Review of the use of cephalosporins in children with anaphylactic reactions from penicillins (original) (raw)
Abstract
OBJECTIVE:
It is a widely accepted practice that children with anaphylaxis from penicillins should avoid cephalosporins. The purpose of the present study was to determine whether there is evidence in the literature to support this practice.
DATA SOURCES:
MEDLINE, EMBASE, Toxline, International Pharmaceutical Abstracts and PubMed were used to search the literature published from 1966 to 2001. The Canadian Medical Protective Association, Health Canada and the Boston Collaborative Drug Surveillance Program were also contacted to determine whether there were any unpublished cases of cross-reactivity between penicillins and cephalosporins.
DATA EXTRACTION:
Cases describing the use of cephalosporins in adults and children with positive penicillin skin tests or anaphylaxis from penicillin were evaluated. Case reports of anaphylaxis from cephalosporins in paediatric patients were identified.
DATA SYNTHESIS:
There have been five reported cases of serious reactions from cephalosporins in patients with a history of anaphylaxis from penicillins. All cases occurred in adults; three developed anaphylaxis from the older, first-generation cephalosporins, cephalothin and cephaloridine; one developed anaphylaxis from cefamandole; and one developed anaphylaxis from cefaclor. There have been 12 other published reports of anaphylaxis from cephalosporins in adults with a history of penicillin allergy or a positive penicillin skin test, but with no history of anaphylaxis from penicillin. In seven studies, in which a total of 158 patients with positive penicillin skin tests were administered cephalosporins, seven had apparent immunoglobulin E-mediated reactions when they were given a cephalosporin. When the class of cephalosporin was able to be determined, none of the reports of reactions from cephalosporins in patients with allergies to penicillin involved third-generation cephalosporins. There have been 13 case reports of anaphylaxis from cephalosporins in paediatric patients.
CONCLUSION:
There are no published case reports of anaphylaxis from cephalosporins in children with anaphylaxis from penicillin, and there are only a small number of such reports in adults. Anaphylaxis from cephalosporins appears to be incredibly rare in children. There is minimal evidence in the literature to support the avoidance of cephalosporins in children with anaphylaxis from penicillins.
Key Words: Anaphylaxis, Antibiotic allergy, Antibiotic hypersensitivity, Cephalosporins, Cross-reactivity, Drug allergy, Drug hypersensitivity, Penicillin
A common problem in paediatrics is determining whether a cephalosporin can be used in a child with a history of a serious reaction from a penicillin, and whether a penicillin can be administered to a child with a history of a serious reaction from a cephalosporin. In many cases, such patients are prescribed antibiotics that are less effective, more toxic, have a broader spectrum or are more expensive than the drug of choice for their condition.
Diagnostic tests for antibiotic allergies are limited and are standardized only for penicillin.
When penicillin is metabolized, the beta-lactam ring opens to form a penicilloyl derivative that, when bound to serum and tissue proteins, accounts for more than 90% of immunologically active penicillin metabolites (the major determinants). However, about 16% of allergic reactions to penicillin involve multiple, different antigens that result from further penicillin metabolism. These antigens are referred to as minor determinants (1). In patients who have a history of penicillin allergy but negative skin test results using major and minor determinants, studies have shown that the chance of a serious allergic reaction to penicillin is negligible (1). Skin testing for cephalosporin allergy is sometimes performed, but is not standardized because the antigenic determinants of a serious allergic reaction have not been established.
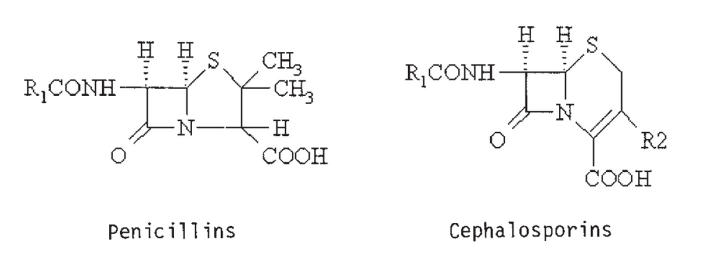
Standard teaching is that patients who have had possible anaphylaxis from penicillin should be given cephalosporins only with extreme caution, because their risk of developing anaphylaxis from cephalosporins is increased (2). Penicillins have a beta-lactam ring attached to a thialazolidine ring with one side chain, while cephalosporins have a beta-lactam ring attached to a dihydrothiazine ring with two side chains (1) (Figure 1). Because of the similar structures, there is a theoretical risk of cross-reactivity between penicillins and cephalosporins.
Figure 1.
Comparison of chemical structure of penicillins and cephalosporins. R Side chain
Soon after cephalosporins were introduced, there were reports of anaphylaxis in patients who were given cephalosporins and who had also experienced anaphylaxis from penicillin. Furthermore, during the initial clinical trials with first-generation cephalosporins and cefamandole, 8.1% of patients with a history of allergy to a penicillin had a possible allergy to a cephalosporin, compared with 4.5% of patients with no such history (3). However, in those initial trials, no attempt was made to ensure that the reaction to the penicillin or to the cephalosporin was truly allergic. The purpose of the present study was to determine whether there is further evidence in the literature to support the avoidance of cephalosporins in children with suspected penicillin allergies. If the risk of cross-reactivity between penicillins and cephalosporins is low in the adult population, the same is probably true for the paediatric population. Therefore, the incidence of case reports of anaphylaxis from cephalosporins in adults or children with anaphylaxis from penicillin or a positive penicillin skin test was studied. The results of cephalosporin challenge in patients with positive penicillin skin tests were also reviewed. If it is exceedingly rare for children to develop anaphylaxis from cephalosporins, it may not be necessary to avoid their use in children who are allergic to penicillins. Therefore, the total number of published case reports of anaphylaxis from cephalosporins in all children was also researched.
DATA AND METHODS
The literature was reviewed for results of cephalosporin challenge in adults or children with anaphylactic reactions from penicillin or with positive penicillin skin tests. Case reports of anaphylaxis from cephalosporins in paediatric patients (up to 16 years of age) were also researched. MEDLINE, EMBASE, Toxline, International Pharmaceutical Abstracts and PubMed were used to search the literature published from 1966 to 2001, using 'anaphylaxis', 'penicillin', 'cephalosporin', 'beta-lactams', 'cross-reactivity', 'cross-sensitivity', 'drug allergy' and 'drug hypersensitivity' as keywords, and by referencing the bibliographies of related papers. Cases with urticaria or life-threatening symptoms (apnea, bronchospasm or hypotension) were considered to be anaphylaxis. We also contacted Health Canada, the Canadian Medical Protective Association (CMPA) and the Boston Collaborative Drug Surveillance Program to determine whether they were aware of any unpublished cases of cross-reactivity between penicillins and cephalosporins, and anaphylaxis from cephalosporins in children.
RESULTS
A review of case reports of anaphylaxis from cephalosporins in adults or children with a history of penicillin allergy or a positive penicillin skin test is presented in Table 1. In five cases (4-8), patients with a history of anaphylaxis from penicillin developed anaphylaxis from a cephalosporin. Three of the cases involved anaphylaxis from the older, first-generation cephalosporins, cephalothin and cephaloridine, while two cases involved anaphylaxis from the second-generation cephalosporins, cefaclor and cefamandole. There were 12 other published reports of anaphylaxis from cephalosporins in adults with a history of penicillin allergy or a positive penicillin skin test but with no history of anaphylaxis from penicillin. In nine of these cases, the class of cephalosporin was known (six were cephalothin, one was both cephalothin and cephalexin, one was cephalexin and one was cefamandole). The CMPA was not aware of any legal action in Canada that resulted from the administration of a cephalosporin to a patient with an allergy to penicillin. Health Canada's Adverse Drug Reaction Monitoring Program and the Boston Collaborative Drug Surveillance Program had no additional unpublished reports of anaphylaxis from cephalosporins in patients who were allergic to penicillin.
Table 1.
Summary of case reports of anaphylaxis to cephalosporins in patients with a history of penicillin allergy or a positive penicillin skin test
| Year, Reference | Age (years) | Sex | Reaction to penicillin | Anaphylaxis to penicillin (yes/no) | Cephalosporin | Reaction to cephalosporin |
|---|---|---|---|---|---|---|
| 1965, Kabins et al (4) | 47 | F | Pruritis and angioneurotic edema | Yes | Cephalothin | Hypotensive, wheezing and unresponsive within 2 min |
| 1966, Rothschild and Doty (23) | 56 | M | Pruritis and urticaria | No | Cephalothin | Apnea and hypotension within minutes |
| 1966, Drug Letter (24) | 40 | F | Rash* | No | Cephalothin | Pruritis, dyspnea and angioneurotic edema |
| 1968, Scholand et al (5) | 65 | M | Urticaria, angioneurotic edema and dyspnea | Yes | Cephalothin | Wheezing and hypotension within 30 s |
| 1968, Girard (6) | 1 patient† | Anaphylaxis* | Yes | Cephaloridine | Mild anaphylactic shock* | |
| 1971, Petz (25) | 2 patients† | Unknown* | No | Cephalothin | Anaphylaxis* | |
| 1974, Spruill et al (10) | 59 | M | Unknown* | Unknown | Cephalothin | Cardiac arrest within 5 min |
| 1980, Zeok and Tsueda (26) | 1 patient† | Urticaria | No | Cephalothin | Hypotension, bradycardia, diminished respiratory excursions and wheezing, generalized edema, urticaria | |
| 1989, Blanca et al (7) | 22 | F | Angioedema of the mouth and eyes | No | Cefamandole | Hypotension |
| 1989, Blanca et al (7) | 50 | F | Hypotension, pruritis of the lips, breathing difficulties | Yes | Cefamandole | Hypotension, dysphonia, generalized pruritis, and upper airway obstruction |
| 1989, Macnab (27) | 35 | F | Unknown* | No | Cephalexin and cephalothin | Urticaria, dyspnea, nausea and severe headaches |
| 1999, Pumphrey and Davis (8) | 76 | F | Anaphylaxis to amoxicillin* | Yes | Cefaclor | Fatal anaphylaxis* |
| 1999, Pumphrey and Davis (8) | 3 patients† | Two allergic to amoxicillin and 1 allergic to penicillin* | Unknown | Unknown | Fatal anaphylaxis* | |
| 1999, Nordt et al (28) | 32 | F | Unknown* | Unknown | Cephalexin | Rapid onset of throat tightness and urticaria |
Table 2 summarizes the studies of cephalosporin challenge in children and adults with positive penicillin skin tests. Only one study (9) focused on paediatric patients. Of a total of 158 patients, there were seven positive challenges with cephalosporins. Four of the seven suspected immunoglobulin E (IgE)-mediated reactions were mild (one reaction to cephaloridine and three reactions in which the cephalosporin was not specified), while three were serious reactions that consisted of anaphylaxis from cephaloridine or cefamandole.
Table 2.
Summary of studies of cephalosporin challenge in patients with positive skin tests for allergies to penicillin
| Reference (year) | Patients challenged with cephalosporins | Patients with negative challenge | Patients with positive challenge | Cephalosporin | Reaction if positive challenge |
|---|---|---|---|---|---|
| Assem and Vickers (29) (1974) | 2* | 0 | 2* | Cephaloridine | Anaphylactic reaction† in first patient, extensive urticaria in second patient |
| Warrington et al (30) (1978) | 3 | 3 | 0 | Not applicable | Not applicable |
| Solley (31) (1982) | 27 | 27 | 0 | Not applicable | Not applicable |
| Rohr (32) (1987) | 62 | 61 | 1 | Not specified | Mild urticaria and bronchospasm† |
| Blanca et al (7) (1989) | 12‡ | 10 | 2 | Cefamandole | In one patient, profound and sustained hypotension; in the other patient, transient hypotension, dysphonia, generalized pruritus, and upper airway breathing difficulties |
| Shepherd and Burton (33) (1993) | 9 | 9 | 0 | Not applicable | Not applicable |
| Pichichero and Pinchichero (9) (1988) | 43§ | 41 | 2 | Not specified | Mild IgE-mediated reactions† |
There were 13 reported cases of anaphylaxis from cephalosporins in children (Table 3). One case involved the old, first-generation cephalosporin cephalothin (10), four cases involved second-generation cephalosporins (cefaclor, cefamandole, cefotetan and cefuroxime) (11-13) and eight cases involved a third-generation cephalosporin (ceftriaxone in seven patients and ceftazidime in one patient) (14-16).
Table 3.
Case reports of anaphylaxis from cephalosporins in children
| Reference | Age (years) | Sex | Cephalosporin | Reaction to cephalosporin |
|---|---|---|---|---|
| Romano et al (14) (1999) | 15 | Male | Ceftriaxone | Urticaria, dyspnea, tachycardia and severe hypotension within 5 min |
| Grouhi et al (11) (1999) | 2 | Male | Cefaclor | Vomiting, generalized hives, shortness of breath, wheezing with grunting and loss of consciousness |
| Wessel (12) (1998) | 6 | Female | Cefamandole | Anaphylactic shock* |
| Spruill et al (10) (1974) | 11 | Male | Cephalothin | Hypotension and shock |
| Romano et al (15) (2000) | 6 | Male | Ceftriaxone | Urticaria |
| 7 | Female | Ceftriaxone | Anaphylactic shock* | |
| 14 | Male | Ceftriaxone | Anaphylactic shock* | |
| 12 | Female | Ceftriaxone | Anaphylactic shock* | |
| 5 | Female | Ceftriaxone | Anaphylactic shock* | |
| 9 | Female | Ceftriaxone | Anaphylactic shock* | |
| Romano et al (16) (2001) | 16 | Female | Ceftazidime | Anaphylactic shock (urticaria, rhinitis, wheezing, dyspnea and hypotension) |
| Health Canada (13) (2001) | 10 | Female | Cefotetan | Flushing, hypotension, atrial arrhythmia and tachycardia |
| Health Canada (13) (2001) | 8 | Female | Cefuroxime | Urticaria and edema |
DISCUSSION
There have been only 17 published case reports of anaphylaxis from cephalosporins in patients with a history of penicillin allergy or a positive penicillin skin test. All cases occurred in adults. In nine of the 14 cases in which the class of cephalosporin could be determined, anaphylaxis was from first-generation cephalosporins, while the other five cases were anaphylaxis from second-generation cephalosporins. However, one limitation of the present study was that the reporting of cases was sporadic. Therefore, the infrequency of case reports may have been due to the fact that physicians are very compliant about not prescribing cephalosporins to patients with suspected penicillin allergies, the cases of cross-reactivity between penicillins and cephalosporins that occur are often not reported, or that it is very rare for cross-reactivity to occur.
There have been seven studies in which 158 patients with positive penicillin skin tests were administered cephalosporins; seven had IgE-mediated reactions when they were administered a cephalosporin. Four of the reactions were mild (one reaction was to cephaloridine and three were in reaction to a cephalosporin that was not specified). There was one serious reaction to cephaloridine and two serious reactions to cefamandole in patients with allergies to penicillin. A possible explanation for the three serious reactions is that of all the cephalosporins, cephaloridine and cefamandole have side chain structures that are most similar to penicillin and, thus, cross-reactivity may have occurred (2).
When the class of the cephalosporin was specified, none of the serious reactions to cephalosporins in patients with positive penicillin skin tests involved third-generation cephalosporins. The risk of serious allergic reactions from cephalosporins in the general population appears to be less than 0.02%, and the risk is lowest for third-generation cephalosporins (possibly because cephalosporins are thought to be only antigenic if the side chain is bound to a serum protein, and free side chains compete with bound side chains for binding to IgE antibodies) (17,18).
Anaphylaxis from cephalosporins appears to be incredibly rare in children, with only 13 case reports in the literature. However, it is likely that not all cases would have been reported or even recognized. It is interesting that eight of those case reports came from Romano (15,16). He also mentioned a six-year-old with an immediate reaction to a cephalosporin in another paper (19), but it is not clear if this was an additional case. A recent review article found only 54 published cases of anaphylaxis to cephalosporins in both adults and children, but did not include all of the paediatric cases found in the present study (12,15,16).
The reason why a high incidence of cross-reactivity between penicillins and cephalosporins was thought to be likely is that both have a beta-lactam ring. However, there is increasing evidence that, in most allergic reactions to cephalosporins, it is the side chain rather than the beta-lactam ring that is the antigen (2). It is only the older, first- and second-generation cephalosporins (cephalothin, cephaloridine and cefamandole) that have a side chain that is similar to that of penicillin, while cephalexin has a side chain that is identical to that of ampicillin. In addition, older, first-generation cephalosporins were sometimes contaminated with penicillin before 1980 (20). These two facts may account for some of the early reports of cross-reactivity between penicillins and cephalosporins. Furthermore, the results of penicillin skin testing are not predictive of cephalosporin allergy, which again suggests that there is limited cross-reactivity (3).
There is evidence that patients with a history of penicillin allergy have three times the rate of patients in control groups of having an allergic reaction to any other antibiotic (17). Therefore, even if the risk of patients with a history of penicillin allergy having a serious reaction to cephalosporins is double that of patients in control groups, this risk may be lower than the risk of them having a serious reaction to any alternative antibiotic (2). It has been suggested that the low incidence of serious allergic reactions to cephalosporins may relate to the lack of stability of immunogenic metabolites (16).
CONCLUSIONS
There is minimal evidence in the literature to support the avoidance of cephalosporins in children with anaphylaxis from penicillins. Despite published guidelines to the contrary (20), a child or adult with a previous mild reaction to a penicillin can probably safely be administered a cephalosporin. If a more serious reaction to a penicillin has occurred, yet the patient has a negative penicillin skin test, it is probably safe to administer a cephalosporin (21). However, if skin testing cannot be done or if a patient is known to have had a positive penicillin skin test, it is not advisable to administer a full dose of an intravenous cephalosporin in the face of standard recommendations against this practice (20). However, it would appear to be safe to administer a supervised, graded challenge with a nonfirst-generation cephalosporin other than cefamandole or to embark on desensitization (22).
References
- 1.Sogn DD, Evans R, Shepherd GM, et al. Results of the National Institute of Allergy and Infectious Diseases collaborative clinical trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med 1992;152:1025-32. [PubMed] [Google Scholar]
- 2.Weiss ME, Adkinson NF. Beta-lactam allergy. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases, 5th edition. Philadelphia: Churchill Livingstone, 2000:299-305. [Google Scholar]
- 3.Kim S, Warrington RJ. Clinical cross-reactivity between penicillins and cephalosporins. Can J Allergy Clin Immunol 1998;3:12-5. [Google Scholar]
- 4.Kabins SA, Eisenstein B, Cohen S. Anaphylactoid reaction to an initial dose of sodium cephalothin. JAMA 1965;193:165-6. [DOI] [PubMed] [Google Scholar]
- 5.Scholand JF, Tennenbaum JI, Cerilli GJ. Anaphylaxis to cephalothin in a patient allergic to penicillin. JAMA 1968;206:130-2. [PubMed] [Google Scholar]
- 6.Girard JP. Common antigenic determinants of penicillin G, ampicillin and the cephalosporins demonstrated in men. Int Arch Allergy 1968;33:428-38. [DOI] [PubMed] [Google Scholar]
- 7.Blanca M, Fernandez J, Miranda A, et al. Cross-reactivity between penicillins and cephalosporins: Clinical and immunologic studies. J Allergy Clin Immunol 1989;83:381-5. [DOI] [PubMed] [Google Scholar]
- 8.Pumphrey RSH, Davis D. Under-reporting of antibiotic anaphylaxis may put patients at risk. Lancet 1999;353:1157-8. [DOI] [PubMed] [Google Scholar]
- 9.Pichichero ME, Pichichero DM. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. J Pediatr 1998;132:137-43. [DOI] [PubMed] [Google Scholar]
- 10.Spruill FG, Minette LJ, Sturner WQ. Two surgical deaths associated with cephalothin. JAMA 1974;229:440-1. [PubMed] [Google Scholar]
- 11.Grouhi M, Hummel D, Roifman CM. Anaphylactic reaction to oral cefaclor in a child. Pediatrics 1999;103:50. [DOI] [PubMed] [Google Scholar]
- 12.Wessel F. Les allergies per-anesthetiques aux antibiotiques. Allergie et Immunologie 1998;30:190-2. [PubMed] [Google Scholar]
- 13.Canadian Adverse Drug Reaction Monitoring Program. Summary of Reported Adverse Drug Reactions. Ottawa: Health Canada, 2001.
- 14.Romano A, Quaratino D, Venemalm L, Torres MJ, Venuti A, Blanca M. A case of IgE-mediated hypersensitivity to ceftriaxone. J Allergy Clin Immunol 1999;104:1113-4. [DOI] [PubMed] [Google Scholar]
- 15.Romano A, Mayorga C, Torres MJ, et al. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J Allergy Clin Immunol 2000;106:1177-83. [DOI] [PubMed] [Google Scholar]
- 16.Romano A, Di Fonso M, Artesani MC, Viola M, Adesi FB, Venuti A. Selective immediate hypersensitivity to ceftazidime. Allergy 2001;56:84-5. [DOI] [PubMed] [Google Scholar]
- 17.Anne S, Reisman RE. Risk of administering cephalosporin antibiotics to patients with histories of penicillin allergy. Ann Allergy Asthma Immunol 1995;74:167-70. [PubMed] [Google Scholar]
- 18.Adkinson NF. Drug allergy. In: Middleton E, Reed CE, Ellis EF, et al, eds. Allergy - Principals and Practice, 5th edn. St Louis: Mosby, 1998:1214-24. [Google Scholar]
- 19.Romano A, Quaratino I, Aimone-Gastin I, et al. Cephalosporin allergy: Characterization of unique and cross-reacting cephalosporin antigens. Int J Immunopathol Pharmacol 1997;10:187-91. [Google Scholar]
- 20.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology, Joint Council of Asthma, Allergy and Immunology. Executive summary of disease management of drug hypersensitivity: A practice parameter. Ann Allergy Asthma Immunol 1999;83:665-700. [PubMed] [Google Scholar]
- 21.Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med 2001;345:804-9. [DOI] [PubMed] [Google Scholar]
- 22.Mendelson LM. Adverse reactions to beta-lactam antibiotics. Immunol Allergy Clin North Am 1998;18:745-57. [Google Scholar]
- 23.Rothschild PD, Doty DB. Cephalothin reaction after penicillin sensitization. JAMA 1966;196:372-3. [Google Scholar]
- 24.Drug letter II. Report of a drug reaction: Allergic reaction to cephalothin in a patient with penicillin hypersensitivity. Bull Johns Hopkins Hosp 1966;118:352-3. [Google Scholar]
- 25.Petz LD. Immunologic reactions of humans to cephalosporins. Postgrad Med J 1971;47:(Suppl):64-9. [PubMed] [Google Scholar]
- 26.Zeok SS, Tsueda K. Failure of a cephalothin test dose to produce anaphylaxis. Anesth Analg 1980;59:393-4. [PubMed] [Google Scholar]
- 27.Macnab KA. Cross allergenicity of penicillins and cephalosporins. Can J Hosp Pharm 1989;42:81-4. [Google Scholar]
- 28.Nordt SP, Cantrell FL, Rodriguez GJ. Anaphylactic reaction to dermal exposure to cephalexin. Am J Emerg Med 1999;17:492-3. [DOI] [PubMed] [Google Scholar]
- 29.Assem ES, Vickers MR. Tests for penicillin allergy in man. II. The immunological cross-reaction between penicillins and cephalosporins. Immunology 1974;27:255-69. [PMC free article] [PubMed] [Google Scholar]
- 30.Warrington RJ, Simons FER, Ho HW, Gorski BA. Diagnosis of penicillin allergy by skin testing: The Manitoba experience. CMAJ 1978;118:787-91. [PMC free article] [PubMed] [Google Scholar]
- 31.Solly GO, Gleich GJ, Van Dellen RG. Penicillin allergy: Clinical experience with a battery of skin-test reagents. J Allergy Clin Immunol 1982;69:238-44. [DOI] [PubMed] [Google Scholar]
- 32.Rohr AS. Cephalosporins. In: Saxon A, moderator. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:210. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd GM, Burton DA. Administration of cephalosporin antibiotics to patients with a history of penicillin allergy. J Allergy Clin Immunol 1993;91:262 (Abst) [Google Scholar]