MDMA-like behavioral effects of N-substituted piperazines in the mouse (original) (raw)
. Author manuscript; available in PMC: 2008 Apr 30.
Published in final edited form as: Pharmacol Biochem Behav. 2007 Jul 6;88(1):18–27. doi: 10.1016/j.pbb.2007.06.007
Abstract
Few studies have characterized the subjective effects of N-substituted piperazines, but these drugs show potential for abuse in humans, and have often been associated with MDMA (“ecstasy”) in this regard. The aim of the present studies was to test the capacity of N-substituted piperazines to induce a head-twitch response, alter locomotor activity, and induce MDMA-like discriminative stimulus effects in mice. Various doses of l-benzylpiperazine (BZP), 1-(3-trifluoromethylphenyl) piperazine (TFMPP), 1-(3-methoxybenzyl) piperazine (m-MeO-BZP) or meta-chlorophenyl piperazine (m-CPP) were administered to mice to determine effects on these behavioral endpoints. BZP, but not its meta-methoxyl analogue, increased locomotor activity in a dose-dependent manner; the phenylpiperazines and m-MeO-BZP only decreased locomotor activity. TFMPP was the only compound active in the head twitch assay, eliciting a moderate head twitch response which was comparable to that previously observed with the MDMA enantiomers. BZP, TFMPP and m-CPP fully substituted in S(+)-MDMA-trained animals, but did not elicit significant drug lever responding in mice trained to discriminate R(−)-MDMA. m-MeO-BZP partially substituted for both training drugs. The present results suggest that BZP has stimulant-like effects, and that TFMPP has hallucinogen-like effects. Their structural analogues, however, do not share these behavioral profiles. Further studies into the relationships between the N-substituted piperazines and MDMA are warranted.
Keywords: MDMA, drug-discrimination, head twitch response, locomotor activity
Introduction
Human users of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) report that the drug elicits a complex cluster of subjective effects, including both stimulant-like and hallucinogen-like actions (Vollenweider et al. 1998). Use of MDMA has been increasing across the globe, particularly among young adults (Landry, 2002), and despite regulatory control of MDMA here in the United States, federal law enforcement officers continue to encounter and confiscate MDMA across the country. In addition to this reasonably widespread use of MDMA itself, adulterated “ecstasy” tablets have been shown to contain a number of compounds, several of which are N-substituted piperazines. Among the most common combination tablets in this regard consist of 1-benzylpiperazine (BZP, Figure 1A) and 1-(3-trifluoromethylphenyl) piperazine (TFMPP, Figure 1C) in a 2:1 ratio, as estimated by the DEA System to Retrieve Information From Drug Evidence (STRIDE) program (US DEA, 2003). Ingestion of this drug combination in an attempt to mimic the effects of MDMA has been reported in Europe (de Boer et al., 2001). Similarly, drug users have posted experiences with meta-chlorophenyl piperazine (m-CPP, Figure 1D) to internet sites specializing in the dissemination of drug information, such as erowid.org and lycaeum.org, and the drug has been used as a positive control for MDMA in human studies (Tancer and Johanson, 2001; Tancer and Johanson, 2003). Interestingly, some cocaine (Buydens-Branchey et al., 1997), alcohol (Benkelfat et al., 1991), and MDMA abusers (McCann et al., 1999) have reported ‘euphoric’ responses to m-CPP, perhaps explaining its recreational use. We are not aware of recreational use of 1-(3-methoxybenzyl) piperazine (m-MeO-BZP, Figure 1B), and literature searches did not return any relevant behavioral or pharmacological data concerning this compound. However, among the phenethylamines (such as amphetamine, MDMA, and mescaline), the addition of multiple oxygen-containing substituents to the phenyl ring typically abolishes stimulant effects and confers a hallucinogen-like behavioral profile. Thus, m-MeO-BZP was synthesized to determine whether similar structure-activity relationships (SAR) hold among the N-substituted piperazines.
Figure 1.
Chemical structures of the drugs used in this study. A. l-benzylpiperazine (BZP), B. 1-(3-Methoxybenzyl)piperazine (m-MeO-BZP), C. trifluoromethylphenyl-piperazine (TFMPP), D. _m_-chlorophenylpiperazine (m-CPP).
We have previously investigated the reinforcing and discriminative stimulus effects of BZP and TFMPP in rhesus monkeys (Fantegrossi et al., 2005a) and rats (Fantegrossi et al., 2004a). In these studies, BZP was self-administered and amphetamine-like in drug discrimination, while TFMPP was not self-administered by rhesus monkeys, but had MDMA-like discriminative stimulus effects in rats. In accordance with these studies, stimulant-like effects of BZP have been demonstrated in humans (Campbell et al., 1972) and rats (Oberlander et al., 1979; Bauman et al., 2005). The binding profile of TFMPP at various serotonin receptors is complex, as similar potencies have been reported for TFMPP at 5-HT1A, 5-HT1B and 5-HT2C receptors (Schoeffter and Hoyer, 1989). Additional studies have suggested that TFMPP may be either an antagonist (Conn and Sanders-Bush, 1987) or a weak partial agonist (Grotewiel et al., 1994) at 5-HT2A receptors as well. Similar to MDMA, TFMPP has also been shown to increase 5-HT in nucleus accumbens dialysate in the rat (Bauman et al., 2005). A previous study (Pettibone and Williams, 1984) also demonstrated that both TFMPP and m-CPP stimulate 5-HT release from hypothalamic slices via a mechanism dependant on serotonin transporters. Thus, based on these results, we have hypothesized that BZP should function as a behavioral stimulant, while the phenylpiperazines should be hallucinogen-like in animal models. Furthermore, consistent with phenethylamine SAR, we expected m-MeO-BZP to be less stimulant-like and more hallucinogen-like, as compared to BZP.
To test these hypotheses, we investigated the effects of BZP and m-MeO-BZP, as well as TFMPP and m-CPP, in a murine assay of drug-elicited head twitch behavior. The head twitch response (HTR) (Corne et al., 1963; Corne and Pickering, 1969) is a selective behavioral model for 5-HT2A agonist activity in the rodent, and several previous studies have established that direct and indirect 5-HT agonists induce this effect (Peroutka et al., 1981; Colpaert and Janssen, 1983; Green et al., 1983; Goodwin and Green, 1985; Darmani et al., 1990a, 1990b, 1992; Fantegrossi et al., 2004b). All N-substituted piperazines were also tested for locomotor stimulant effects in a modified open field apparatus. Finally, mice trained to discriminate either S(+)-MDMA or R(−)-MDMA from saline were tested for generalization to all of the N-substituted piperazines described above. In the mouse, S(+)-MDMA stimulates locomotor activity, while R(−)-MDMA does not (Fantegrossi et al., 2003). Similarly, the behavioral effects of S(+)-MDMA in the mouse are dependent on monoamine release, while those of R(−)-MDMA are related to agonist effects at 5-HT receptors (Fantegrossi et al., 2005b). Finally, in the rat, S(+)-MDMA dose-dependently inhibited [3H]dopamine uptake into striatal synaptosomes, while the R(−)- enantiomer was inactive in this regard (Steele et al., 1987). Thus, stimulant-like drugs may be more likely to substitute for the discriminative cue induced by S(+)-MDMA, while hallucinogen-like compounds may be more apt to substitute for R(−)-MDMA.
Materials and Methods
Animals
Male Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, MA) weighing approximately 20–30 g were housed 5 animals per 44.5 × 22.3 × 12.7 cm Plexiglas cage in a temperature-controlled room within the Yerkes National Primate Research Center. The rodent vivarium was maintained at an ambient temperature of 22±2°C at 45–50% humidity, and lights were set to a 12-h light/dark cycle. Animals were fed Lab Diet rodent chow (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St. Louis, MO) and water ad libitum until immediately before testing. Mice were not used in experiments until at least 2 days after arrival in the laboratory, and there was no specific handling regimen employed in these studies. All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health, and experimental protocols were approved by the Animal Care and Use Committee at Emory University.
Procedure
MDMA-like discriminative stimulus effects
Six small animal test chambers (Med-Associates Model ENV-008), each equipped with a house light and an exhaust fan, and housed in larger lightproof Malaguard sound attenuating cubicles (Med-Associates Model ENV-022M) were used for these experiments. Each chamber contained two retractable levers mounted on opposite sides of one wall. Centered between the levers was a spout that delivered approximately 0.02 ml of non-dairy vanilla-flavored liquid diluted 1:1 with tap water. Chambers have been bisected to allow for the simultaneous testing of two animals at a time, and the stainless steel bar flooring was overlaid with chickenwire. Animals tested simultaneously in the same chamber are conspecifics within the same home cage during periods when they are not being tested, and are separated from each other within the chamber by an opaque polycarbonate wall during experimental sessions.
Six subjects were trained to discriminate S(+)-MDMA (1.5 mg/kg) from saline, and six mice were trained to discriminate R(−)-MDMA (1.5 mg/kg) from saline under a modified “errorless training” procedure using the MED-PC version IV behavioral programming application. After injection, mice we placed into the experimental chambers for 5 minutes, during which time both levers were retracted and all lights were off. After this pretreatment period, house lights were illuminated and both levers were extended into the chambers. Emission of a single injection-inappropriate response immediately retracted that lever, was quantified as an error, extinguished the house light, and imposed a 30 second timeout. During this timeout, responses on the remaining (injection-appropriate) lever had no programmed consequences. After the timeout elapsed, the house light was again illuminated, and the emission of 10 responses on the remaining lever (FR10) retracted that lever, lit a red stimulus light mounted above the spout, and delivered liquid reinforcement. Two seconds later, all lights were extinguished for 10 seconds. After this inter-reinforcement timeout, the house light was illuminated and both levers were reintroduced. When both levers were present, completion of 10 consecutive responses on the injection-appropriate lever retracted both levers and delivered liquid reinforcement as described above. In this manner, the percent of responses on the injection-appropriate lever was always 90% or more of the total responses emitted. As such, our metric for discriminative performance was the number of reinforcers received, divided by the sum of errors plus reinforcers received, referred to as percent correct choices. Sessions lasted for 30 minutes, or until 20 reinforcers were received (whichever came first.)
Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, animals made 80% or more corrected choices. After stimulus control was established with the training agents, tests with the various N-substituted piperazines were conducted once per week in each animal so long as performance did not fall below the criterion level of 80% correct responding in any one of the previous three training sessions. Half of the test sessions were conducted the day after saline training sessions with the remainder following drug training sessions. During test sessions, a multiple component cumulative dosing procedure was used, and no responses were reinforced. Each component was terminated after the emission of ten responses on either lever. Mice were then removed from the chamber, administered the next cumulative dose, then returned to the chamber. Five minutes later, levers were re-extended into the experimental space. In this manner, four doses of drug could be tested in a single session. The distribution of responses between the two levers was expressed as a percentage of total responses emitted on the drug-appropriate lever. Response rate was calculated for each session by dividing the total number of responses emitted on both levers by the elapsed time prior to 10 responses on either lever.
Criteria for discriminative performance were those we have previously employed in collaborative studies in the rat (e.g., Fantegrossi et al., 2006; Fantegrossi et al., 2005c). Complete generalization of a training drug to a test drug is said to be present when (a) a mean of 80% or more of all test responses occurs on the drug-appropriate lever; (b) there is no statistically significant difference between the response distributions of the training drug and the test drug; and (c) there is a statistically significant difference between the response distributions of the test drug and saline control sessions. An intermediate degree of generalization is defined as being present when response distributions after a test drug are less than 80% drug-appropriate, and are significantly different from both training conditions. Finally, when the response distribution after a test drug is not statistically significantly different from that in saline control sessions, an absence of generalization of the training drug to the test drug is assumed. Failure to complete an FR10 on either lever within a component terminated the sessions and indicated disruption of schedule-controlled behavior. In such cases, only data from animals completing the component are included in figures and statistical analyses.
Upon completion of all substitution trials, mice were tested in the open field assay with 30.0 mg/kg BZP and in the head twitch assay with 10.0 mg/kg TFMPP. The purpose of these experiments was to determine whether discrimination training and the concomitant exposure to MDMA rendered these animals more or less sensitive to stimulant-like or hallucinogen-like effects of the N-substituted piperazines. These studies were conducted as described in the appropriate sections below.
Drug-elicited head-twitch response
On experimental days, groups of six mice were weighed, marked, and returned to the home cage. Doses were then calculated and prepared for injection. Individual animals were subsequently removed from the home cage, injected, and placed into a 15.24 × 25.40 × 12.70 cm Plexiglas mouse cage. Methods for measuring drug-elicited head twitch behavior have been previously described (Corne et al. 1963; Corne and Pickering 1969; Fantegrossi et al. 2004b). For the present experiments, mice were injected with various doses (between 1.0 and 30.0 mg/kg) of BZP, m-CPP, TFMPP, m-MeO-BZP or saline, then returned to the small observation cage. Five minutes after this injection, a camera mounted above the observation cage began recording behavior, and continued to do so for 10-min. Videotapes were later scored by blind observers for twitch behavior, here defined as a rapid rotational jerk of the head that is not contiguous with any grooming or scratching behaviors. All such experiments were conducted in a proximate behavioral laboratory at an ambient temperature of 22±2°C, and neither food nor water were available during the tests.
Effects on locomotor behavior
All animals (n = 6 per group) were handled and weighed prior to initiation of locomotor experiments. Following injection, animals were directly placed into the locomotor chambers and activity was monitored and quantified for 2 h using a modified open field activity system under low light conditions (San Diego Instruments, San Diego, CA), but mice were not habituated to the locomotor chambers prior to injection. Each clear Lexan chamber (40 cm × 40 cm × 40 cm) was fitted with textured polycarbonate flooring and isolated from the rest of the laboratory by a zippered opaque curtain. Chambers were surrounded with a 16 × 16 photobeam grid located 2.5 cm from the floor of the chamber (to quantify ambulatory behavior) and by a second array of 16 photobeams mounted 7.0 cm above the floor (to quantify rearing behavior). Beam breaks were recorded as an index of locomotor activity, and repetitive interruptions of the same beam were tracked as behavioral stereotypy. Doses studied for effects on locomotor activity were chosen based on their capacity to suppress responding in the drug discrimination experiments described below.
Data analysis
Graphical presentation of all data depicts mean ± SEM. Drug discrimination data are expressed as percent drug-appropriate responding, which is the number of responses emitted on the drug-appropriate lever as a percentage of the total number of responses emitted. Response rates are expressed as the number of responses per minute, calculated for each session by dividing the total number of responses emitted (prior to the emission of 10 responses on either lever) by elapsed time. Data for any subjects failing to emit 10 responses within the constraints of the 10-min test session were not considered in the calculation of the percent drug-appropriate responding or response rates. Generalization was said to occur if 80% or more of the responses were on the drug-appropriate lever. The statistical significance of the generalization of a training drug to the N-substituted piperazines was determined using one-way ANOVA to compare the two training conditions with the test drug. Subsequent multiple comparisons were made by the method of Student-Newman-Keuls. Control data were repeated for each comparison and statistical analyses were applied using the appropriate control sessions. However, for purposes of clarity, mean values for control data are shown in all figures. Data from HTR experiments and total locomotor activity were compared to values obtained from equivolume saline controls using one way ANOVA and Tukey’s post-hoc tests. Locomotor timecourse data were compared by two way repeated measures ANOVA with time and injection as factors. All statistical tests were performed using commercially available software, and significance was judged at P<0.05.
Drugs
S(+)-MDMA, R(−)-MDMA, and BZP were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC), TFMPP and m-CPP were purchased from Sigma (St. Louis, MO), and m-MeO-BZP was synthesized at the Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, (Baltimore, MD) and provided as a generous gift. All compounds were dissolved in 0.9% physiological saline, and all injections were administered intraperitoneally at a volume of 1.0 ml/100g.
Results
MDMA-like discriminative stimulus effects
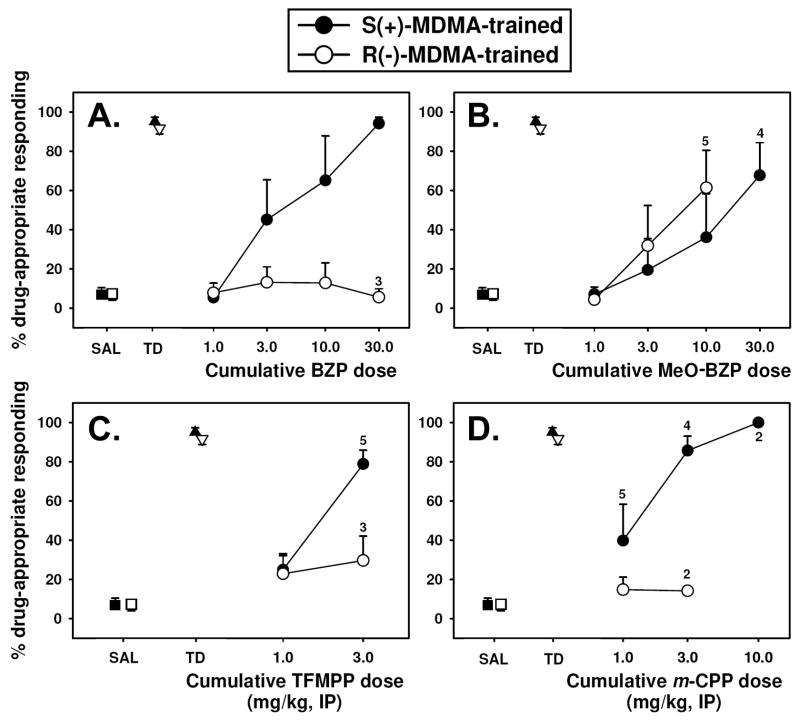
BZP dose-dependently and fully substituted for S(+)-MDMA (Figure 2A, closed circles) at a dose of 30.0 mg/kg, and reduced response rates (Table 1). The ED50 for BZP in mice trained to discriminate S(+)-MDMA from saline was approximately 3.0 mg/kg. Interestingly, BZP did not elicit drug-appropriate responding in mice trained to discriminate R(−)-MDMA from saline (Figure 2A, open circles) up to doses which suppressed responding (Table 2). The complete suppression of responding in 3 of 6 mice trained with R(−)-MDMA as a discriminative stimulus at a dose of 30.0 mg/kg BZP prevented the testing of higher doses in this group, thus, an ED50 could not be determined in these animals.
Figure 2.
Effects of substituted piperazines in mice trained with 1.5 mg/kg S (+)-MDMA (closed circles) or 1.5 mg/kg R (−)-MDMA (open circles) as a discriminative stimulus (N=6 per group), then tested with BZP (A.), m-MeO-BZP (B.), TFMPP (C.), or m-CPP (D.). All points represent the mean ± SEM, and any points without error bars indicate instances in which the SEM is encompassed by the data point. Abscissae: Dose of drug expressed as mg/kg and plotted on a log scale. The points at SAL and TD represent saline and MDMA training sessions, respectively. Ordinates: Percent MDMA-appropriate responding. A numeral adjacent to a symbol indicates the number of animals completing the test, if less than 6.
Table 1.
Rates of responding (per second) following injection with various drugs and doses in mice trained to discriminate 1.5 mg/kg S (+)-MDMA from saline.
| Injection | Rate | SEM | # completing |
|---|---|---|---|
| Saline | 1.57 | 0.43 | 6/6 |
| S(+)-MDMA | 1.11 | 0.45 | 6/6 |
| 1.0 BZP | 0.45 | 0.15 | 6/6 |
| 3.0 BZP | 0.55 | 0.26 | 6/6 |
| 10.0 BZP | 0.45 | 0.14 | 6/6 |
| 30.0 BZP | 0.05 | 0.01 | 6/6 |
| 1.0 m-MeO-BZP | 0.41 | 0.14 | 6/6 |
| 3.0 m-MeO-BZP | 0.46 | 0.18 | 6/6 |
| 10.0 m-MeO-BZP | 0.31 | 0.14 | 6/6 |
| 30.0 m-MeO-BZP | 0.05 | 0.01 | 4/6 |
| 1.0 TFMPP | 0.13 | 0.06 | 6/6 |
| 3.0 TFMPP | 0.14 | 0.08 | 5/6 |
| 1.0 m-CPP | 0.14 | 0.04 | 5/6 |
| 3.0 m-CPP | 0.02 | 0.00 | 4/6 |
| 10.0 m-CPP | 0.02 | 0.01 | 2/6 |
Table 2.
Rates of responding (per second) following injection with various drugs and doses in mice trained to discriminate 1.5 mg/kg R (−)-MDMA from saline.
| Injection | Rate | SEM | # completing |
|---|---|---|---|
| Saline | 1.29 | 0.51 | 6/6 |
| R(−)-MDMA | 1.08 | 0.50 | 6/6 |
| 1.0 BZP | 0.60 | 0.23 | 6/6 |
| 3.0 BZP | 0.43 | 0.20 | 6/6 |
| 10.0 BZP | 0.21 | 0.12 | 6/6 |
| 30.0 BZP | 0.08 | 0.08 | 3/6 |
| 1.0 m-MeO-BZP | 0.34 | 0.15 | 6/6 |
| 3.0 m-MeO-BZP | 0.37 | 0.14 | 6/6 |
| 10.0 m-MeO-BZP | 0.21 | 0.18 | 5/6 |
| 1.0 TFMPP | 0.32 | 0.15 | 6/6 |
| 3.0 TFMPP | 0.12 | 0.07 | 3/6 |
| 1.0 m-CPP | 0.35 | 0.23 | 6/6 |
| 3.0 m-CPP | 0.12 | 0.07 | 2/6 |
m-MeO-BZP dose-dependently and partially substituted for S(+)-MDMA (Figure 2B, closed circles) and for R(−)-MDMA (Figure 2B, open circles). At the highest dose tested, responding was completely suppressed in two of six mice in the R(−)-MDMA group (Table 1). The ED50 for m-MeO-BZP in mice trained to discriminate S(+)-MDMA from saline was approximately 17.0 mg/kg, while the ED50 in R(−)-MDMA-trained animals was approximately 5.6 mg/kg. Interestingly, m-MeO-BZP was the only drug to elicit drug-appropriate responding in mice trained to discriminate R(−)-MDMA from saline.
TFMPP dose-dependently and fully substituted for S(+)-MDMA (Figure 2C, closed circles) at a dose of 3.0 mg/kg, and completely suppressed responding in all animals at 10.0 mg/kg (Table 1). The ED50 for TFMPP in mice trained to discriminate S(+)-MDMA from saline was approximately 1.7 mg/kg. TFMPP did not elicit drug-appropriate responding in mice trained to discriminate R(−)-MDMA from saline (Figure 2C, open circles) and completely suppressed responding in all six animals at doses above 3.0 mg/kg (Table 2). Thus, an ED50 for this compound could not be determined in this group of animals.
m-CPP dose-dependently and fully substituted for S(+)-MDMA (Figure 2D, closed circles) at doses of 3.0 and 10.0 mg/kg, and suppressed responding in all animals at 30.0 mg/kg (Table 1). The ED50 for m-CPP in mice trained to discriminate S(+)-MDMA from saline was approximately 1.0 mg/kg. No dose of m-CPP elicited drug-appropriate responding in mice trained to discriminate R(−)-MDMA from saline (Figure 2D, open circles), and doses above 3.0 mg/kg completely suppressed responding in all animals (Table 2), precluding an ED50 determination for this compound.
Drug-elicited head-twitch response
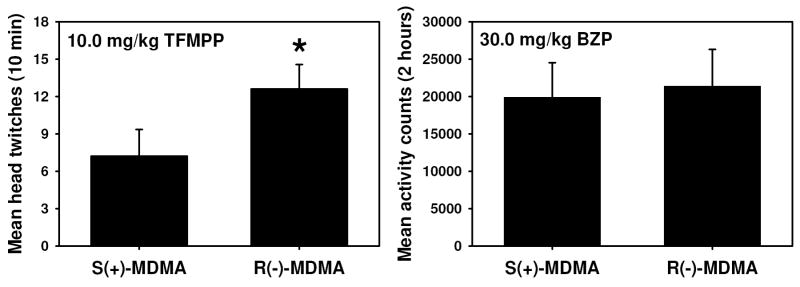
Administration of BZP (Figure 3, inverted filled triangles, F=1.90, P= 0.386), m-MeO-BZP (Figure 2, filled circles, F=2.20, P= 0.141), or m-CPP (Figure 3, open diamonds, F=1.10, P= 0.371) did not elicit significant HTR at any of the doses tested. However, TFMPP (Figure 3, open triangles) induced a dose-dependent and significant (F=18.00, P<0.05) HTR in mice, producing a maximum of approximately 5 twitches during the 10 min observation period at a dose of 10.0 mg/kg. By way of comparison, this is approximately the same maximal HTR previously reported for the MDMA enantiomers (Fantegrossi et al., 2004b; 2005b). Only the 10.0 mg/kg dose of TFMPP elicited significantly more head twitch behavior than did saline, and the HTR induced by this dose was also significantly different from that produced by all other TFMPP doses. Mice trained to discriminate S(+)-MDMA or R(−)-MDMA from saline also exhibited marked head twitch behavior when when injected with 10.0 mg/kg TFMPP (Figure 4, left panel). This dose of TFMPP induced a significantly greater HTR in mice trained to discriminate R(−)-MDMA than in drug-naïve mice (F=4.863, P<0.05), suggesting that repeated administration of R(−)-MDMA rendered these animals more sensitive to the effects of TFMPP. In contrast, the HTR elicited in S(+)-MDMA-trained animals was not different than that observed in either the drug-naïve (P=0.70) or R(−)-MDMA-trained mice (P=0.112).
Figure 3.
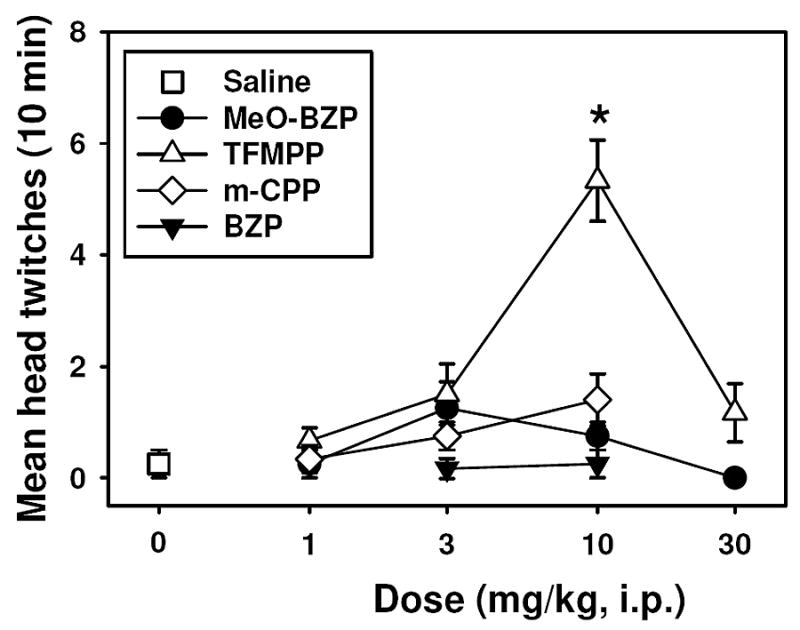
Effects of substituted piperazines on head twitch behavior. All points represent the mean ± SEM (N = 6 mice per group), and any points without error bars indicate instances in which the SEM is encompassed by the data point. Abscissa: Dose of drug expressed as mg/kg on a log scale. Ordinate: Mean head twitches recorded over a 10 minute observation period. Asterisks indicate significant differences from saline controls (open squares) (P<0.05) by ANOVA and Tukey’s post hoc test.
Figure 4.
Left panel: Effects of 10.0 mg/kg TFMPP on head twitch behavior in mice trained to discriminate S(+)-MDMA or R(−)-MDMA from saline. Graph properties as described in Figure 3. Asterisks indicate significant differences from drug-naive controls receiving this dose (open triangles in Figure 3) (P<0.05) by ANOVA and Tukey’s post hoc test. Right panel: Effects of 30.0 mg/kg BZP on locomotor activity summed over 2 hours in mice trained to discriminate S(+)-MDMA or R(−)-MDMA from saline. Graph properties as described in Figure 5.
Effects on locomotor behavior
BZP dose-dependently increased locomotor activity in the modified open field, as compared to equivolume saline controls. Examining the total ambulatory activity across the 2 hour cycle (Figure 5, A.) indicates that drug administration induced significantly more locomotor behavior than did saline (F=39.94, P<0.05). Administration of 30.0 mg/kg BZP approximately doubled locomotor activity compared to saline controls, while mice injected with 100.0 mg/kg BZP were approximately 3.5-fold more active than saline controls. Analysis of timecourse data (Figure 5, B.) indicates significant main effects of time (F= 11.79, P<0.05) and injection (F= 27.79, P<0.05), as well as a significant interaction (F= 2.39, P<0.05). Locomotor activity induced by 30.0 mg/kg BZP was similar to that elicited by saline injection for approximately the first 45 minutes of testing, but for the remainder of the experimental period, mice receiving this dose of BZP were more active than saline controls. In contrast, 100.0 mg/kg BZP induced hyperlocomotion within 10 minutes after administration, and this effect persisted for the remainder of the experimental interval. By way of comparison, S(+)-MDMA, but not R()-MDMA, also increased locomotor activity in the mouse (Fantegrossi et al., 2004b, 2006b). Mice trained to discriminate S(+)-MDMA or R(−)-MDMA from saline behaved similarly to drug-naïve animals when injected with 30.0 mg/kg BZP in the open field apparatus (Figure 4, right panel), indicating that the repeated injections of MDMA received during discrimination training produced neither sensitization nor tolerance to the locomotor stimulant effects of BZP.
Figure 5.
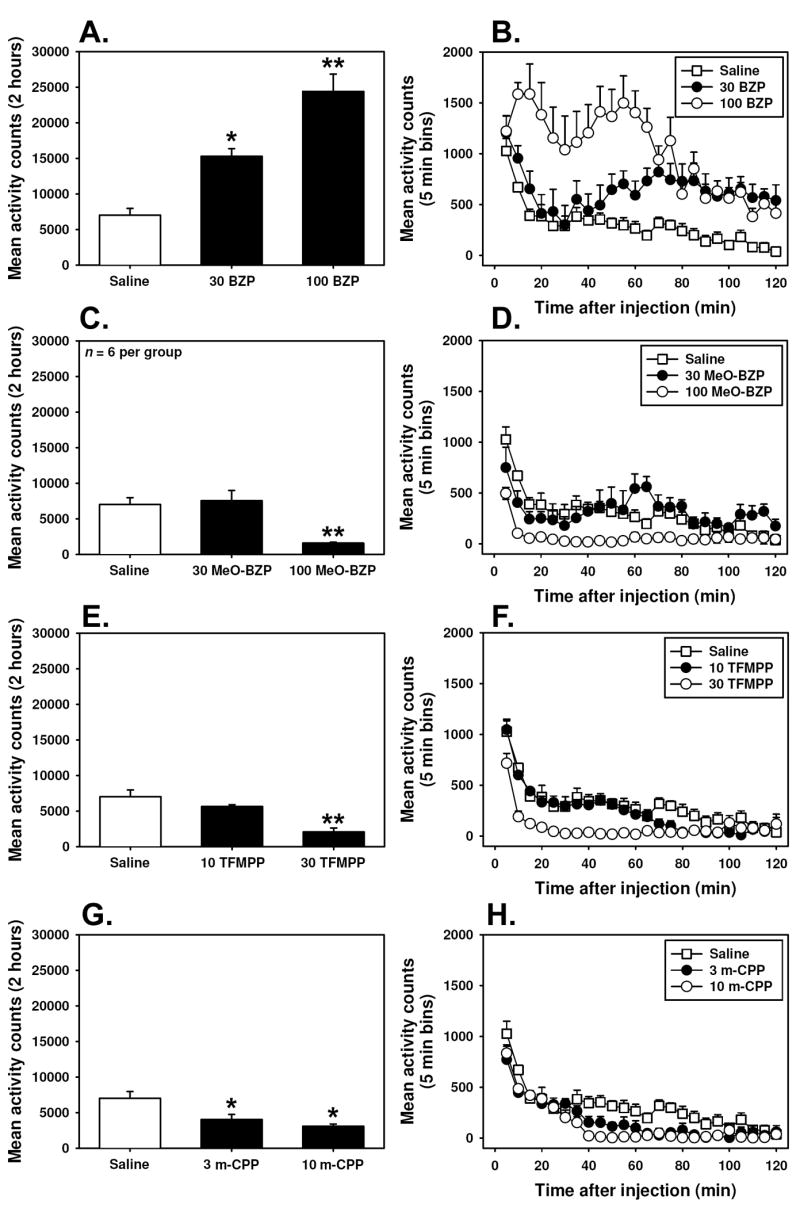
Effects of substituted piperazines on locomotor behavior summed over 2 hours (left panels) or expressed as mean ambulatory counts across successive 5 minute bins (right panels, any points without error bars indicate instances in which the SEM is encompassed by the data point). Abscissae: Dose of drug expressed as mg/kg (left panels) or time after injection in minutes (right panels). Ordinates: Mean total ambulatory activity recorded over the entire 2 hour experiment (left panels) or mean ambulatory activity per 5 minute interval (right panels). Asterisks in left panels indicate significant differences from saline controls (open bars) (P<0.05) by ANOVA and Tukey’s post hoc test; double asterisks indicate significant differences from both saline controls and the lower drug dose using the same statistical tests. To preserve figure readability, indicators of statistical significance have been omitted from graphs in the right panels, but have been outlined in the Results section.
Administration of m-MeO-BZP did not increase locomotor activity in the modified open field, and decreased ambulatory activity at the highest dose tested. Examining the total ambulatory activity across the 2 hour cycle (Figure 5, C.) indicates that drug administration induced significantly less locomotor behavior than did saline (F=13.31, P<0.05), and post-hoc testing revealed that this effect was significant at the 100.0 mg/kg dose, but not at 30.0 mg/kg. Analysis of timecourse data (Figure 5, D.) indicates significant main effects of time (F= 12.37, P<0.05) and injection (F= 13.31, P<0.05), as well as a significant interaction (F= 2.83, P<0.05). Locomotor activity induced by 30.0 mg/kg m-MeO-BZP, a dose sufficient to abolish responding in a majority of the drug discrimination animals, was similar to that elicited by saline injection throughout the duration of the experimental period. In contrast, 100.0 mg/kg m-MeO-BZP induced hypolocomotion almost immediately after administration, and this effect persisted for the remainder of the experimental interval.
TFMPP did not increase locomotor activity in the modified open field, and decreased ambulatory behavior at the highest dose tested. Examining the total ambulatory activity across the 2 hour cycle (Figure 5, E.) indicates that drug administration induced significantly less locomotor behavior than did saline (F=19.11, P<0.05), and post-hoc testing revealed that this effect was significant at the 30.0 mg/kg dose, but not at 10.0 mg/kg. Analysis of timecourse data (Figure 5, F.) indicates significant main effects of time (F= 46.82, P<0.05) and injection (F= 19.44, P<0.05), as well as a significant interaction (F= 4.83, P<0.05). Locomotor activity induced by a 10.0 mg/kg dose of TFMPP, which completely suppressed responding in all drug discrimination animals, was similar to that elicited by saline injection for approximately the first hour of testing, but steadily decreased throughout the duration of the experimental period. In contrast, 30.0 mg/kg TFMPP induced hypolocomotion almost immediately after administration, and this effect persisted for the remainder of the experimental interval.
Injection of m-CPP decreased locomotor activity in the modified open field. Examining the total ambulatory activity across the 2 hour cycle (Figure 5, G.) indicates that drug administration induced significantly less locomotor behavior than did saline (F= 10.48, P<0.05), and post-hoc testing revealed that this effect was significant at the 30.0 mg/kg dose, but not at 10.0 mg/kg. Analysis of timecourse data (Figure 5, H.) indicates significant main effects of time (F= 46.94, P<0.05) and injection (F= 20.24, P<0.05), as well as a significant interaction (F= 2.67, P<0.05). Locomotor activity induced by 3.0 or 10.0 mg/kg doses of m-CPP, which completely suppressed responding in 50% or more of all drug discrimination animals, was similar to that elicited by saline injection for approximately the first 40 minutes of testing, but steadily decreased throughout the duration of the experimental period.
Discussion
Of the N-substituted piperazines tested, only BZP exhibited a clear stimulant-like pattern of behavioral effects (see Table 3 for a summary of all drugs in all assays). BZP lacked hallucinogen-like actions in the test of drug-elicited HTR, induced a dose-dependent locomotor stimulant effect in the open field, and fully substituted for the stimulant-like S(+)- enantiomer of MDMA. As would be predicted by the phenethylamine SAR, the stimulant effects of BZP were attenuated by the addition of a methoxy group on the phenyl ring. m-MeO-BZP did not stimulate locomotor activity in the open field, and only decreased activity at the highest dose tested. The notion that this structural change would result in a more hallucinogen-like pattern of behavioral effects was neither confirmed nor rejected by these data. Although m-MeO-BZP was the only compound to substitute (albeit partially) for the hallucinogen-like R(−)- enantiomer of MDMA, it was inactive in the head twitch assay. m-MeO-BZP, and perhaps other novel oxygenated benzylpiperazines, should be tested in animals trained to discriminate more traditional hallucinogens, such as the phenethylamine 2,5-dimethoxy-4-iodoamphetamine (DOI) or the ergoline lysergic acid diethylamide (LSD), as MDMA and its enantiomers have some novel effects which are not shared by classical hallucinogens (Nichols, 1986), which could influence generalization tests in the drug discrimination assay.
Table 3.
Summary of experimental results.
| Drug | Head Twitch | Locomotor | S(+)-MDMA Discrimination | R(−)-MDMA Discrimination |
|---|---|---|---|---|
| BZP | no | increase | Full | None |
| m-MeO-BZP | no | decrease | Partial | Partial |
| TFMPP | yes | decrease | Full | None |
| m-CPP | no | decrease | Full | None |
Our hypotheses regarding the behavioral effects of phenylpiperazines were only partially confirmed by these experiments. Although TFMPP was active in the head twitch assay and was approximately as effective as MDMA in this regard (Fantegrossi et al., 2004b; 2005b), it substituted for the stimulant-like S(+)- enantiomer of MDMA, but not for the hallucinogen-like R(−)-MDMA. Furthermore, m-CPP was devoid of effects on head twitch behavior, but shared a similar profile with TFMPP in terms of discriminative stimulus effects. In agreement with previous research (e.g., Bauman et al., 2005), neither TFMPP nor m-CPP stimulated locomotor activity. Indeed, at high doses, both compounds suppressed locomotor behavior, an effect which seems to be consistent with the locomotor slowing and ataxia induced in rhesus monkeys administered high doses of TFMPP (Fantegrossi et al., 2005a). Nevertheless, in previous rodent experiments (Schechter, 1989; Fantegrossi et al., 2004a), rats trained to discriminate racemic MDMA from saline dose-dependently generalized to the interoceptive cue elicited by TFMPP. The present studies would suggest that this generalization was driven more by the interoceptive effects of S(+)-MDMA than by the stimulus effects of R(−)-MDMA. Interestingly approximately 50% of humans trained to discriminate amphetamine from m-CPP reported racemic MDMA to be m-CPP-like, while the remainder reported racemic MDMA to be more similar to amphetamine (Johanson et al., 2005). In an earlier study from this same laboratory, m-CPP did not function as a reinforcer in humans, although it did share subjective effects with racemic MDMA (Tancer and Johanson, 2003). The relationship between the effects of the phenylpiperazines and MDMA seem to warrant further study.
In all cases, mice trained to discriminate S(+)-MDMA from saline were less sensitive to the disruptive effects of N-substituted piperazines on operant behavior than were animals trained with R(−)-MDMA. The relative resistance of the subjects in the S(+)-MDMA group to the rate-decreasing effects of the N-substituted piperazines invokes the phenomenon of behavioral tolerance (Schuster, 1978; Dews, 1978), whereby functional antagonism to the effects of a drug develops as a result of prior exposure to the drug (or to a related drug, in which case the phenomenon is denoted as cross-tolerance.) Since S(+)-MDMA elicits both stimulant-like effects on locomotor behavior and hallucinogen-like effects on head twitch behavior in the mouse (Fantegrossi et al., 2004b; 2005b), it seems plausible to propose that mice trained to discriminate this enantiomer might come to display cross-tolerance to other stimulant- and hallucinogen-like drugs. Similarly, R(−)-MDMA induces a HTR (Fantegrossi et al., 2004b; 2005b) but does not stimulate locomotor activity (Fantegrossi et al., 2002) in the mouse, perhaps indicating that repeated exposure to this enantiomer might result in the development of cross-tolerance to the effects of hallucinogen-like but not stimulant-like compounds. Our results suggest that neither group of discrimination animals were tolerant to the locomotor stimulant effects of BZP, as a dose of 30.0 mg/kg of this compound produced an identical ambulatory response in both groups which was not different from that induced by this dose in drug-naïve mice. With regards to head twitches, we also can not confirm tolerance to the hallucinogen-like effects of TFMPP. Indeed, mice trained to discriminate R(−)-MDMA from saline were more sensitive to the effects of TFMPP on head twitch behavior than were mice trained to discriminate the S(+)- enantiomer, or drug-naïve mice. An explanation for the increased propensity of the R(−)-MDMA-trained mice to be behaviorally disrupted by substitution of N-substituted piperazines clearly does not involve cross-tolerance.
In summary, the present data confirm previous characterizations of BZP as a behavioral stimulant (Campbell et al., 1972; Oberlander et al., 1979; Fantegrossi et al., 2005a), and hint that structural modifications to the molecule known to attenuate psychostimulant effects among the phenethylamines may have similar results with N-substituted piperazines. These studies demonstrate hallucinogen-like effects of TFMPP in the head twitch assay, but these effects were not observed with the related phenylpiperazine m-CPP. Finally, these studies imply differences in the subjective effects of the MDMA enantiomers, as BZP substituted for the discriminative cue elicited by S(+)-MDMA, but not for that induced by R(−)-MDMA. As recreational use of N-substituted piperazines continues, further study will likely be necessary to understand the complex behavioral and pharmacological actions of these drugs. An approach involving behavioral and molecular assays would likely be most informative in this regard.
Acknowledgments
These studies were funded by US PHS Grants DA19634 (A.C.) and DA020645 (W.E.F.), by the College on Problems of Drug Dependence, and by the Yerkes Base Grant RR-00165. The authors wish to thank the Yerkes Animal Care staff for expert animal husbandry services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or ‘Ecstasy’) Neuropsychopharmacology. 2005;30:550–560. doi: 10.1038/sj.npp.1300585. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Murphy DL, Hill JL, George DT, Nutt D, Linnoila M. Ethanol-like properties of the serotonergic partial agonist m-chlorophenylpiperazine in chronic alcoholic patients. Arch Gen Psychiat. 1991;48:383. doi: 10.1001/archpsyc.1991.01810280099018. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Fergeson P, Hudson J, McKernin C. The meta-chlorophenylpiperazine challenge test in cocaine addicts: hormonal and psychological responses. Biol Psychiat. 1997;41:1071–1086. doi: 10.1016/S0006-3223(96)00182-5. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PAJ. The head twitch response to intraperitoneal injection of 5-hydroxytryptophan in the rat: Antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, an LSD antagonist. Neuropharmacology. 1983;22(8):993–1000. doi: 10.1016/0028-3908(83)90215-0. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Brit J Pharmacol. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioral response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Withdrawal from chronic treatment with (±)-DOI causes supersensitivity to 5-HT2 receptor-induced head-twitch behavior in mice. Eur J Pharmacol. 1990a;186:115–118. doi: 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- Darmani NR, Martin bR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990b;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J Pharmacol Exp Ther. 1992;262(2):692–698. [PubMed] [Google Scholar]
- de Boer D, Bosman IJ, Hidvegi E, Manzoni C, Benko AA, dos Reys LJ, Maes RA. Piperazine-like compounds: a new group of designer drugs-of-abuse on the European market. Forensic Sci Int. 2001;121:47–56. doi: 10.1016/s0379-0738(01)00452-2. [DOI] [PubMed] [Google Scholar]
- Dews PB. Behavioral tolerance. NIDA Res Monogr. 1978;18:18–26. [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology. 2003;166(3):202–11. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G, McMahon LR, France CP, Woolverton WL, Winter JC, Cunningham KA. Progress report from the testing program for stimulant and depressant drugs (2003). In: Dewey WL, editor. National Institute on Drug Abuse Research Monograph #184; Problems of Drug Dependence, 2003: Proceeding of the 65th Annual Scientific Meeting; The College on Problems of Drug Dependence, Inc.. Washington D.C: U.S. Government Printing Office; 2004a. pp. 205–231. [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, Martin CV, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology. 2004b;173(3–4):270–7. doi: 10.1007/s00213-003-1741-2. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Winger G, Woods JH, Woolverton WL, Coop A. Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend. 2005a;77(2):161–8. doi: 10.1016/j.drugalcdep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, De la Garza R, 2nd, Woods JH. Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology. 2005b;181(3):529–36. doi: 10.1007/s00213-005-0005-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005c;181(3):496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83(1):122–9. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Green AR. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985;84(3):743–53. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, O’Shaughnessy K, Hammond M, Schachter M, Grahame-Smith DG. Inhibition of 5-hydroxytryptamine-mediated behaviours by the putative 5-HT2 receptor antagonist pirenperone. Neuropharmacology. 1983;22:573–578. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81(1):27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Landry MJ. MDMA: a review of epidemiologic data. J Psychoactive Drugs. 2002;34:163–169. doi: 10.1080/02791072.2002.10399950. [DOI] [PubMed] [Google Scholar]
- McCann UD, Eligulashvili V, Mertl M, Murphy DL, Ricaurte GA. Altered neuroendocrine and behavioral responses to m-chlorophenylpiperazine in 3,4-methylenedioxymethamphetamine (MDMA) users. Psychopharmacology. 1999;147:56–65. doi: 10.1007/s002130051142. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs. 1986;18:305–313. doi: 10.1080/02791072.1986.10472362. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science. 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Williams M. Serotonin-releasing effects of substituted piperazines in vitro. Biochem Pharmacol. 1984;33:1531–1535. doi: 10.1016/0006-2952(84)90424-6. [DOI] [PubMed] [Google Scholar]
- Schuster CR. Theoretical basis of behavioral tolerance: implications of the phenomenon for problems of drug abuse. NIDA Res Monogr. 1978;18:4–17. [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GKW. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36(14):2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72(1):33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Johanson CE. The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend. 2001;65(1):97–101. doi: 10.1016/s0376-8716(01)00146-6. [DOI] [PubMed] [Google Scholar]
- United States Department of Justice/Drug Enforcement Administration. System to Retrieve Information from Drug Evidence II (STRIDE) 2003 [Google Scholar]
- Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short-term sequela of MDMA (‘ecstasy’) in MDMA-naïve healthy volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]