Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart (original) (raw)
. Author manuscript; available in PMC: 2008 Oct 1.
Abstract
Objective:
Insulin regulates both glucose uptake and postnatal cardiac growth. The anabolic effects of insulin are mediated by the mammalian target of rapamycin (mTOR), an evolutionarily conserved kinase which is also a convergence point between nutrient sensing and cell growth. We postulated that mTOR signalling in the heart requires the metabolism of glucose.
Methods:
We interrogated the insulin-mediated mTOR signalling pathway in response to different metabolic interventions regulating substrate metabolism in the isolated working rat heart and in isolated cardiomyocytes.
Results:
Although insulin enhanced Akt activity, phosphorylation of mTOR and its downstream targets (p70S6K and 4EBP1) required the addition of glucose. Glucose-dependent p70S6K phosphorylation was independent of the hexosamine biosynthetic pathway, the AMP kinase pathway, and the pentose phosphate pathway. However, inhibition of glycolysis downstream of hexokinase markedly enhanced p70S6K phosphorylation. Furthermore, 2-deoxyglucose activated p70S6K suggesting that phosphorylation of glucose is required for carbohydrate-mediated mTOR signalling in the heart. Lastly, we also found enhanced p70S6K phosphorylation in the hearts of diabetic rats.
Conclusion:
Phosphorylation of glucose is necessary for insulin-dependent mTOR activity in the heart, suggesting a link between intermediary metabolism and cardiac growth.
Introduction
The target of rapamycin (TOR) is an evolutionarily conserved kinase which is vital for the regulation of cell growth in response to nutrients in all eukaryotic cells 1, 2. Originally discovered in yeast, TOR regulates cell growth by initiating protein translation and inducing ribosomal biogenesis in response to amino acid availability2-6. In mammalian tissues mammalian TOR (mTOR) is a downstream protein in the insulin/insulin-like growth factor-1 signalling cascade stimulating growth largely by activating the protein translational machinery in the cell3-5, 7. Specifically, the growth promoting effects of mTOR are mediated by two downstream proteins which regulate protein translation3, 6. p70S6K, when phosphorylated by mTOR on threonine 389, phosphorylates the ribosomal protein S6 and leads to the translation of mRNA which encode for ribosomal proteins and elongation factors3, 6. 4EBP1 is a binding protein which releases the initiation factor eIF4E when phosphorylated by mTOR. eIF4E forms a complex with other initiation factors and the 40S ribosome to start protein translation3, 6.
In the heart, mTOR is an important regulator of cardiac hypertrophy. Rapamycin, an inhibitor of mTOR, can attenuate load-induced cardiac hypertrophy in mice8. Furthermore, overexpression of Akt/PKB, an upstream regulator of mTOR, results in cardiac hypertrophy9. Recent work has revealed that mTOR, like yeast TOR, is activated directly by nutrients3, 6. In skeletal muscle and liver, amino acids are capable of eliciting the phosphorylation of p70S6K and 4EBP110-15. However, the regulation of mTOR in the heart by glucose is not known.
We proposed that glucose metabolism regulates the mTOR pathway in the heart in response to insulin. In both the isolated working rat heart and in isolated cardiomyocytes, we found that glucose is required for the activation of mTOR by insulin. Our findings suggest that the anabolic and metabolic actions of insulin are linked and that glycolytic intermediates serve as signals for pathways regulating cardiac growth.
Methods
Animals
We obtained male Sprague-Dawley rats (300-350g), Zucker lean (ZL), and Zucker diabetic fatty (ZDF) rats (age 8 wk) in each group from Harlan (Indianapolis, IN, USA) subsequently kept in the Animal Care Center at the University of Texas Medical School at Houston under controlled conditions (23±1°C; 12 h light/12 h dark cycle), receiving standard laboratory food and water ad libitum. Animals were killed between 700 and 900 am, the hearts were removed working rat heart experiments. Neonatal Sprague Dawley rats (1-to-2 days old) rats were obtained from Harlan and sacrificed for isolated cardiomyocyte experiments. The use of animals and the animal protocol were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston, and conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Isolated working rat heart
Details of the apparatus and specific technique have been published previously 16. Male Harlan Sprague-Dawley rats were anesthetized by intraperitoneal injection of sodium pentobarbital (10 mg/100 g body wt). All experiments were performed between 9 am and noon. Heparin (100 U) was injected into the femoral vein. The heart was rapidly removed from the animal, transferred into cold saline, and mounted on a cannula assembly. After several minutes of retrograde perfusion with warm perfusate, the heart was switched to a working mode by opening a line to the left atrium. The filling pressure was 15 cm H2O and the afterload was 100 cm H2O. The perfusate was recirculated and oxygenated as previously described16. After 30 minutes of perfusion and nutrient manipulation, while the heart was still beating vigorously, both ventricles were freeze clamped below the atria with a pair of Wollenberger tongs cooled in liquid nitrogen. Protein extraction and nutrient uptake assays were performed on frozen samples which were powdered under liquid nitrogen.
Working heart perfusion protocol
Hearts were perfused for 30 minutes with recirculating Krebs-Henseleit bicarbonate saline (200 ml) with the addition of propionyl-L-carnitine (2mM) and acetoacetate (7.5mM) with and without insulin (40μU/ml). These perfusions were used as control experiments because both substrates enter mitochondrial oxidative pathways without upstream modification. Propionyl-L-carnitine directly enters the mitochondria where it is oxidized via β-oxidation, and acetoacetate is converted into acetyl CoA in the mitochondria and enters the TCA cycle. Therefore, propionyl-L-carnitine/acetoacetate are fuels allowing the heart to function without the accumulation of metabolic intermediates from exogenous substrates which may participate in cellular signalling. Subsequent experimental groups are listed in Table 1. All perfusions were performed with an afterload of 100 cm H2O and preload of 15 cm H2O. When insulin was used the concentration was always 40 μU/ml. Changes in nutrients and insulin had no effect on cardiac output in the perfusions (data not shown). In one series of experiments, rapamycin (4mg/kg/day) or vehicle (propylene glycol) was gavaged for 7 days in rats prior to the hearts being perfused with glucose (5mM) as described above.
Table 1.
Heart Perfusions
| Carbohydrate Metabolism PLC/AcAc ± Insulin glucose (5mM, 15mM) + PLC/AcAc ± Insulin amino acids + Gluc (5mM) + PLC/AcAc ± Insulin lactate (0.5mM)/pyruvate (0.05mM)+ PLC/AcAc ± Insulin glucosamine (5mM, 15mM) + PLC/AcAc + Insulin xylitol (0.5mM, 1mM) + PLC/AcAc + Insulin 2-deoxyglucose (5mM, 15mM) + PLC/AcAc + Insulin |
|---|
| Modulators of Enzyme Activity AICAR ( 0.4 mM) + glucose (5mM, 15mM) + PLC/AcAc + Insulin Azaserine (10mM, 20mM, 100mM) + glucose + PLC/AcAc + insulin |
Neonatal cardiomyocyte cell culture
Neonatal Sprague Dawley rats (1-to-2 days old) were used. Each isolation was a single experimental replicate. Newborn rats were briefly cleaned with 70% ethanol. They were then sacrificed by decapitation with heads dropped immediately into liquid nitrogen. Hearts were removed and minced in an enzyme solution containing collagenase type II (Worthington, Lakewood, NJ, USA) and pancreatin (Sigma, St. Louis, MO, USA). Minced tissue and solution were placed in a trypsinizing flask and shaken at 37.5°C for 5 minutes to allow digestion. The supernatant was collected and discarded. Ten milliliters of enzyme solution was added again to the flask and shaken at 37.5°C for 20 minutes. The supernatant fraction this time was retained in 50 ml tubes and centrifuged at 660rpm for 5 minutes. The pellet was resuspended in media containing 5% neonatal calf serum (NCS) and placed in an incubator at 37.0°C with 5% CO2. This digestion step was repeated 4 more times for 25, 25, 15, and 15 minutes. Each time the supernatant fraction was retained, spun down, resuspended in NCS. The isolates were then spun again at 660rpm, resuspended in Ham's F-10 media with 10% fetal bovine serum (FBS), and filtered into a new 50 ml tube. The cells were pre-plated in 60 mm dishes and kept in the incubator for 1 hour. Then, using a hemocytometer and trypan blue dye, the cell harvest number was determined and the cells were plated in 60 mm dishes for 24 to 48 hours prior to experiments.
Cardiomyocyte experiments
Isolated cardiomyocytes were serum starved in Ham's F-10 media with 0.1% FBS. After serum starvation, the cells were incubated for 30 minutes in either PBS or media with the additions shown in Table 2. Phosphate buffered saline (PBS) ± insulin (1μg/ml) were used as control experiments. After 30 minute incubation, cells were washed twice with PBS and proteins isolated as previously described17.
Table 2.
Cardiomyocyte Experiments
| Carbohydrate Metabolism PBS + Insulin glucose (5mM, 10mM, 15mM) + PBS + Insulin 3-O-Methylglucose (5mM, 10mM, 15mM) + PBS + Insulin 2-dooxyglucose (5mM, 10mM, 15mM) + PBS + Insulin |
|---|
| Modulators of Enzyme Activity AICAR (1mM) + PBS/Media + Insulin Azaserine (10mM) + PBS/Media + insulin Iodoacetate(10mM) + PBS/Media + Insulin |
Protein expression
Protein extraction from the frozen powdered heart tissue has been described previously18. Samples were homogenized in extraction buffer and centrifuged (30 min at 15000g), and the supernatant was isolated. Western blots were performed using the antibodies for mTOR, p70S6K1, 4EBP1, AMP kinase, GSK-3β and Akt1/2 (Cell Signalling, Beverly, MA) as previously described19. The antibodies for phospho-Akt1/2 (Ser473), phospho-mTOR (Ser2448), phospho-p70S6K (Thr389), phospho-GSK-3β (Ser9), phospho-AMPkinase (Thr172), phospho-ACC (Ser79) were also purchased from Cell Signalling (Danvers, Mass.). We used antibody for GAPDH or β-tubulin to normalize for protein loading (Research Diagnostics, Concord, Mass.).
Statistical analysis
Gels were scanned and analyzed for area and density with background subtraction by N.I.H. Scion image analyzer for densitometry. All protein expression data were analyzed using a two-tailed Student's t-test. All numerical data were displayed as a mean and SEM. A p<0.05 was considered significant.
Results
Regulation of mTOR by insulin
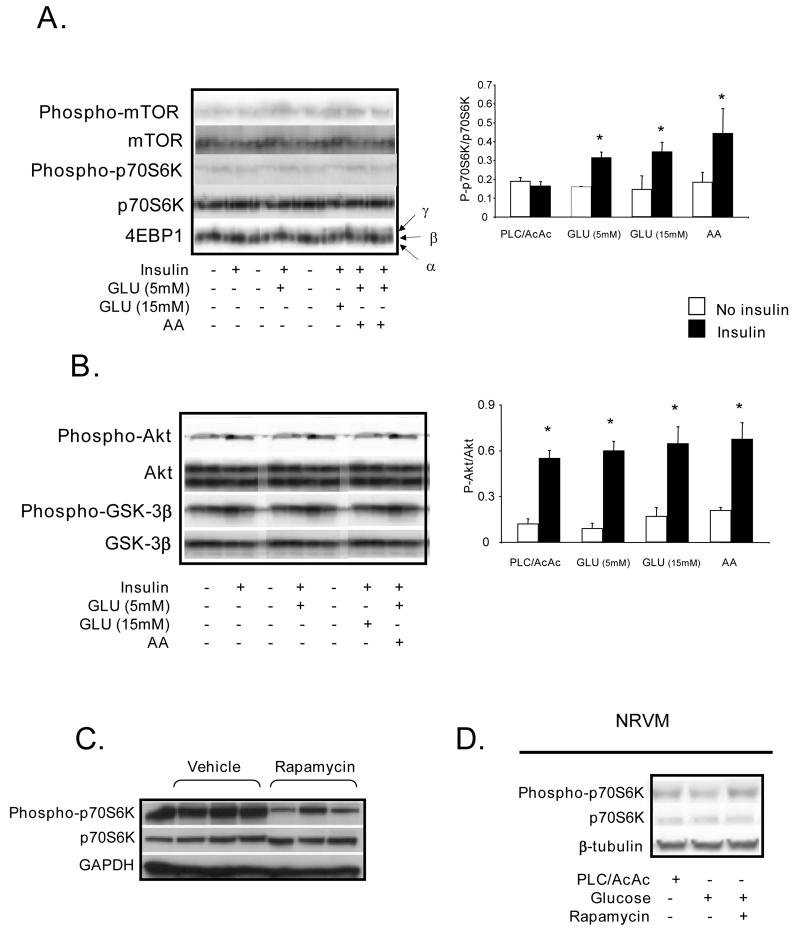
Upon binding to its receptor, insulin activates phosphotidyl-inositol-3-kinase (PI3 kinase), which subsequently phosphorylates its downstream target Akt. Akt is a multifunctional kinase that regulates both glucose uptake (by translocation of GLUT4) and also cell size. For example, overexpression of Akt induces cardiac hypertrophy9. The regulation of cell size by Akt is thought to be mediated by its phosphorylation and by the subsequent downstream phosphorylation of mTOR on the serine 2448. The latter leads to increased protein synthesis via phosphorylation of downstream targets p70S6K and 4EBP13. Here, we demonstrate in the isolated rat heart that phosphorylation of mTOR and both its downstream targets (p70S6K and 4EBP1) required exogenous glucose (Fig. 1A). As mentioned earlier, amino acids have been shown to activate mTOR in skeletal muscle3, 6. We also confirmed the activation of mTOR by amino acids in the heart. (Fig. 1A). In contrast, Akt was phosphorylated by insulin independent of nutrient availability (Fig. 1B). Furthermore, the increased activity of Akt was reflected by the phosphorylation of GSK-3β, a kinase downstream of Akt that regulates glycogen metabolism. Phosphorylation of p70S6K by glucose was attenuated by rapamycin indicating that mTOR is required for glucose-dependent signalling (Fig. 1C). In isolated cardiomyocytes, we confirmed that glucose induced p70S6K phosphorylation and that rapamycin attenuated this phosphorylation indicating mTOR involvement (Fig. 1D).
Figure 1. Immunoblots and densitometry ratios.
A. Immunoblots (n=5) of mTOR signalling proteins in isolated working rat hearts perfused glucose (GLU) at 5mM and 15mM, and amino acids (AA) at physiologic plasma concentrations. Densitometry of the ratio of phosphorylated and total p70S6K (n=5). B. Immunoblots (n=5) of phosphorylated and total Akt and GSK 3β in isolated working rat hearts perfused, with GLU (5mM and 15mM), and AA. C. p70S6K signalling in working rat hearts perfused GLU (5mM) after animals were gavaged for 7 days with either vehicle or rapamycin. D. Immunoblot (n=3) of p70S6K signalling in neonatal cardiomyocytes incubated in PBS and insulin (1μg/ml) ± rapamycin (0.1μM), with the addition of glucose (5mM).* p< 0.05 compared to no insulin
Metabolism of glucose
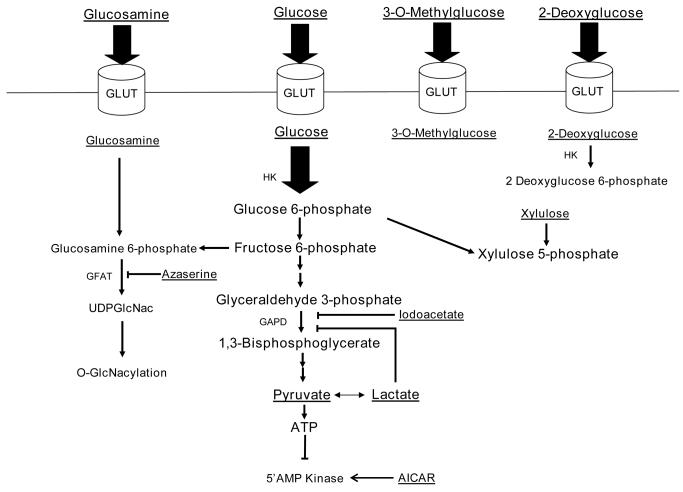
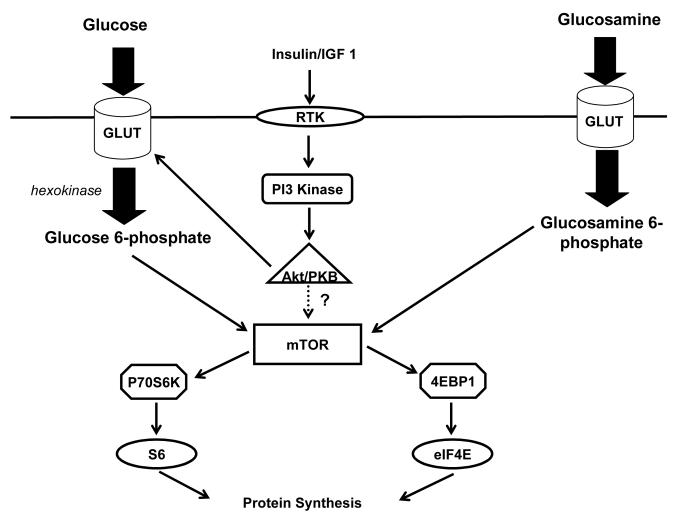
Once glucose is transported into the cardiomyocyte via glucose transporters, it is phosphorylated by hexokinase and may enter a number of metabolic pathways. Several of these pathways may be involved in intracellular signalling in the heart and were interrogated. Figure 2 is a schematic drawing which provides the rationale of our experimental strategy.
Figure 2. Ratoinale for the experimental strategy.
Glucose is taken up by glucose transporters (GLUT) and phosphorylated inside the myocyte by hexokinase. Glucose 6-phosphate may and enter a number of pathways. Glycolysis and glucose oxidation result in ATP generation which may inhibit AMP kinase. Fructose 6-phosphate is converted to glucosamine-6-phosphate and enters the hexosamine biosynthetic pathway ultimately leading to O-GlcNacylation of proteins. Glucose 6-phosphate can also enter the pentose phosphate pathway and generate xylulose-5-phophate. In our experiments we utilized several substrates, activators, or inhibitors to assess whether glucose 6-phosphate regulates mTOR activation. These are underlined. Inhibitors or activators are in italics as well. AICAR 5-aminoimidazole-4-carboxamide ribonucleotide. GFAT glutamine:fructose 6-phosphate amidotransferase, HK hexokinase.
Hexosamine biosynthetic pathway
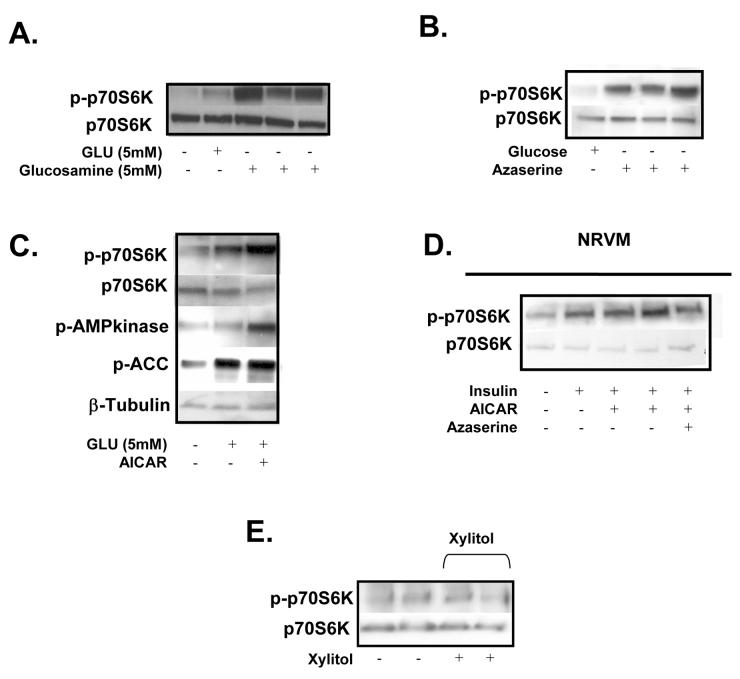
After phosphorylation by hexokinase, glucose 6-phosphate is converted to fructose 6-phosphate in the next step of glycolysis. Fructose 6-phosphate can be converted into UDP-N-acetylglucosamine (UDPGlcNac) by glutamine:fructose 6-phosphate-amidotransferase (GFAT), the first and rate-limiting enzyme of the hexosamine biosynthetic pathway. UDPGlcNac serves as the substrate for O-GlcNacylation of proteins by the enzyme O-GlcNac transferase (OGT). O-GlcNacylation has been shown to compete for phosphorylation sites of signalling proteins and transcriptions factors modifying activity20. We found that glucosamine strongly induced the phosphorylation of p70S6K (Fig. 3A). However, inhibition of GFAT by azaserine did not attenuate carbohydrate-mediated p70S6K phosphorylation either in the working rat heart (Fig 3B) or in isolated cardiomyocytes (Fig. 3D). These observations suggest that, although glucosamine can activate mTOR, the hexosamine biosynthetic pathway is not involved in p70S6K phosphorylation by glucose.
Figure 3. Immunoblots.
A. Phosphorylation of p70S6K (n=3) in working rat hearts perfused with GLU (5mM), insulin (40μU/ml), Glucosamine (5mM). B. p70S6K signalling in rat hearts perfused (n=6) with GLU (5mM) + insulin (40μU/ml) with the addition of azaserine (10 mM and 20 mM). C. Immunoblot of p70S6K, AMP kinase, ACC phosphorylation in rat hearts perfused with GLU (5mM) + Insulin with the addition of AICAR (0.4mM). D. Immunoblot (n=3) of p70S6K phosphorylation in neonatal cardiomyocytes incubated in Ham's F-10 media + insulin (1μg/ml) and AICAR (1mM) or azaserine (10mM). E. p70S6K phosphorylation in rat hearts perfused with PLC/AcAc + Insulin (40μU/ml) with the addition of xylitol (0.5mM) (n=3).
5′AMP kinase
Glycolysis and glucose oxidation produce ATP, which decreases the AMP/ATP ratio subsequently resulting in the decreased activity of 5′AMP kinase21. In recent studies, 5′AMP kinase has been shown to inhibit mTOR22. When working rat hearts were perfused with glucose and 5-aminoimidazole 4-carboxamide 1-beta-D ribofuranoside (AICAR), an activator of AMP kinase, there was a modest increase in the phosphorylation of p70S6K (Fig 3C). Increase in 5′AMP kinase activity was confirmed by an increase in the phosphorylation of 5′AMP kinase itself and also its downstream target acetyl CoA carboxylase (ACC) (Fig 3C). In isolated cardiomyocytes, activation of AMP kinase did not affect the phosphorylation of p70S6K by nutrients (Fig 3D and 4B). Therefore, our observations indicate glucose-mediated phosphorylation of p70S6K is independent of AMP kinase.
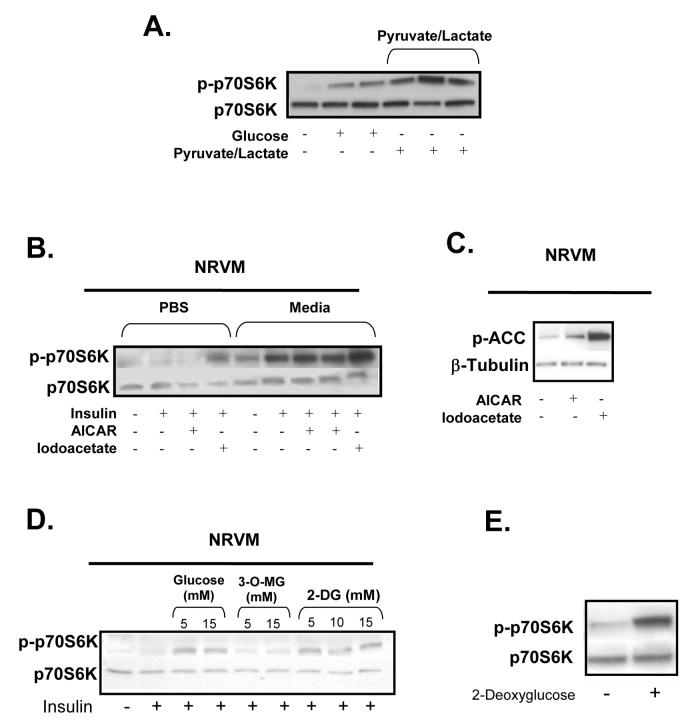
Figure 4. Immunoblots.
A. p70S6K phosphorylation in rat hearts perfused with insulin (40μU/ml) with the addition of pyruvate (0.05mM)/lactate (0.5mM) (n=3). B. Immunoblot (n=3) of p70S6K signalling in neonatal cardiomyocytes incubates in PBS or media ± insulin (1μg/ml) with the addition of AICAR (1mM) or iodoacetate (10mM). C. ACC phosphorylation in the cardiomyocytes exposed to AICAR and iodoacetate. D. Immunoblot (n=3) of p70S6K signalling in neonatal cardiomyocytes incubates in PBS+ insulin (1μg/ml) with the addition of GLU (5 mM and 15mM), 3-O methylglucose (3-O-MG) (5mM and 15mM), or 2-deoxyglucose (2-DG) (5mM and 15mM). E. Phosphorylation of p70S6K (n=3) in rat hearts perfused with PLC/AcAc + insulin (40μU/ml) with the addition of 2-DG (5mM).
Pentose phosphate pathway
We have previously shown that 20% of glucose taken up by the heart enters the pentose phosphate pathway23. The non-oxidative arm of the pentose phosphate pathway produces xylulose 5-phosphate, which has been shown to induce the expression of several genes in the liver (e.g. pyruvate kinase) and may modify other signalling pathways24. However, working rat heart perfusions with xylitol (which is converted to xylulose-5-phophate in the heart) failed to induce the phosphorylation of p70S6K suggesting that the pentose phosphate pathway does not contribute to nutrient-dependent mTOR activation (Fig. 3E).
Glycolysis
We next examined if glycolysis (or a glycolytic intermediate) regulates carbohydrate-dependent mTOR activity in the heart. Unexpectedly, lactate/pyruvate, the metabolites formed at the end of glycolysis, strongly induced the phosphorylation of p70S6K in the heart (Fig. 4A). However, lactate also inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an intermediate enzyme in glycolysis25. This inhibition of glycolysis by lactate/pyruvate could lead to the accumulation of upstream glycolytic intermediates which modulate mTOR activity. In order to determine whether lactate itself or inhibition of glycolysis induces p70S6K phosphorylation, another inhibitor of GAPDH, iodoacetate, was utilized. We found that inhibition of GAPDH strongly induced the phosphorylation of p70S6K in cardiomyocytes even in the absence of exogenous nutrients (Fig. 4B). This observation suggests that an upstream glycolytic intermediate is involved in carbohydrate-dependent mTOR signalling. Not surprisingly, inhibition of glycolysis with iodoacetate (which should increase AMP concentrations) also strongly induced the phophorylation of ACC indicating increased 5′AMP kinase activity (Fig. 4C).
Next we examined the first committed step of glycolysis, the conversion of glucose to glucose 6-phosphate by hexokinase. 3-O-methyl glucose, a glucose analogue which can enter the cell but not be phosphorylated, could not elicit p70S6K phosphorylation suggesting that metabolism of glucose is necessary for mTOR activation (Fig. 4D). In contrast, when cardiomyocytes where exposed to 2-deoxyglucose, a glucose analogue which is phosphorylated but not metabolized further in glycolysis, there was phosphorylation of p70S6K (Fig. 4D). The phosphorylation of p70S6K by 2-deoxyglucose was confirmed in the working rat heart (Fig. 4E). Therefore, our findings suggest that glucose 6-phosphate is a key glycolytic intermediate which regulate carbohydrate-mediated mTOR activity.
Activation of mTOR in the heart in diabetic rat
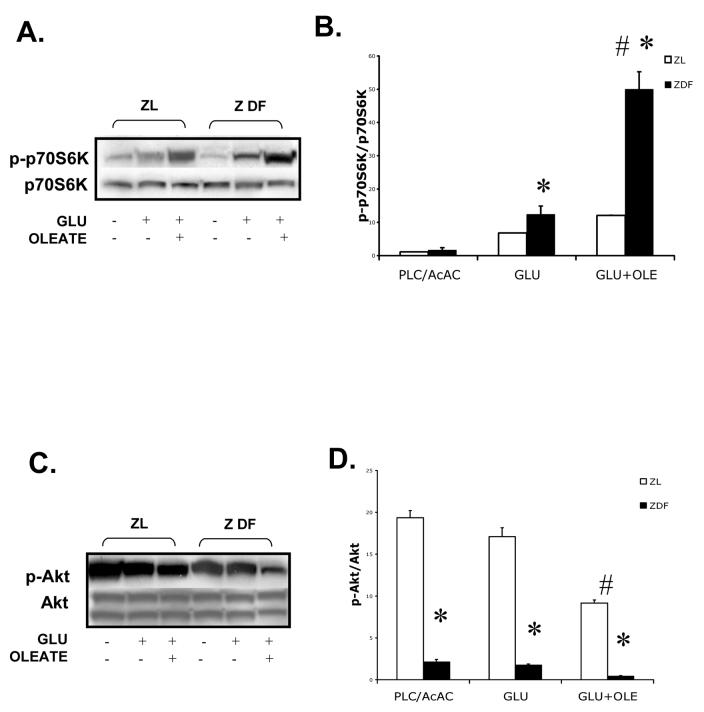
In the normal heart, fatty acids inhibit glycolysis to a greater extent than glucose uptake -- a phenomenon termed the Randle effect26. Furthermore, in type 2 diabetes mellitus (DM) glucose oxidation and glycolysis are inhibited to a greater extent than glucose uptake and phosphorylation27. If our hypothesis is correct, this preferential inhibition of glycolysis over glucose uptake by fatty acids would result in the accumulation of upstream glycolytic intermediates (including glucose 6-phosphate), subsequently leading to increased mTOR activation. Here, we demonstrate that not only p70S6k phosphorylation is enhanced in the Zucker diabetic fatty (fa/fa) rat hearts compared to Zucker lean (fa/+) rat hearts, but also that this phosphorylation is further enhanced by the addition of oleate to the perfusate. (Fig. 5A and B). Although the Akt phosphorylation was decreased in Zucker diabetic fatty (ZDF) rat hearts, interestingly, the addition of oleate further decreased Akt phosphorylation in the ZDF rat hearts (Fig. 5C and D).
Figure 5. Immunoblots and densitometry ratios.
A. p70S6K phosphorylation in ZDF rat hearts perfused with insulin (40μU/ml) with the addition of glucose (5 mM) alone or with glucose (5 mM) and sodium oleate (0.4mM) (n=3). B. Densitometry of the ratio of phosphorylated and total p70S6K (n=3) C. Akt phosphorylation in ZDF rat hearts perfused with insulin (40μU/ml) with the addition of glucose (5 mM) alone or with glucose (5 mM) and sodium oleate (0.4mM) (n=3). D. Densitometry of the ratio of phosphorylated and total Akt (n=3). * p<0.01 between ZDF and ZL rat hearts. # p<0.05 between GLU and GLU+OLE
Discussion
We tested the hypothesis that glucose regulates mTOR signalling in the heart in response to insulin. The three main findings of our study are: 1) Glucose is required for the activation of mTOR in response to insulin. 2) A glycolytic intermediate, most likely glucose 6-phosphate, is the key regulator of carbohydrate-mediated mTOR activity. 3) There is increased glucose-dependent p70S6K phosphorylation in the diabetic rat heart. Our findings provide evidence that nutrients form the link between metabolic effects of insulin and downstream signalling pathways that regulate cardiac growth (Fig. 6).
Figure 6. The proposed scheme for glucose 6-phosphate mediated mTOR signalling in the heart.
Insulin activates PI3 kinase and Akt, which increases the uptake of glucose (by translocation of GLUT4 to the cell surface). Once it is phosphorylated, glucose induces mTOR activation. Glucosamine is phosphorylated to glucosamine 6-phosphate, which likely participates in mTOR signalling. Glycogen breakdown provides alternative carbohydrate source for mTOR activation. It is unknown whether Akt/PKB directly participates in mTOR signalling in the heart. Abbreviations: GLUT, glucose transporter; RTK, receptor tyrosine kinase; IGF1, insulin-like growth factor 1; PKB, protein kinase B; mTOR, mammalian target of rapamycin.
Insulin activation of mTOR requires glucose
Hormones that regulate growth also have a substantial effect on metabolism. For example, insulin not only enhances glucose uptake, but also regulates tissue growth via the mTOR signalling pathway4, 6, 7. In the heart, deletion of the insulin receptor in the early postnatal life results in small hearts demonstrating the importance of insulin in postnatal cardiac growth28. However, the anabolic and metabolic effects of hormones are thought to be mediated by distinctly separate pathways. We found that despite phosphorylation of Akt by insulin, mTOR signalling requires the presence of glucose. Because other downstream targets of Akt (i.e. GSK-3β) were phosphorylated by insulin in the absence of glucose, we suspect that mTOR signalling is unique in its ability to respond to glucose metabolites.
As mentioned earlier the mTOR pathway has been implicated in growth by the activation of protein synthesis and ribosomal biogenesis. Because protein synthesis and ribosomal biogenesis are energy requiring process, the ability of mTOR to sense glucose may allow cells to detect adequate nutrient availability prior to stimulating growth. Because of the lack of consistency in 4EBP1 phosphorylation, p70S6K was used as downstream marker for mTOR activation. Therefore, one limitation in this study is that our findings may be specific for p70S6K and another mechanism for glucose mediated activation of 4EBP1 may exist.
Glucose 6-phosphate regulates carbohydrate-mediated mTOR signalling
In this study, we focused on the metabolic pathway and/or intermediate involved in glucose-mediated mTOR signalling. The mechanism by which mTOR physically detect nutrients is the area of active investigation in our laboratory. Although there are multiple metabolic pathways capable of eliciting glucose-mediated cellular signalling, none of these pathways participated in carbohydrate-mediated mTOR signalling in the heart. We suggest that a glycolytic intermediate (glucose 6-phosphate) that can induce the phosphorylation of p70S6K. Although we did not measure glucose 6-phosphate levels directly, we have previously shown that glucose 6-phophate levels increase significantly during activation of glycolysis, together with increased levels of lactate and alanine.29
The hexosamine biosynthetic pathway can modulate insulin signalling30. However, inhibition of GFAT, the rate-limiting enzyme in this pathway, did not attenuate glucose induced p70S6K phosphorylation. Surprisingly, glucosamine potently induced the phosphorylation of p70S6K. When glucosamine enters the cell it is phosphorylated to glucosamine 6-phosphate. Similar to glucose 6-phosphates and 2-deoxyglucose 6-phosphate, we speculate that glucosamine 6-phosphate directly modulates mTOR activity. Because we demonstrate at least 3 hexoses (glucose, 2-deoxyglucose, and glucosamine) activating mTOR, it is possible that all hexose 6-phophates may participate in mTOR signalling. Whether other hexose-6-phosphates (e.g. fructose 6-phosphate, galactose 6-phosphate) activate mTOR is an area of active research in our laboratory.
Recently, 5′AMP kinase has been shown to phosphorylate mTOR and inhibit its activity21. In isolated cardiomyocytes, Chan et al found that activation of AMP kinase decreased p70S6K phosphorylation in response to phenylephrine and Akt activation31. Our findings do not support the role of 5′AMP kinase in carbohydrate-mediated mTOR signalling. The observation that inhibition of glycolysis (which also markedly increased 5′AMP kinase activity) induced p70S6K phosphorylation also strongly suggests that 5′AMP kinase does not play role in carbohydrate-mediated phosphorylation of p70S6K. The discrepancy between our findings and those of Chan et al31 may be explained by the fact that different stimuli were used to induce p70S6K phosphorylation.
Glucose 6-phosphate can modulate enzyme activity. For example, glucose 6-phosphate allosterically inhibits hexokinase. Glucose 6-phosphate can also enhance gene expression in the liver32. We speculate that under normal conditions, flux through glycolysis is rapid enough to buffer any increases in exogenous glucose concentrations and prevent the accumulation of hexose 6-phosphates. However, there are multiple conditions (e.g. diabetes, heart failure) in which glucose oxidation is inhibited to greater extent than glucose uptake26, 33. This mismatch between uptake and oxidation of carbohydrates leads to the accumulation of glucose intermediates (including glucose 6-phosphate) and may have an important effect in the development of cardiac hypertrophy.
Enhanced p70S6K phosphorylation in the hearts of diabetic rats
As mentioned above, there are several a condition in which glycolysis is inhibited to a greater extent than glucose uptake. Here, we show p70S6K phosphorylation is enhanced in the ZDF rat heart and that addition of oleate to glucose in the perfusate results in further enhancement of p70S6K phosphorylation. It is well known that fatty acids inhibit glucose oxidation. We speculate that the inhibition of glycolysis by oleate results in the accumulation of glucose 6-phosphate which activates mTOR. Interestingly, despite significantly decreased Akt phosphorylation in the ZDF rat heart (presumably due to insulin resistance), there is enhanced p70S6K activity suggesting a minimal permissive effect of Akt signalling. Because in type 2 DM glycolsis is inhibited more than glucose uptake27, we postulate that accumulation of glycolytic intermediates may contribute to this increased p70S6K phosphorylation in the ZDF rat heart.
Clinical Implications
Our findings indicate that glucose is not only a substrate for energy metabolism, but also acts as an intracellular signal regulating protein synthesis and ultimately growth. From a physiologic prospective, the availability of energy substrates is essential for protein synthesis and growth since these are energy consuming processes. Therefore, the coupling of glucose metabolism with protein synthesis seems logical. In type 2 diabetes mellitus, glucose oxidation is inhibited to greater extent than glucose uptake26, 33 resulting in the accumulation of glucose intermediates and, thus, the activation of mTOR. We speculate that glucose-mediated activation of mTOR in the hearts of diabetic patients may contribute to the development of cardiac hypertrophy. In the failing heart, there is a downregulation of fatty acid gene expression resulting in a switch in substrate utilization from predominately fatty acids to glucose. Furthermore, heart failure is associated with myocardial and systemic insulin resistance34. We hypothesize that these derangements in myocardial metabolism also result in mTOR activation in the failing heart contributing to cardiac hypertrophy. The role of nutrient mediate mTOR activation in the diabetic and failing heart is an active area of research in our laboratory.
Conclusion
In the heart, glucose is necessary for insulin-dependent mTOR signalling. Specifically hexose-6-phosphate induce the phosphorylation of p70S6K. Furthermore, we demonstrate p70S6K phosphorylation is increased in the diabetic rat heart. Therefore, glucose is not only a metabolic substrate but also is an intracellular signal potentially regulating cardiac growth.
Acknowledgements
Research is supported by grants from the National Institutes of Health (5T32 HL 07591 and R01 HL 43133). The authors wish to acknowledge Dr. Leonard Golfman and Rebecca Salazar for technical assistance, and Roxy Ann Tate for editorial help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rohde J, Heitman J, Cardenas ME. The tor kinases link nutrient sensing to cell growth. J Biol Chem. 2001;276:9583–6. doi: 10.1074/jbc.R000034200. [DOI] [PubMed] [Google Scholar]
- 2.Crespo JL, Hall MN. Elucidating tor signaling and rapamycin action: Lessons from saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2002;66:579–91. doi: 10.1128/MMBR.66.4.579-591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proud CG. Role of mtor signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–44. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/igf-i-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–71. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- 5.Moule SK, Denton RM. Multiple signaling pathways involved in the metabolic effects of insulin. Am J Cardiol. 1997;80:41A–49A. doi: 10.1016/s0002-9149(97)00457-8. [DOI] [PubMed] [Google Scholar]
- 6.Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–49. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmelzle T, Hall MN. Tor, a central controller of cell growth. Cell. 2000;103(2):253–62. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 8.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–70. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 9.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase b promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eif4f formation. J Nutr. 2000;130:139–45. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 11.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 12.Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol. 1999;277(6 Pt 1):E1077–86. doi: 10.1152/ajpendo.1999.277.6.E1077. [DOI] [PubMed] [Google Scholar]
- 13.Kimball SR, Jefferson LS. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:43–5. doi: 10.1097/00075197-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mrna translation but not global rates of protein synthesis in the liver of rats. J Nutr. 2001;131:1171–6. doi: 10.1093/jn/131.4.1171. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 s6 kinase and multiple translation factors. Biochem J. 1998;334:261–7. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980;186:701–11. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, et al. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–30. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, et al. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–75. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 19.Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–41. doi: 10.1161/01.CIR.0000096481.45105.13. [DOI] [PubMed] [Google Scholar]
- 20.McClain DA. Hexosamines as mediators of nutrient sensing and regulation in diabetes. Diabetes Complications. 2002;16:72–80. doi: 10.1016/s1056-8727(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto K, Goodyear LJ. Invited review: Intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–83. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mtor) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–22. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin GW, Cohen DM, Taegtmeyer H. [5-3H]glucose overestimates glycolytic flux in isolated working rat heart: Role of the pentose phosphate pathway. Am J Physiol Endocrinol Metab. 2001;280:E502–E08. doi: 10.1152/ajpendo.2001.280.3.E502. [DOI] [PubMed] [Google Scholar]
- 24.Doiron B, Cuif MH, Chen R, Kahn A. Transcriptional glucose signaling through the glucose response element is mediated by the pentose phosphate pathway. J Biol Chem. 1996;271:5321–24. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- 25.Rovetto M, Lamberton W, Neely J. Mechanisms of glycolytic inhibition in ischemic rat heart. Circ Res. 1975;37:742–51. doi: 10.1161/01.res.37.6.742. [DOI] [PubMed] [Google Scholar]
- 26.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–89. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 27.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part i: General concepts. Circulation. 2002;105:1727–33. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 28.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taegtmeyer H, Roberts AF, Raine AE. Energy metabolism in reperfused heart muscle: Metabolic correlates to return of function. J Am Coll Cardiol. 1985;6:864–70. doi: 10.1016/s0735-1097(85)80496-4. [DOI] [PubMed] [Google Scholar]
- 30.Andreozzi F, D'Alessandris C, Federici M, Laratta E, Del Guerra S, Del Prato S, et al. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on ser307 and ser612 and impairs the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin insulin biosynthetic pathway in rin pancreatic beta-cells. Endocrinology. 2004;145:2845–57. doi: 10.1210/en.2003-0939. [DOI] [PubMed] [Google Scholar]
- 31.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of amp-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–9. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 32.Towle HC, Kaytor EN, Shih HM. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu Rev Nutr. 1997;17:405–33. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 33.Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–76. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]