maes B, Martin-Subero JI, Siebert R, Pauwels P, De Wolf-Peeters C, Hagemeijer A. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominance Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood. 2003;101:706–710. doi: 10.1182/blood-2002-05-1592. [DOI] [PubMed] [Google Scholar]
- Martin-Subero JI, Harder L, Gesk S, Schlegelberger B, Grote W, Martinez-Climent JA, Dyer MJ, Novo FJ, Calasanz MJ, Siebert R. Interphase FISH assays for the detection of translocations with breakpoints in immunoglobulin light chain loci. Int J Cancer. 2002;98:470–474. doi: 10.1002/ijc.10169. [DOI] [PubMed] [Google Scholar]
- Sanchez-Izquierdo D, Siebert R, Harder L, Marugan I, Gozzetti A, Price HP, Gesk S, Hernandez-Rivas JM, Benet I, Sole F, Sonoki T, Le Beau MM, Schlegelberger B, Dyer MJ, Garcia-Conde J, Martinez-Climent JA. Detection of translocations affecting the BCL6 locus in B cell non-Hodgkin’s lymphoma by interphase fluorescence in situ hybridization. Leukemia. 2001;15:1475–1484. doi: 10.1038/sj.leu.2402207. [DOI] [PubMed] [Google Scholar]
- Martin-Subero JI, Chudoba I, Harder L, Gesk S, Grote W, Novo FJ, Calasanz MJ, Siebert R. Multicolor-FICTION: expanding the possibilities of combined morphologic, immunophenotypic, and genetic single cell analyses. Am J Pathol. 2002;161:413–420. doi: 10.1016/S0002-9440(10)64197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Subero JI, Gesk S, Harder L, Grote W, Siebert R. Interphase cytogenetics of hematological neoplasms under the perspective of the novel WHO classification. Anticancer Res. 2003;23:1139–1148. [PubMed] [Google Scholar]
- Avet-Loiseau H, Brigaudeau C, Morineau N, Talmant P, Lai JL, Daviet A, Li JY, Praloran V, Rapp MJ, Harousseau JL, Facon T, Bataille R. High incidence of cryptic translocations involving the Ig heavy chain gene in multiple myeloma, as shown by fluorescence in situ hybridization. Genes Chromosom Cancer. 1999;24:9–15. doi: 10.1002/(sici)1098-2264(199901)24:1<9::aid-gcc2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Barrans SL, O’Connor SJ, Evans PA, Davies FE, Owen RG, Haynes AP, Morgan GJ, Jack AS. Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br J Haematol. 2002;117:322–332. doi: 10.1046/j.1365-2141.2002.03435.x. [DOI] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Zuhlke-Jenisch R, Gesk S, Martin-Subero JI, Schaller C, Van Roost D, Wiestler OD, Deckert M, Siebert R. Interphase cytogenetic analysis of lymphoma-associated chromosomal breakpoints in primary diffuse large B-cell lymphomas of the central nervous system. J Neuropathol Exp Neurol. 2002;61:926–933. doi: 10.1093/jnen/61.10.926. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Franssila KO, Saxen E. Hodgkin’s disease, lymphocytic predominance nodular. Increased risk for subsequent non-Hodgkin’s lymphomas. Cancer. 1983;51:2293–2300. doi: 10.1002/1097-0142(19830615)51:12<2293::aid-cncr2820511221>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hansmann ML, Stein H, Fellbaum C, Hui PK, Parwaresch MR, Lennert K. Nodular paragranuloma can transform into high-grade malignant lymphoma of B type. Hum Pathol. 1989;20:1169–1175. doi: 10.1016/s0046-8177(89)80007-3. [DOI] [PubMed] [Google Scholar]
- Ong ST, Le Beau MM. Chromosomal abnormalities and molecular genetics of non-Hodgkin’s lymphoma. Semin Oncol. 1998;25:447–460. [PubMed] [Google Scholar]
- Chaganti SR, Chen W, Parsa N, Offit K, Louie DC, Dalla-Favera R, Chaganti RS. Involvement of BCL6 in chromosomal aberrations affecting band 3q27 in B-cell non-Hodgkin lymphoma. Genes Chromosom Cancer. 1998;23:323–327. [PubMed] [Google Scholar]
- Tashiro S, Takechi M, Asou H, Takauchi K, Kyo T, Dohy H, Kikuchi M, Kamada N, Tsujimoto Y. Cytogenetic 2; 18 and 18; 22 translocation in chronic lymphocytic leukemia with juxtaposition of bcl-2 and immunoglobulin light chain genes. Oncogene. 1992;7:573–577. [PubMed] [Google Scholar]
- Falzetti D, Crescenzi B, Matteuci C, Falini B, Martelli MF, Van Den Berghe H, Mecucci C. Genomic instability and recurrent breakpoints are main cytogenetic findings in Hodgkin’s disease. Haematologica. 1999;84:298–305. [PubMed] [Google Scholar]
- Wlodarska I, Stul M, De Wolf-Peeters C, Hagemeijer A. Heterogeneity of BCL6 rearrangements in nodular lymphocyte predominant Hodgkin’s lymphoma. Haematologica. 2004;89:965–972. [PubMed] [Google Scholar]
- Akasaka T, Lossos IS, Levy R. BCL6 gene translocation in follicular lymphoma: a harbinger of eventual transformation to diffuse aggressive lymphoma. Blood. 2003;102:1443–1448. doi: 10.1182/blood-2002-08-2482. [DOI] [PubMed] [Google Scholar]