Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue during Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration following Highly Active Antiretroviral Therapy (original) (raw)
Abstract
Gut-associated lymphoid tissue (GALT) harbors the majority of T lymphocytes in the body and is an important target for human immunodeficiency virus type 1 (HIV-1). We analyzed longitudinal jejunal biopsy samples from HIV-1-infected patients, during both primary and chronic stages of HIV-1 infection, prior to and following the initiation of highly active antiretroviral therapy (HAART) to determine the onset of CD4+ T-cell depletion and the effect of HAART on the restoration of CD4+ T cells in GALT. Severe depletion of intestinal CD4+ T cells occurred during primary HIV-1 infection. Our results showed that the restoration of intestinal CD4+ T cells following HAART in chronically HIV-1-infected patients was substantially delayed and incomplete. In contrast, initiation of HAART during early stages of infection resulted in near-complete restoration of intestinal CD4+ T cells, despite the delay in comparison to peripheral blood CD4+ T-cell recovery. DNA microarray analysis of gene expression profiles and flow-cytometric analysis of lymphocyte homing and cell proliferation markers demonstrated that cell trafficking to GALT and not local proliferation contributed to CD4+ T-cell restoration. Evaluation of jejunal biopsy samples from long-term HIV-1-infected nonprogressors showed maintenance of normal CD4+ T-cell levels in both GALT and peripheral blood. Our results demonstrate that near-complete restoration of mucosal immune system can be achieved by initiating HAART early in HIV-1 infection. Monitoring of the restoration and/or maintenance of CD4+ T cells in GALT provides a more accurate assessment of the efficacy of antiviral host immune responses as well as HAART.
The efficacy of highly active antiretroviral therapy (HAART) in the treatment of human immunodeficiency virus type 1 (HIV-1) infection is measured by viral suppression and increased CD4+ T-cell numbers in peripheral blood (3, 8). However, T lymphocytes in peripheral blood represent only 2 to 5% of the total lymphocytes in the body, while the majority of the lymphocytes are sequestered in lymphoid tissues. Because pathological processes occurring in lymphoid organs during HIV-1 infection may strongly influence disease progression, examination of lymphoid tissues will be important to gain a better understanding of the efficacy of HAART (2, 5).
Gut-associated lymphoid tissue (GALT) harbors the vast majority of lymphoid tissue in the body and has been shown to be a persistent viral reservoir as well as an important site for host-pathogen interactions in HIV-1 infection (4, 7, 14). Since intestinal biopsy samples from patients can be obtained repeatedly, GALT can serve as an accessible source of mucosal lymphoid tissue to examine the direct effects of HAART on the kinetics of CD4+ T-cell homeostasis and suppression of viral loads. Studies of simian immunodeficiency virus (SIV)-infected rhesus macaque models have demonstrated that severe CD4+ T-cell depletion in GALT and intestinal dysfunction occur during primary SIV infection (6, 10, 20, 22, 23). Moreover, clinically asymptomatic HIV-infected patients consistently show a more pronounced depletion of CD4+ T cells in intestinal mucosa than in peripheral blood (19). Information on the timing of the onset of intestinal CD4+ T-cell depletion and/or disruption in T-cell homeostasis as well as the pathogenic mechanisms in HIV infection is not available.
Long-term HIV-1-infected nonprogressors (LTNPs) generate antiviral immune responses that are effective in suppressing viral replication. These patients maintain normal levels of CD4+ T cells and suppress viral loads to undetectable levels in peripheral blood, thus maintaining the integrity of the immune system. In contrast, HIV-1-infected patients who fail to develop strong antiviral immune responses that are capable of controlling viral replication show high viral loads and progressive decline of CD4+ T cells in peripheral blood. It is not known if efficient antiviral host immune responses in LTNPs maintain the levels of CD4+ T cells in the intestinal mucosa.
Individuals who received HAART during primary HIV-1 infection consistently developed strong HIV-1 Gag-specific CD4+ and CD8+ T-cell responses as well as rapid increases in CD4+ T-cell numbers in peripheral blood (16-18). However, it has not been fully determined whether initiation of HAART early in HIV-1 infection would be more beneficial than starting therapy after CD4+ T-cell numbers in peripheral blood have declined below 300 cells/mm3. It has been suggested that HAART should not be initiated in early HIV-1 infection in order to prevent the emergence of drug-resistant viral variants and to allow the immune system to generate strong and diverse antiviral cellular and immune responses capable of suppressing viremia. Thus, examination of lymphoid tissues, in addition to peripheral blood, will be important to better evaluate the effect of the initiation of HAART during early or later stages of viral infection on CD4+ T-cell restoration and viral suppression in GALT of HIV-1-infected patients.
In order to gain insights into the kinetics of CD4+ T-cell depletion and restoration in GALT during HIV-1 infection, longitudinal jejunal biopsy samples and peripheral blood samples of HIV-1-infected patients were analyzed, during both primary and chronic stages of infection, prior to and following the initiation of HAART. Our results showed that the onset of severe CD4+ T-cell depletion in GALT occurred during primary HIV-1 infection. Increased cell proliferation, presumably in response to viral infection, was also observed in GALT during early stages of HIV-1 infection. The loss of proliferating cells by either activation-induced cell death (AICD) or direct viral infection may account for the severe depletion of intestinal CD4+ T cells during primary HIV-1 infection. Incomplete and substantially delayed restoration of intestinal CD4+ T cells, despite a near-complete suppression of viral loads and the rebound of CD4+ T cells in peripheral blood, was observed when HAART was initiated during chronic HIV-1 infection. However, early intervention with HAART resulted in near-complete restoration of CD4+ T cells in GALT, despite the delay. Restoration of intestinal CD4+ T cells following HAART was attributed to trafficking and not to local proliferation, as demonstrated by increased expression of cell trafficking mediators and decreased expression of genes associated with cell cycle progression and proliferation. The impairment of cell proliferation and increased trafficking in GALT during HIV-1 infection may strongly influence the rate and extent of CD4+ T-cell restoration during HAART and may be fundamental to HIV pathogenesis.
MATERIALS AND METHODS
Human subjects, antiretroviral therapy, and sample collection.
Jejunal biopsy samples and peripheral blood samples were obtained from 11 HAART-naïve, HIV-1-seropositive individuals (two females and nine males) with CD4+ T-cell numbers of <300/mm3 and six HIV-1-seronegative healthy individuals (four females and two males). Additionally, samples were obtained from two individuals during primary HIV-1 infection and three HIV-1-infected LTNPs who had never received antiviral therapy. Samples were also obtained from one HIV-1-infected patient who had been receiving HAART for 5 years. Antiviral therapy consisted of combinations of two reverse transcriptase inhibitors and one protease inhibitor (see Table 2). Absolute CD4+ T-cell numbers were obtained from complete blood counts from whole-blood samples. HIV-1 viral loads were determined by a bDNA assay (Bayer Diagnostics, Emeryville, Calif.). Studies were performed under an Institutional Review Board-approved protocol.
TABLE 2.
Viral loads and absolute CD4+ T-cell counts in longitudinal peripheral blood samples of HIV-1-infected patients receiving antiviral therapy
| Patient and sample no. | Time post-HAART (mo) | CD4+ absolute counts (cells/mm3) | Plasma viral loads (copies/ml) | Antiretroviral therapya |
|---|---|---|---|---|
| 120 | ||||
| 1 | 0 | 8b | >500,000b | |
| 2 | 3 | 97 | 347 | Trizivir, RTV, APV |
| 3 | 6 | 97 | 50 | |
| 4 | 12 | 190 | 50 | |
| 123 | ||||
| 1 | 0 | 46b | >500,000b | |
| 2 | 2 | 233 | 6,682 | Trizivir, RTV, APV |
| 3 | 6 | 250 | 50 | |
| 4 | 12 | 300 | 50 | |
| 104 | ||||
| 1 | 0 | 216b | 4,130b | |
| 2 | 24 | 571 | 50 | D4T, 3TC, EFV |
| 101 | ||||
| 1 | 0 | 161b | 2,708b | |
| 2 | 6 | 459 | 50 | ABC, 3TC, EFV |
| 3 | 24 | 658 | 50 | |
| 116 | ||||
| 1 | 0 | 881b | 1,076b | |
| 2 | 4 | 840 | 50 | ABC, 3TC, NFV |
| 3 | 8 | 884 | 50 | |
| 4 | 14 | 1,146 | 50 |
Isolation of intestinal lymphocytes.
Jejunal biopsy samples were collected by upper gastrointestinal endoscopy from patients under moderate sedation. Samples were processed by using collagenase digestion to isolate intestinal lymphocyte populations according to previously published procedures (10, 20). Briefly, jejunal biopsy samples were placed in lymphocyte isolation medium containing RPMI 1640 (Invitrogen/Gibco BRL, Grand Island, N.Y.), 5% fetal calf serum (Gibco BRL), 100 U of penicillin (Gibco BRL) per ml, 100 U of streptomycin (Gibco BRL) per ml, and 100 U of collagenase type II (Sigma Chemical Co., St. Louis, Mo.) per 100 ml and subjected to rapid shaking at 37°C for 30 min. Cell suspensions were centrifuged over a 35%:60% (vol/vol) isotonic discontinuous Percoll (Sigma) density gradient for lymphocyte enrichment. Cell viability (>95%) was determined by trypan blue exclusion.
Antibodies.
The following monoclonal antibodies were used in this study: fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated CD3, CD4-PE, and integrin β7-PE (clone FIB504) (Pharmingen, San Diego, Calif.); CD4-TC and CD8-APC (Caltag Laboratories, South San Francisco, Calif.); and Ki67-FITC, MIB-1 clone (Coulter Immunotech, Miami, Fla.). The isotype controls included FITC-conjugated immunoglobulin G1 (IgG1), IgG1-PE, IgG1-Tri Color (TC), and IgG1-allophycocyanin (APC) antibodies (Becton Dickinson, Mountain View, Calif., and Caltag).
Flow-cytometric analysis of isolated lymphocytes.
Immunophenotypic analysis of T lymphocytes was performed as previously described (10, 20). Briefly, isolated cells were incubated with mouse anti-human monoclonal antibodies for 30 min at 4°C. After incubation, cells were washed and fixed with 1% paraformaldehyde. Isotype-matched IgG1 antibodies were used as negative controls. For flow-cytometric detection of Ki67, cells were stained for cell surface markers, fixed, permeabilized, and stained with FITC-conjugated anti-human Ki67. Negative control samples were stained with matched isotype control monoclonal antibodies. Sample data were acquired on a FACSCalibur (Becton Dickinson). A minimum of 100,000 total events was collected for each sample, and data were analyzed with CellQuest software (Becton Dickinson) and Flow Jo (Tree Star Inc., San Carlos, Calif.).
Microarray analysis.
Total RNA was isolated from jejunal biopsy samples using RNeasy kit (Qiagen, Valencia, Calif.). Fifteen micrograms of RNA was used to synthesize cDNA, which was then amplified by in vitro transcription to cRNA according to recommended protocols (Affymetrix, Santa Clara, Calif.). The cRNA was fragmented, and 15 μg was used to hybridize to a HuGene U95-AV2 oligonucleotide microarray (Affymetrix). Arrays were scanned by a laser scanner (Agilent Technologies, Palo Alto, Calif.). Microarray suite 4.0 (Affymetrix) and DNA-Chip Analyzer (dChip) version 1.1 (Harvard University) were used for data analysis. Comparative analysis was performed with samples obtained from HIV-1-infected patients before and after the initiation of HAART. Gene expression profiles were also determined for jejunal biopsy samples from five uninfected healthy individuals for comparative analysis.
Immunohistochemistry.
CD4+ T cells were detected in jejunal tissue sections by immunohistochemistry with anti-CD4 antibody (Nova Castra Laboratories, Newcastle, England). Biopsy samples were fixed in Strecks tissue fixative (Streck Laboratories, Omaha, Nebr.). Slides were counterstained lightly with hematoxylin, and coverslips were mounted with Permount (Fisher Scientific, Fair Lawn, N.J.).
RESULTS
Patient characteristics.
Jejunal biopsy and peripheral blood samples from HAART-naïve HIV-1-infected men (n = 13) and women (n = 3) were collected to compare the effects of HIV-1 infection on intestinal and peripheral T-cell depletion (Table 1). Two of the individuals had primary HIV-1 infection. Another three individuals were clinically identified as LTNPs. Additionally, samples from healthy HIV-1-seronegative men (n = 2) and women (n = 4) were included as negative controls.
TABLE 1.
Patient characteristicsa
| Status | Patient | Gender | Age (yrs) | Time diagnosedb | CD4+ absolute counts (cells/mm3) | Plasma viral loads (copies/ml)c |
|---|---|---|---|---|---|---|
| HIV-1 positive, | 115 | M | 27 | 7 mo | 248 | 970 |
| HAART-naïve | 124 | M | 43 | 8 mo | 294 | 452,717 |
| 120 | M | 37 | 1 yr | 8 | >500,000 | |
| 121 | M | 36 | 1 yr | 46 | >500,000 | |
| 119 | F | 49 | <2 yr | 8 | 727,897 | |
| 104 | M | 46 | 2.5 yr | 216 | 4,130 | |
| 102 | M | 42 | <3 yr | 762 | 8,221 | |
| 101 | F | 30 | 3.5 yr | 161 | 2,708 | |
| 106 | F | 37 | 5.5 yr | 289 | 73,774 | |
| 111 | M | 50 | 10.5 yr | 219 | 200,810 | |
| 103 | M | 45 | 11.5 yr | 42 | 1,253 | |
| LTNP | 126 | M | 43 | 5.5 yr | 750 | <50 |
| 122 | M | 49 | 13.5 yr | 910 | <50 | |
| 135 | M | 46 | 18 yr | 689 | <50 | |
| HIV-1 negative | 114 | M | 22 | NA | 1,346 | NA |
| controls | 117 | M | 24 | NA | 1,465 | NA |
| 118 | F | 23 | NA | 1,236 | NA | |
| 130 | F | 35 | NA | 1,046 | NA | |
| 131 | F | 47 | NA | 632 | NA | |
| 132 | F | 53 | NA | 1,203 | NA | |
| Primary HIV-1 | 134 | M | 35 | 4 wk | 752 | >500,000 |
| infection | 116 | M | 36 | 6 wk | 881 | 41,464 |
| HIV-1 positive, 5 yr of HAART | 129 | M | 43 | 14 yr | 800 | <50 |
Most HAART-naïve HIV-1-infected individuals had absolute CD4+ T-cell counts below 300 cells/mm3 (Table 1) except for patient 102 (762 cells/mm3). All LTNPs, as well as patients 134 and 116, had absolute CD4+ T-cell numbers in peripheral blood within the normal range. Patients 134 and 116 had been recently exposed to HIV-1 (4 and 6 weeks, respectively), which may explain the relatively normal absolute CD4+ T-cell numbers in the peripheral blood of these individuals. Plasma viral loads ranged from 970 to >5 × 106 RNA copies/ml, as determined by a bDNA assay (Table 1).
Severe depletion of CD4+ T-cell subsets and CD8+ T lymphocytosis in GALT occurs during primary HIV-1 infection.
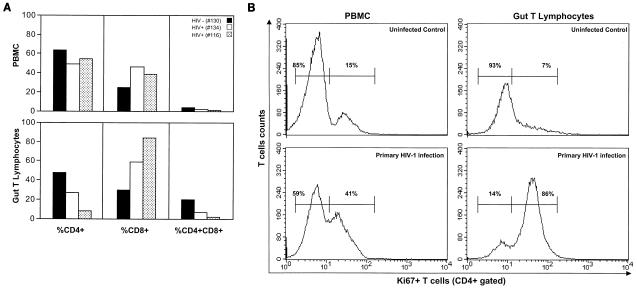
To determine alterations in T-cell homeostasis during primary HIV-1 infection, jejunal biopsy samples and peripheral blood samples were obtained from two individuals who had recently been exposed to HIV-1. Four weeks following a suspected high-risk HIV-1 exposure, a significant decline in CD4+ and CD4+CD8+ T-cell percentages was observed in GALT of patient 134 (27 and 7%, respectively) (Fig. 1A). Samples were obtained from another individual, patient 116, at 6 weeks following a suspected high-risk HIV-1 exposure (Fig. 1A). Patient 116 also demonstrated severe depletion of CD4+ and CD4+ CD8+ T-cell percentages (8 and 2%, respectively). Both patients had relatively normal levels of CD4+ T cells in peripheral blood (Fig. 1A). These results clearly demonstrated that the onset of severe CD4+ T-cell depletion in GALT occurred during primary HIV-1 infection.
FIG. 1.
(A) Onset of severe depletion of CD4+ T-cell subsets in GALT occurs in primary HIV-1 infection. Prevalence of CD4+, CD8+, and CD4+CD8+ T-cell subsets in gut T lymphocytes and peripheral blood mononuclear cells (PBMC) of two HAART-naïve individuals (patients 134 and 116) during primary HIV-1 infection was analyzed by flow cytometry and compared with that in uninfected healthy controls. (B) Increased proliferation of intestinal CD4+ T cells in response to primary HIV-1 infection. Flow-cytometric analysis was performed to determine Ki67 expression in CD4+ T cells in peripheral blood and gut T lymphocytes 4 weeks after a suspected high-risk exposure to HIV-1 infection (patient 134), in comparison to a healthy uninfected control.
Increased CD4+ T-cell proliferation in GALT in response to primary HIV-1 infection.
To examine whether there was an expansion of intestinal CD4+ T cells in response to primary HIV-1 infection, the expression of the nuclear antigen Ki67 (cell proliferation marker) was analyzed. It was of interest that at 4 weeks following a suspected high-risk HIV-1 exposure, more than 80% of the intestinal CD4+ T lymphocytes in patient 134 expressed Ki67, indicating a selective expansion of intestinal CD4+ T cells early in infection (Fig. 1B). In contrast, less than 10% of intestinal CD4+ T cells expressed Ki67 in GALT of healthy uninfected individuals (Fig. 1B) and two chronically HIV-1-infected patients (data not shown). Proliferating CD4+ T cells may be more sensitive to HIV-1 infection and AICD. The rapid loss of these cells may contribute to the severe CD4+ T-cell depletion observed in GALT during primary HIV-1 infection.
LTNPs maintain normal levels of intestinal CD4+ T cells, in contrast to HAART-naïve chronically HIV-1-infected patients.
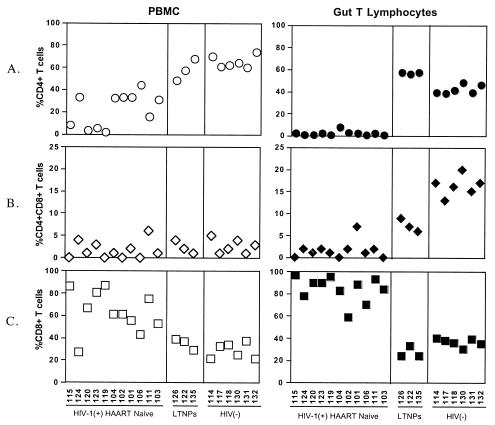
Jejunal biopsy samples and peripheral blood samples were evaluated from 11 HAART-naïve chronically HIV-1-infected patients. These patients were known to have been HIV-1 seropositive for 7 months to 11.5 years. Our results showed that the level of depletion of CD4+ and CD4+CD8+ T-cell subsets in these patients was more pronounced in GALT than in peripheral blood, compared to uninfected healthy controls (Fig. 2A and B). The percentage of CD8+ T cells, in both GALT and peripheral blood, in all HAART-naïve HIV-1-infected individuals was increased, and this may be attributed to a homeostatic response to compensate for the loss of CD4+ T cells (Fig. 2C).
FIG. 2.
Alterations in CD4+ and CD8+ T-cell subsets in GALT of HIV-1-infected individuals with diverse clinical outcomes. Flow-cytometric analysis was performed to determine changes in percentages of CD3-gated CD4+, CD4+CD8+, and CD8+ T cells in gut T lymphocytes and peripheral blood mononuclear cells (PBMC) from HAART-naïve chronically HIV-1-infected individuals (n = 11), LTNPs (n = 3), and uninfected healthy controls (n = 6). Absolute CD4+ T-cell numbers, viral loads, and length of infection at the time of sample collection are presented in Table 1.
Alterations in T-cell subsets were examined in jejunal biopsy samples and peripheral blood samples from three HAART-naïve LTNPs (patients 126, 122, and 135) and compared to those in HAART-naïve chronically HIV infected patients and healthy uninfected controls. All LTNPs had undetectable plasma viral loads and CD4+ T-cell numbers in peripheral blood that were comparable to those in healthy uninfected controls, despite the fact that the patients had been seropositive for several years (Table 1). Intriguingly, the percentages of intestinal CD4+ T cells in these patients were slightly higher (patient 126, 57%; patient 122, 56%; and patient 135, 57%) than those in uninfected healthy controls (Fig. 2A). However, the CD4+CD8+ T-cell subsets were slightly depleted (patient 126, 9%; patient 122, 7%; and patient 135, 6%) compared to those in uninfected controls (Fig. 2B). The CD8+ T-cell percentages in LTNPs were within the normal range in both peripheral blood and GALT (Fig. 2C). Taken together, these data suggested that preservation of intestinal CD4+ T cells in HIV-1-infected individuals might correlate with a better clinical outcome. The maintenance of high levels of CD4+ T cells in GALT may be reflective of the strong antiviral immune responses that provide effective control of viral infection in LTNPs.
Delayed and incomplete restoration of intestinal CD4+ and CD4+CD8+ T-cell subsets in chronically HIV-1-infected patients receiving HAART.
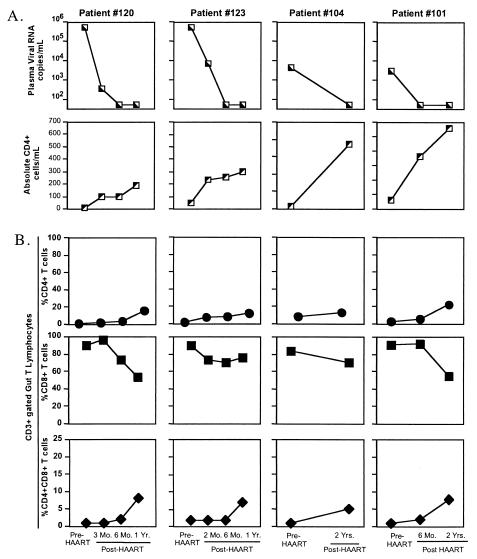
To compare the efficacy of HAART on the restoration of CD4+ T cells in GALT and peripheral blood, longitudinal jejunal biopsy samples and peripheral blood samples from chronically HIV-1-infected patients (patients 120, 123, 104, and 101) were collected prior to and following the initiation of HAART (Table 2). All patients displayed suppression of viral loads and a gradual increase in CD4+ T-cell numbers in peripheral blood following the initiation of HAART (Fig. 3A).
FIG. 3.
Delayed and incomplete restoration of CD4+ T-cell subsets in GALT compared to peripheral blood in chronically HIV-1-infected patients receiving HAART. (A) Longitudinal analysis of viral loads and absolute CD4+ T-cell numbers in peripheral blood of HIV-1-infected patients (n = 4) prior to and following HAART. (B) Flow-cytometric analysis was performed to determine alterations in percentages of CD3-gated CD4+, CD8+, and CD4+CD8+ T cells in longitudinal jejunal biopsy samples from HIV-1-infected patients prior to and following HAART.
Our results showed incomplete and significantly delayed restoration of intestinal CD4+ T cells among all patients, even after 2 years of HAART (patient 120, 1 to 16%; patient 123, 2 to 12%; patient 104, 8 to 13%; patient 101, 3 to 22%) compared to peripheral blood (Fig. 3B). A similar pattern was observed for the CD4+CD8+ T-cell subset (Fig. 3B). However, the elevated CD8+ T-cell percentages observed in GALT prior to therapy were slightly reduced following HAART but remained elevated compared to percentages in HIV-negative controls (Fig. 3B). Initiation of HAART during chronic infection did not effectively restore T-cell homeostasis in GALT of HIV-1-infected individuals compared to that in peripheral blood.
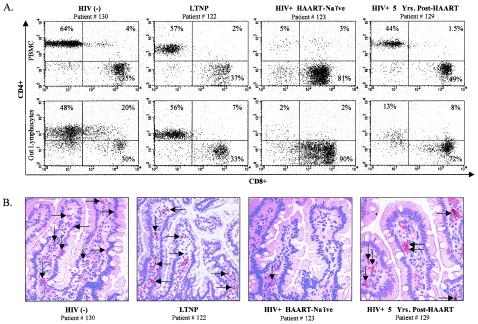
In order to evaluate the efficacy of long-term HAART on the restoration of intestinal CD4+ T cells, jejunal biopsy and peripheral blood samples were obtained from one HIV-1-infected patient who had been receiving HAART for 5 years (patient 129). Intestinal CD4+ and CD4+CD8+ T cells in GALT of this individual were not fully restored (13 and 8%, respectively), despite 5 years of antiviral therapy (Fig. 4A). Immunohistochemical analysis of jejunal tissue biopsy samples also demonstrated lack of intestinal CD4+ T-cell restoration compared to uninfected controls and LTNPs (Fig. 4B). Taken together, these data showed that despite the restoration of CD4+ T cells in peripheral blood, HAART did not result in comparable levels of immune restoration in GALT of HIV-1-infected individuals.
FIG. 4.
Flow-cytometric and immunohistochemical analysis of CD4+ T-cell depletion in GALT of HIV-1-infected individuals. (A) Representative dot plots of CD3-gated CD4+ and CD8+ T cells in peripheral blood and GALT; (B) immunohistochemical detection of CD4+ cells of an HIV-negative healthy control, an LTNP, a HAART-naïve chronically HIV-1-infected patient, and an HIV-1-infected patient who had received HAART for 5 years.
Initiation of HAART during primary HIV-1 infection is effective in restoring CD4+ T-cell populations in GALT.
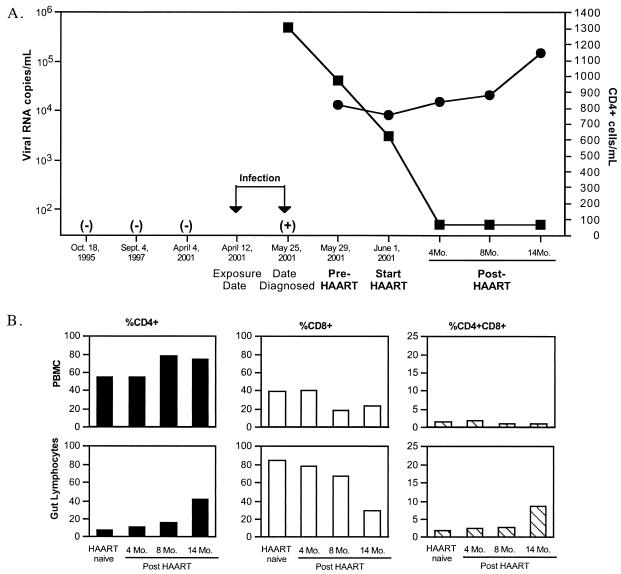
To determine whether initiation of HAART during primary HIV-1 infection resulted in improved CD4+ T-cell restoration in GALT, longitudinal jejunal biopsy and peripheral blood samples from one recently HIV-1-infected individual (patient 116) were evaluated (Fig. 5A). Blood samples from this individual were negative for HIV-1 RNA for over 6 years (1995 to 2001). On 12 April 2001, patient 116 had a potential exposure to HIV-1 (Fig. 5A). Plasma samples collected on 25 May 2001 showed >500,000 copies of viral RNA. Thus, the time of viral infection was presumed to be between 12 April and 25 May 2001.
FIG. 5.
Initiation of HAART during primary HIV-1 infection results in near-complete restoration of CD4+ T cells in GALT. (A) Time line, plasma viral loads, and absolute CD4+ T-cell numbers in peripheral blood of patient 116 during early HIV-1 infection and following the initiation of HAART. ▪, viral RNA copies; •, CD4+ cells. (B) Alterations in percentages of CD4+, CD4+CD8+, and CD8+ T cells in longitudinal jejunal biopsy samples and peripheral blood samples of patient 116, prior to and following HAART. −, HIV-1 seronegative; +, plasma HIV-1 RNA positive.
At the time of diagnosis, patient 116 opted to immediately initiate HAART. Jejunal biopsy and peripheral blood samples were collected prior to the initiation of HAART and at 4, 8, and 14 months post-HAART (Table 2). Quantitative analysis of plasma viral loads showed a decrease in viral burden from 25 May to 1 June 2001, prior to the initiation of HAART, suggesting that the generation of innate antiviral immune responses during primary HIV-1 infection was leading to the reduction in the viral loads (Fig. 5A). Initiation of HAART led to the suppression of viral loads to undetectable levels and increases in CD4+ T-cell numbers in peripheral blood (Fig. 5A).
Patient 116 had peripheral blood CD4+ T-cell numbers within the normal range (881 cells/mm3), with severe CD4+ T-cell depletion in GALT (8%) at the time of the initiation of HAART. The restoration of intestinal CD4+ T cells of patient 116 followed a pattern similar to that observed among chronically HIV-1-infected patients receiving HAART. However, patient 116 reached near-complete restoration of CD4+ T cells in GALT after 14 months of HAART (Fig. 5B). This observation contrasted with the lack of complete restoration observed in chronically HIV-1-infected patients who had been on HAART for similar lengths of time but initiated therapy after their peripheral CD4+ T-cell numbers dropped below 300 cells/mm3. We propose that the early initiation of HAART allowed near-complete restoration of CD4+ T-cell subsets in GALT by decreasing the viral loads and supporting the restoration of the mucosal immune system.
Cell trafficking but not local cell proliferation contributes to CD4+ T-cell restoration in GALT of HIV-1-infected patients receiving HAART.
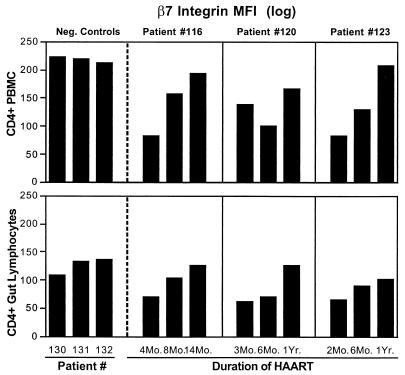
To determine the mechanisms (lymphocyte trafficking or cell proliferation) contributing to the restoration of CD4+ T cells in GALT during HAART, CD4+ T cells from longitudinal jejunal biopsy samples were analyzed the for the expression of integrin β7, which plays an important role in lymphocyte homing to the intestinal mucosa. Samples were also analyzed for the expression of Ki67, a cell proliferation marker. Our results showed a gradual increase in the density of expression of integrin β7 (expressed as mean fluorescent intensity) in both peripheral and intestinal CD4+ T cells following HAART (patients 116, 120, and 123), in comparison to uninfected controls (Fig. 6). These data suggested that the modest restoration of intestinal CD4+ T cells could be attributed to lymphocyte homing from the periphery. There was no increase in Ki67-expressing CD4+ T cells in the longitudinal jejunal biopsy samples of HIV-1-infected patients receiving HAART (data not shown). These results suggested that local cell proliferation was not a mechanism that significantly contributed to restoration of CD4+ T cells in GALT during the first year of HAART.
FIG. 6.
Increased expression of lymphocyte homing marker on repopulating CD4+ T cells in GALT following HAART. Flow-cytometric analysis of integrin β7 expression was performed on CD4+ T cells in longitudinal jejunal and peripheral blood samples of three healthy uninfected controls and three HIV-1-infected patients following HAART.
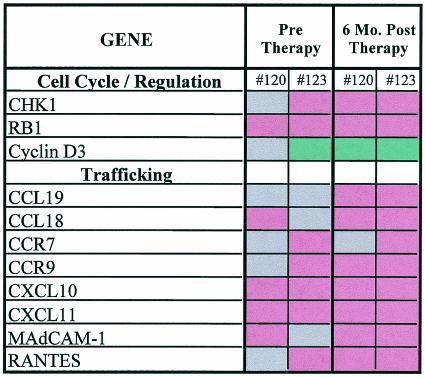
We hypothesized that changes observed at the cellular level in GALT will be preceded by changes at the molecular level. Therefore, oligonucleotide-based high-density microarray analysis was performed to determine the alterations in the expression of genes associated with lymphocyte trafficking in GALT during HAART. Gene expression profiles were generated from longitudinal jejunal biopsy samples collected from two chronically HIV-1-infected patients (patients 120 and 123), prior to and following HAART, and compared to gene expression profiles from five healthy uninfected controls (Fig. 7). Increased expression of genes involved in cellular trafficking (CXCL10, CXCL11, CCR9, and CCR7) was observed in both patients following the initiation of HAART, in comparison to uninfected controls (Fig. 7). CXCL10 and CXCL11 are known to mediate T-cell migration, whereas CCR9 and CCR7 interact with integrin α4β7 (expressed on the surfaces of T cells) to induce trafficking to GALT. Increased expression of the chemoattractants RANTES and CCL19 as well as mucosal addressin cell adhesion molecule (MAdCAM) 1 (involved in T-cell migration to intestinal epithelium) was also observed in both patients following HAART. The increase in expression of genes associated with cell trafficking supported our flow-cytometric analysis demonstrating that lymphocyte homing plays an important role in the restoration of intestinal CD4+ T cells in GALT following HAART.
FIG. 7.
DNA microarray analysis of gene expression profiles in longitudinal jejunal biopsy samples of HIV-1-infected patients prior to and following HAART. Red indicates increased gene expression compared to five HIV-1-negative controls, green indicates decreased gene expression, and grey indicates no change in the level of gene expression.
The expression of genes associated with cell cycle progression was also evaluated (Fig. 7). Our results showed increased expression of the checkpoint suppressor CHK-1 and retinoblastoma-1 as well as down regulation of cyclin D3 expression in GALT of patients 120 and 123, suggesting a block in the G1-to-S phase transition. HAART did not restore the expression of these genes to normal levels. Taken together, the increased gene expression of cell trafficking-associated molecules and the dysregulation of cell cycle mediators suggested that increased lymphocyte homing from the periphery may be the mechanism contributing to the restoration of intestinal CD4+ T cells following HAART.
DISCUSSION
This study is the first to demonstrate that the onset of severe depletion of CD4+ and CD4+CD8+ T-cell subsets, accompanied by CD8+ T lymphocytosis in GALT, occurs during primary HIV-1 infection. These alterations in intestinal T-cell homeostasis were not reflected in peripheral blood. Chronically infected HAART-naïve patients showed persistent CD4+ T-cell depletion, indicating that the loss of CD4+ T cells was not reversed in the absence of therapy. Our previous studies have shown that severe CD4+ T-cell depletion occurred in GALT of SIV-infected rhesus macaques during primary infection and that CD4+ T cells were not restored in the absence of antiviral therapy (10, 20, 23). Depletion of CD4+ T cells in GALT during primary HIV-1 infection may be attributed to increased susceptibility to viral infection due to dual surface expression of the HIV-1 coreceptors CCR5 and CXCR4 on human intestinal CD4+ T lymphocytes (12). The direct cytopathic effect of viral infection, cell cycle dysregulation mediated by viral antigens, cell-mediated cytopathic activity, and AICD may be mechanisms contributing to the severe CD4+ T-cell depletion associated with HIV-1 infection (1, 13).
A high percentage of Ki67-expressing CD4+ T cells was observed during primary HIV-1 infection, in contrast to uninfected controls, indicating an early expansion of CD4+ T cells in GALT in response to viral infection. Proliferating CD4+ T cells may be more susceptible to HIV-1 infection and may become the major contributors to the viral reservoir in the tissue. In addition, these activated CD4+ T cells may be also more susceptible to AICD. Taken together, this may explain the profound intestinal CD4+ T-cell depletion in primary HIV-1 infection. The CD4+ T-cell depletion in GALT could possibly impair effective immune responses against HIV-1 and other opportunistic infections, thus contributing to HIV-1 pathogenesis.
Our results, for the first time, demonstrate that LTNPs maintain normal levels of CD4+ T cells in GALT. This finding correlated with undetectable viral loads and normal CD4+ T-cell numbers in peripheral blood and healthy clinical status. These LTNPs were HAART-naïve and presumably had antiviral immune responses that were effective in maintaining plasma viral loads at undetectable levels. This is in contrast to HAART-naive chronically HIV-1-infected patients with high plasma viral loads and CD4+ T-cell depletion in peripheral blood and GALT. Thus, the maintenance of intestinal CD4+ T cells may reflect the ability of the host antiviral responses to control viral infection and maintain the integrity of the mucosal immune system.
A substantial delay in CD4+ T-cell restoration was seen in GALT of HIV-1-infected patients who initiated HAART during either primary HIV-1 infection or chronic HIV-1 infection despite undetectable plasma viral loads and increased peripheral blood CD4+ T-cell numbers. One HIV-1-infected patient showed incomplete CD4+ T-cell restoration in GALT despite receiving HAART for 5 years. In contrast, initiation of HAART during primary HIV-1 infection led to near-complete restoration of CD4+ T cells, as opposed to patients who initiated HAART in later stages of HIV-1 infection. The functionality of the repopulating CD4+ T cells was not assayed in this study. However, studies with the SIV model have indicated that repopulating CD4+ T cells in GALT of animals initiating antiviral therapy in later stages of infection were found to have a reduced capacity to produce interleukin-2 compared to CD4+ T cells in uninfected animals (9). Our findings suggest that the initiation of HAART during early stages of HIV-1 infection is more effective in restoring gut mucosal immune system and may be reflective of a better immune recovery.
The mechanisms of intestinal CD4+ T-cell restoration following HAART involve primarily CD4+ T-cell trafficking to GALT from the periphery and not local cell proliferation. Gene expression analysis provided evidence of increased expression of mediators associated with lymphocyte homing, such as integrin β7, CCR9, CCR7, CXCL10, CXCL11, and MAdCAM, as well as the chemoattractant molecules CCL19 and RANTES. While the expression of homing receptors was upregulated, we speculate that increased CD4+ T-cell trafficking may not be sufficient to completely repopulate GALT. Secondly, the CD4+ T cells homing to GALT may be readily infected with HIV-1 and eliminated by cytopathic effect of direct viral infection or by the virus-specific cytotoxic CD8+ T cells. Cell cycle dysregulation in CD4+ T cells may also contribute to the lack of local CD4+ T-cell expansion. Another mechanism may include dysregulation of cell cycle-associated genes such as those for cyclin D3, the checkpoint suppressor CHK-1, and retinoblastoma-1 in GALT, which can lead to a block in G1-to-S phase transition, contributing to the lack of local expansion (24).
We propose that the increased prevalence of CD8+ T cells may lead to suppression of local CD4+ T-cell proliferation and contribute to the delay in intestinal CD4+ T-cell restoration. Studies in the mouse model have shown that staphylococcus enterotoxin A-activated CD8+ T cells suppressed the CD4+ proliferative responses and induced CD4+ T-cell apoptosis through Fas/FasL (15). Thus, activated CD8+ T cells have the potential to mediate CD4+ T-cell AICD. Additionally, increased production of proinflammatory cytokines and chemokines by CD8+ T cells (11, 21) may compromise the gut mucosal epithelial cell barrier integrity and digestive and absorptive functions by causing injury to lymphoid and epithelial cells, resulting in gastrointestinal disturbances commonly observed in HIV-1-infected individuals.
In summary, our studies emphasize the importance of evaluation of the gut mucosal immune system in determining the efficacy of HAART and gaining better insights into the fundamental mechanisms of HIV-1 pathogenesis. We demonstrated that the CD4+ T-cell subset in GALT is an early target of HIV-1 infection. Initiation of HAART in early stages of infection was highly effective in restoring CD4+ T cells in GALT, and the mechanism of restoration was attributed to CD4+ T-cell homing and not to local cell proliferation. The severity of the initial CD4+ T-cell depletion as well as the local impairment of cell proliferation within the intestinal mucosa may play a role in the delay of CD4+ T-cell restoration in GALT during HAART. Our studies support the initiation of HAART early in HIV-1 infection, which appears to result in more complete restoration in the peripheral and gut mucosal immune systems. Immune restoration of gut mucosa provides an excellent correlate of effective antiviral immune responses and therapy.
Acknowledgments
This study was supported by NIH grant DK61297 and by CARES and Cable Positive.
We thank Thomas Ndolo and Michael George for critical reviews of the manuscript. We also thank Claudette Gay and Thresha Reed for invaluable assistance in the study.
REFERENCES
- 1.Ameisen, J. C., and A. Capron. 1991. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol. Today 12**:**102-105. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, J., T. E. Fehniger, B. K. Patterson, J. Pottage, M. Agnoli, P. Jones, H. Behbahani, and A. Landay. 1998. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS 12**:**F123-F129. [DOI] [PubMed] [Google Scholar]
- 3.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277**:**112-116. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P. 1989. Overview of the mucosal immune system. Curr. Top. Microbiol. Immunol. 146**:**13-25. [DOI] [PubMed] [Google Scholar]
- 5.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17**:**625-656. [DOI] [PubMed] [Google Scholar]
- 6.Heise, C., C. J. Miller, A. Lackner, and S. Dandekar. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169**:**1116-1120. [DOI] [PubMed] [Google Scholar]
- 7.Kotler, D. P., S. Reka, A. Borcich, and W. J. Cronin. 1991. Detection, localization, and quantitation of HIV-associated antigens in intestinal biopsies from patients with HIV. Am. J. Pathol. 139**:**823-830. [PMC free article] [PubMed] [Google Scholar]
- 8.Lederman, M. M., E. E. Connick, A. Landay, D. R. Kuritzkes, J. Spritzler, M. St. Clair, B. L. Kotzin, L. Fox, M. H. Chiozzi, J. M. Leonard, F. Rousseau, M. Wade, J. D'Arc Roe, A. Martinez, and H. Kessler. 1998. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS clinical trials group protocol 315. J. Infect. Dis. 178**:**70-79. [DOI] [PubMed] [Google Scholar]
- 9.Mattapallil, J. J., Z. Smit-McBride, P. Dailey, and S. Dandekar. 1999. Activated memory CD4+ T helper cells repopulate the intestine early following antiretroviral therapy of simian immunodeficiency virus-infected rhesus macaques but exhibit a decreased potential to produce interleukin-2. J. Virol. 73**:**6661-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattapallil, J. J., Z. Smit-McBride, M. McChesney, and S. Dandekar. 1998. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1β expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J. Virol. 72**:**6421-6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan, I., S. G. Radford, and D. P. Jewell. 1994. Cytokine gene expression in HIV-infected intestinal mucosa. AIDS 8**:**1569-1575. [DOI] [PubMed] [Google Scholar]
- 12.Meng, G., M. T. Sellers, M. Mosteller-Barnum, T. S. Rogers, G. M. Shaw, and P. D. Smith. 2000. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J. Infect. Dis. 182**:**785-791. [DOI] [PubMed] [Google Scholar]
- 13.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257**:**217-219. [DOI] [PubMed] [Google Scholar]
- 14.Nannini, E. C., and P. C. Okhuysen. 2002. HIV1 and the gut in the era of highly active antiretroviral therapy. Curr. Gastroenterol. Rep. 4**:**392-398. [DOI] [PubMed] [Google Scholar]
- 15.Noble, A., G. A. Pestano, and H. Cantor. 1998. Suppression of immune responses by CD8 cells. I. Superantigen-activated CD8 cells induce unidirectional Fas-mediated apoptosis of antigen-activated CD4 cells. J. Immunol. 160**:**559-565. [PubMed] [Google Scholar]
- 16.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407**:**523-526. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278**:**1447-1450. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg, E. S., L. LaRosa, T. Flynn, G. Robbins, and B. D. Walker. 1999. Characterization of HIV-1-specific T-helper cells in acute and chronic infection. Immunol. Lett. 66**:**89-93. [DOI] [PubMed] [Google Scholar]
- 19.Schneider, T., H. U. Jahn, W. Schmidt, E. O. Riecken, M. Zeitz, R. Ullrich, et al. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut 37**:**524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72**:**6646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smit-McBride, Z., J. J. Mattapallil, F. Villinger, A. A. Ansari, and S. Dandekar. 1998. Intracellular cytokine expression in the CD4+ and CD8+ T cells from intestinal mucosa of simian immunodeficiency virus infected macaques. J. Med. Primatol. 27**:**129-140. [DOI] [PubMed] [Google Scholar]
- 22.Stone, J. D., C. C. Heise, C. J. Miller, C. H. Halsted, and S. Dandekar. 1994. Development of malabsorption and nutritional complications in simian immunodeficiency virus-infected rhesus macaques. AIDS 8**:**1245-1256. [DOI] [PubMed] [Google Scholar]
- 23.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280**:**427-431. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, H., J. L. Watkins, and H. Piwnica-Worms. 2002. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. USA 99**:**14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]