FGF-23–Klotho signaling stimulates proliferation and prevents vitamin D–induced apoptosis (original) (raw)
Abstract
Fibroblast growth factor 23 (FGF-23) and Klotho are secretory proteins that regulate mineral-ion metabolism. Fgf-23−/− or Klotho−/− knockout mice exhibit several pathophysiological processes consistent with premature aging including severe atrophy of tissues. We show that the signal transduction pathways initiated by FGF-23–Klotho prevent tissue atrophy by stimulating proliferation and preventing apoptosis caused by excessive systemic vitamin D. Because serum levels of active vitamin D are greatly increased upon genetic ablation of Fgf-23 or Klotho, we find that these molecules have a dual role in suppression of apoptotic actions of vitamin D through both negative regulation of 1α-hydroxylase expression and phosphoinositide-3 kinase–dependent inhibition of caspase activity. These data provide new insights into the physiological roles of FGF-23 and Klotho.
Introduction
FGF-23 is a recently discovered member of the FGF family of signaling proteins. It is a circulating factor derived from osteocytes that acts to regulate kidney function and osteogenesis (Yamashita, 2005; Yu and White, 2005). FGF-23 is essential for maintaining phosphate homeostasis and has been linked to regulation of vitamin D (1,25(OH)2D3) metabolism (Yu and White, 2005; Liu et al., 2006; Razzaque and Lanske, 2006). Although these phenomena have been well established, the signaling mechanisms of FGF-23 and potential target genes have yet to be investigated in detail. FGF-23 alone has minimal ability to promote intracellular signaling. Recent studies have shown that the function of FGF-23 is dependent on interaction with a hormone cofactor, Klotho (Kurosu et al., 2006), which is a trans-membrane protein that binds FGF-23 (Torres et al., 2007). Interaction of Klotho with the FGF receptor 1c (FGFR1c) converts it into a receptor specific for FGF-23 function (Urakawa et al., 2006). Upon formation of a ternary complex with FGF-23 and FGFR1c, phosphorylation of the receptor is achieved (Kurosu et al., 2006). However, little is known about the downstream effects caused by FGF-23–Klotho. In fact, the only significant molecular changes that have currently been observed from FGF-23–Klotho function include phosphorylation of extracellular signal–regulated kinase (ERK), sodium phosphate transport regulation, and increased Egr-1 (early growth responsive 1) gene expression (Yu and White, 2005; Kurosu et al., 2006).
The phenotypes observed from both Fgf-23−/− and Klotho−/− mice are consistent with premature aging. These include shortened life span, severe atrophy of most tissues/organs, soft tissue calcifications, infertility, pulmonary emphysema, osteoporosis, and arteriosclerosis (Kuro-o et al., 1997; Razzaque et al., 2005, 2006; Torres et al., 2007). Fgf-23−/− and Klotho−/− mice also have increased serum phosphate levels. FGF-23–Klotho has been found to inhibit phosphate transport in renal proximal tubule epithelial cells (PTEC) by direct regulation of the sodium phosphate cotransporters (Yu and White, 2005).
Both Fgf-23−/− and Klotho−/− mice have unusually high serum levels of 1,25(OH)2D3 (Tsujikawa et al., 2003; Shimada et al., 2004; Sitara et al., 2004). Although vitamin D precursor molecules are steroid derived (Johnson et al., 2002), an enzyme called 1α-hydroxylase (CYP27B1) is essential for the final synthesis of active vitamin D (Dardenne et al., 2001). In fact, targeted inactivation of 1α-hydroxylase in mouse models (1α-hydroxylase−/−) results in undetectable serum levels of 1,25(OH)2D3 (Dardenne et al., 2001). Therefore, it is reasonable to suggest that elevated serum levels of 1,25(OH)2D3 in Fgf-23−/− or Klotho−/− mice might be caused by the ability of these molecules to regulate expression of the 1α-hydroxylase gene. The correlation between elevated levels of active vitamin D and the premature aging–like phenotype in Fgf-23−/− and Klotho−/− mice makes it tempting to hypothesize that excessive active vitamin D might have cytotoxic effects on various tissues, resulting in the observed atrophy. Studies in prostate and breast cancer cells have shown that exposure of cells to high levels of active vitamin D can have apoptotic effects (Narvaez et al., 2001; Johnson et al., 2002, 2006). Excessive activation of the vitamin D receptor causes transcription of genes associated with mitochondrial export of cytochrome c and subsequent cleavage of caspase-9. This action induces cleavage of caspase-3, which promotes DNA fragmentation causing apoptosis (Demay, 2006).
In this study, we show that FGF-23–Klotho stimulates mitogenic and cell survival pathways to prevent atrophy of tissues caused by excessive production of active vitamin D. High levels of 1,25(OH)2D3 are suppressed by FGF-23–Klotho signaling through inhibited expression of renal 1α-hydroxylase, an enzyme responsible for synthesis of active vitamin D. Furthermore, we show that vitamin D–induced caspase activity is inhibited by signaling pathways initiated by FGF-23–Klotho, thus directly preventing apoptosis.
Results and discussion
FGF-23–Klotho signal transduction
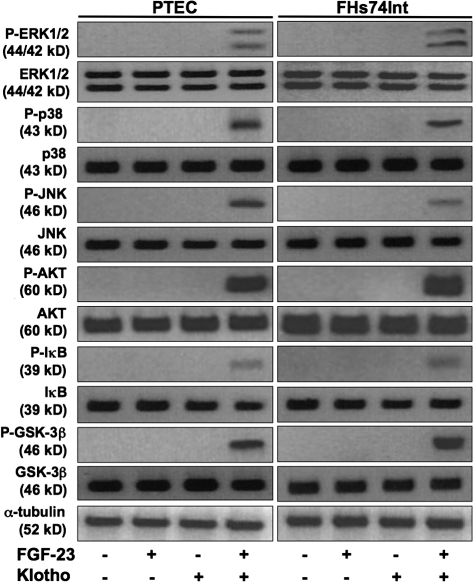
To investigate the role of FGF-23 and Klotho in regulation of systemic vitamin D and tissue atrophy, we first performed protein analyses by immunoblotting. We found that exposure of renal PTEC or small intestine epithelial cells (FHs74Int) to exogenous FGF-23 or Klotho alone had minimal effects on protein phosphorylation of ERK1/2, p38, JNK, AKT, inhibitor κB (IκB), and GSK-3β (glycogen synthase kinase-3β) compared with control (vehicle treated) cells. However, exposure to both FGF-23 and Klotho caused significant up-regulations in phosphorylation of all of these proteins (Fig. 1). These data were confirmed by multiplex ELISA, which also showed increases in CREB (cAMP response element binding), p70S6K, and STAT3 (signal transducer and activator of transcription 3) phosphorylation by FGF-23–Klotho. Addition of a small molecule Ras inhibitor before FGF-23 and Klotho treatment prevented increased phosphorylation of CREB, ERK1/2, JNK, p38, p70S6K, and STAT3. Addition of a small molecule inhibitor against phosphoinositide-3 kinase (PI3K) prevented increased phosphorylation of IκB, p70S6K, AKT, and GSK-3β. Combined effects of both Ras and PI3K inhibitors lowered all FGF-23–Klotho-induced phosphorylations to background levels (Fig. S1, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200803024/DC1).
Figure 1.
Signaling events induced by FGF-23–Klotho. Immunoblotting showing that FGF-23 or Klotho alone have no effect on kinase activity in PTEC or FHs74Int cells. Combined effects of FGF-23 and Klotho show increased phosphorylation of ERK1/2, p38, JNK, AKT, IκB, and GSK-3β. α-Tubulin was used as a loading control.
No evidence of endogenous Klotho was observed in either PTEC or FHs74Int cells by immunoblotting (Fig. S1 C). Because Klotho has previously been described to act as a glucuronidase (Tohyama et al., 2004), we sought to establish if this activity has a role in controlling FGF-23 function. We substituted Klotho with β-glucuronidase to assess potential effects on FGF-23–induced signaling. Immunoblotting showed that β-glucuronidase had no effects on phosphorylation of ERK1/2 or AKT when combined with FGF-23 (unpublished data), confirming the unique role of Klotho in FGF-23 signaling.
FGF-23–Klotho signaling stimulates proliferation
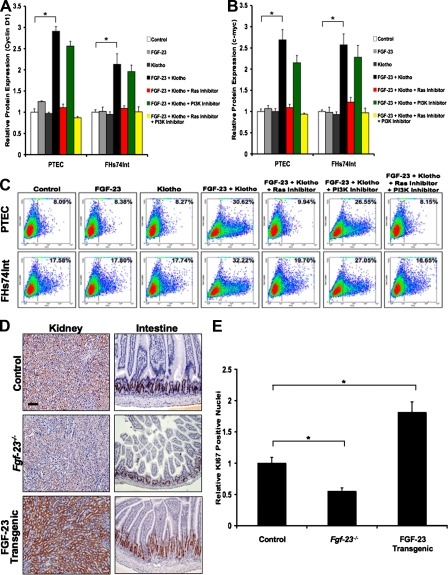
Because the observed phosphorylation patterns are known to be associated with proliferation signaling pathways (Bennasroune et al., 2004), we performed ELISA to detect potential effects of FGF-23–Klotho on expression of the cell cycle proteins Cyclin D1 and c-myc. Consistent with the phosphorylation patterning, we found that levels of Cyclin D1 and c-myc were significantly increased with the addition of FGF-23 and Klotho to cell cultures compared with control cells. As expected, FGF-23 or Klotho alone had no significant effect on Cyclin D1 or c-myc expression. Ras inhibitor was sufficient to abolish most of the increased expression of these proteins under the influence of FGF-23–Klotho, whereas PI3K inhibitor had only a minimal effect (Fig. 2, A and B).
Figure 2.
FGF-23–Klotho signaling promotes cell proliferation. (A and B) ELISA analysis showing increased expression of the cell cycle proteins Cyclin D1 (A) and c-myc (B) upon exposure of cells to FGF-23 and Klotho. FGF-23 or Klotho alone had little effect on expression of these proteins. Addition of a small molecule Ras inhibitor nearly abolished up-regulation of these proteins by FGF-23–Klotho, whereas PI3K inhibitor had minimal effect. Graphs represent mean ± SD (n = 3). *, P < 0.001. (C) Flow cytometry analysis for BrdU incorporation under these conditions showing that cell proliferation correlated with the observed expression patterns for Cyclin D1 and c-myc. (D and E) Ki67 immunostaining showing that expression was lower in Fgf-23−/− mice and higher in FGF-23 transgenic mice. Intestinal tissue showed intense nuclear Ki67 staining (E), whereas the kidney tissue only stained in the cytoplasm. Bar, 100 μm. Graphs represent mean ± SD (n = 3). *, P < 0.05.
To observe potential alterations in cell proliferation mediated by FGF-23–Klotho, we stained PTEC and FHs74Int cells in suspension for BrdU incorporation. No significant changes in proliferation were observed upon treatment of cells with FGF-23 or Klotho alone compared with control cells. However, exposure to both FGF-23 and Klotho greatly increased levels of BrdU incorporation. Introduction of Ras inhibitor to cultures nearly abolished increases in proliferation caused by FGF-23–Klotho, whereas PI3K inhibitor had only a minimal effect. Addition of both inhibitors completely prevented this increased proliferation (Fig. 2 C).
Fgf-23−/− and FGF-23 transgenic mice were generated as previously described (Larsson et al., 2004; Sitara et al., 2004). Kidneys and intestines from these mice at 6 wk of age were sectioned and stained for Ki67 expression to measure proliferation in vivo. Tissues from wild-type mice at 6 wk of age were used as a comparative control. In the intestine, we found that the number of Ki67-positive nuclei was significantly reduced in Fgf-23−/− mice, whereas in FGF-23 transgenic mice it was greatly increased compared with the wild type (Fig. 2, D and E). Similar results were observed in skin and spleen tissues (unpublished data). Interestingly, in the kidney, there were dramatic changes in levels of cytoplasmic Ki67 but no positive staining for nuclear Ki67. We also attempted BrdU incorporation, but there was no positive staining in the nuclei from kidney sections (unpublished data). These data suggest that the role of FGF-23–Klotho is not to control proliferation in the kidney in vivo but rather to regulate mineral-ion homeostasis as previously described (Yu and White, 2005).
FGF-23–Klotho signaling prevents vitamin D–induced apoptosis
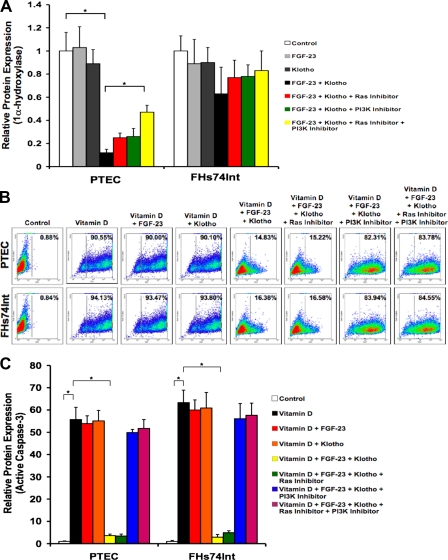
Because active vitamin D has recently been described to have anti-cancer effects (Johnson et al., 2002, 2006) and serum levels of 1,25(OH)2D3 are excessively high in Fgf-23−/− or Klotho−/− mice (Tsujikawa et al., 2003; Shimada et al., 2004; Sitara et al., 2004), we hypothesized that these elevated levels might promote apoptosis, thus causing the observed atrophy of tissues. We first showed the direct effect of FGF-23–Klotho on expression of 1α-hydroxylase, the enzyme responsible for final synthesis of active vitamin D metabolite. Protein levels of 1α-hydroxylase were observed by ELISA, showing that FGF-23 and Klotho together were able to greatly reduce expression of this enzyme in PTEC cells compared with the control, whereas FGF-23 or Klotho alone had little effect. Addition of Ras and PI3K inhibitors showed significant rescue of 1α-hydroxylase expression, however full restoration was not achieved. These data suggest that other signaling pathways may also be involved in regulation of 1α-hydroxylase expression. Interestingly, no statistically significant changes in expression of 1α-hydroxylase were found with treatment of FHs74Int cells (Fig. 3 A). This is consistent with the fact that PTEC of the kidney are the primary site of 1α-hydroxylase activity for synthesis of active vitamin D (Dardenne et al., 2001) not small intestinal epithelium.
Figure 3.
FGF-23–Klotho prevents vitamin D–induced apoptosis. (A) ELISA analysis of 1α-hydroxylase expression showing no significant changes in PTEC cells exposed to FGF-23 or Klotho alone but greatly decreased levels when exposed to both FGF-23 and Klotho. Small molecule inhibitors against Ras and PI3K were sufficient to provide marginal rescue of this decrease in expression. No significant changes were found for treatment of FHs74Int cells. Graphs represent mean ± SD (n = 3). *, P < 0.05. (B) Flow cytometry analysis for TUNEL staining of cells exposed to exogenous vitamin D showing that it caused extremely high levels of apoptosis. Addition of FGF-23 and Klotho was sufficient to rescue most of the vitamin D–induced apoptosis, whereas FGF-23 or Klotho alone did not. PI3K inhibitor prevented this rescue, whereas Ras inhibitor had no effect. (C) ELISA for active caspase-3 levels, showing the same patterns as observed with the TUNEL analysis. Graphs represent mean ± SD (n = 3). *, P < 0.001.
To assess effects of 1,25(OH)2D3 on PTEC or FHs74Int cells, we established a dose-dependent curve of active vitamin D treatment with steadily increasing amounts added to cultures. Cells were stained in suspension for apoptosis by TUNEL and subjected to flow cytometry. We found that a dose of 100 nM of vitamin D had little effect on levels of apoptosis compared with vehicle-treated control cells. Although a 200-nM concentration showed significant but relatively low cell death, a 300-nM dose heavily increased levels of apoptosis. 400 nM of vitamin D caused cell death of nearly the entire populations of PTEC and FHs74Int cells (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200803024/DC1). In the presence of 400 nM of vitamin D, FGF-23 or Klotho alone showed no differences in levels of apoptosis. However, upon addition of both FGF-23 and Klotho, there was a substantial rescue of vitamin D–induced apoptosis. Ras inhibitor had no effect on this rescue, but PI3K inhibitor prevented the FGF-23–Klotho-induced rescue of apoptosis caused by excessive active vitamin D (Fig. 3 B). We performed ELISA for detection of levels of activated (or cleaved) caspase-3 (Fig. 3 C) with expression patterns nearly identical to those observed by TUNEL staining.
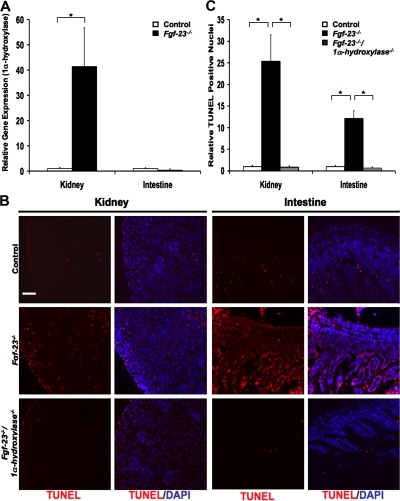
To directly observe whether kidneys of Fgf-23−/− mice express higher levels of 1α-hydroxylase, thus resulting in the previously described high levels of serum vitamin D, we performed real-time quantitative PCR using RNA isolated from these tissues compared with those of wild-type mice. As expected, we found levels of 1α-hydroxylase to be far higher in kidney tissue of knockout mice compared with that of wild-type mice. We also found that there was no significant change in 1α-hydroxylase mRNA in intestinal tissue (Fig. 4 A). Histology of kidney and intestinal tissues from Fgf-23−/− mice show high levels of apoptosis compared with those of wild-type mice, as observed by TUNEL staining. Most significantly, tissues from Fgf-23−/−/1α-hydroxylase−/− mice show a complete rescue of the apoptosis seen in Fgf-23−/− mice (Fig. 4, B and C). Similar results were found in other organs including skin, spleen, liver, lung, ovaries, and testes (unpublished data). These data suggest that systemically elevated active vitamin D is the cause of tissue atrophy observed in Fgf-23−/− mice.
Figure 4.
Genetic ablation of 1α-hydroxylase prevents apoptosis and atrophy in tissues of Fgf-23−/− mice. (A) Real-time quantitative PCR analysis showing that gene expression levels of 1α-hydroxylase from kidney tissues of Fgf-23−/− mice is much higher than those of wild-type (control) mice. Graphs represent mean ± SD (kidney, n = 6; intestine, n = 5 ). *, P < 0.01. (B and C) TUNEL staining of tissue sections from kidney and intestine of Fgf-23−/− mice showing far higher levels of apoptosis than those of wild-type mice. Fgf-23−/−/1α-hydroxylase−/− double knockout mice show a complete rescue of the apoptosis seen in tissues of Fgf-23−/− mice. Bar, 100 μm. Graphs represent mean ± SD (n = 3). *, P < 0.001.
This study provides a foundation for understanding the signal transduction mechanisms initiated by FGF-23 and Klotho, processes that until now were relatively unknown. We have also provided insight into the physiological functions of these proteins, which regulate cell survival, proliferation, and vitamin D metabolism. These discoveries go beyond the traditional dogma of FGF-23 and Klotho being responsible for maintaining mineral-ion homeostasis (Yu and White, 2005). It is now clear that two major signal transduction pathways are initiated by FGF-23–Klotho. Ras signaling promotes downstream kinase activities that cause up-regulation of cell cycle genes to induce proliferation, whereas PI3K signaling counter regulates apoptotic pathways through its downstream activation of AKT (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200803024/DC1).
Perhaps the most interesting aspect of our study is that although virtually all internal organs showed evidence of vitamin D–induced apoptosis in Fgf-23−/− mice, only select tissues (intestine, spleen, and skin) showed effects of FGF-23–Klotho on proliferation. Although kidneys of Fgf-23−/− mice showed no evident change in proliferation, in vitro studies of kidney PTEC demonstrated greatly increased proliferation as a result of exposure to exogenous FGF-23 and Klotho. These data suggest that there are distinct regulatory factors controlling in vivo proliferation for each organ that may render FGF-23–Klotho ineffective for this purpose. However, it is clear that FGF-23–Klotho does inhibit expression of 1α-hydroxylase in kidney cells (in vitro and in vivo) and subsequent production of active vitamin D. Although the concentrations of active vitamin D used for our in vitro studies may not be the same as those reached in vivo in Fgf-23−/− mice, evidence of apoptotic rescue by genetic knockout of 1α-hydroxylase suggests that active vitamin D is the cause of the observed tissue atrophy.
The potential use of FGF-23–Klotho as an antiaging therapy is tempting; however, there is great risk. Although excessive serum levels of active vitamin D are clearly responsible for several aging-like phenotypes, including tissue atrophy, moderate production of active vitamin D is still essential for normal bone mineralization (Christakos et al., 2006). Also, lack of systemic vitamin D has been linked to increased risk in development of various forms of cancer including colorectal, breast, lung, ovarian and prostate carcinomas, as well as non-Hodgkin's lymphoma. Furthermore, there is strong evidence that vitamin D deficiency increases the risk of developing other pathological conditions such as multiple sclerosis, type 1 and type 2 diabetes mellitus, rheumatoid arthritis, osteoarthritis, hypertension, and stroke (Grant, 2006; Peterlik and Cross, 2006). Pharmacological doses of vitamin D analogues have been used as potential therapies against these conditions (Hayes, 2000; Holick, 2006; Deeb et al., 2007). The ability of FGF-23–Klotho signaling to inhibit synthesis of active vitamin D through inhibitory control of 1α-hydroxylase gene expression, as well as to induce signaling pathways that counter-regulate vitamin D–induced apoptotic signaling, could potentially slow the aging process. However, it would also likely increase the risk of developing diseases associated with vitamin D deficiency. Therefore, it is evident that a balance of moderate levels of systemic active vitamin D is essential for maintaining overall health and longevity.
Materials and methods
Cell culture
Human renal PTEC were obtained from Clontech Laboratories, Inc. FHs74Int human small intestine epithelial cells were acquired from the American Type Culture Collection. PTEC cells were grown in culture using REBM (Clontech Laboratories, Inc.) + 10% FBS + 1% penicillin/streptomycin. FHs74Int were grown in HybriCare medium (American Type Culture Collection) + 10% FBS + 1% penicillin/streptomycin. FBS was removed 24 h before all experimental conditions. Recombinant FGF-23 and Klotho proteins (generated as previously described [Kurosu et al., 2006; Yu et al., 2005]) were added to the culture medium for all relative experiments at concentrations of 10 ng/ml and 200 pM, respectively. Cells were treated for 15 min to assess kinase activity or for 12 h to measure proliferation. Vitamin D (Sigma-Aldrich) was used at a cytotoxic concentration of 400 nM for 24 h. Small molecule Ras (FTI-276; provided by M. Azam, Harvard Medical School, Boston, MA) and PI3K (LY294002; Cell Signaling Technology) inhibitors were added 1 h before experiments at a concentration of 1 μM. β-glucuronidase (Sigma-Aldrich) was used at a concentration of 200 ng/ml. 500 ng of the pcDNA3.1/V5-His-TOPO-Klotho (provided by D.E. Arking, Johns Hopkins University, Baltimore, MD) plasmid was transfected into cells using Lipofectamine and Plus reagents (Invitrogen) according to the manufacturer's guidelines. All experiments for this study were performed in triplicate.
Immunoblotting
Western blotting was performed using the following antibodies at concentrations (and using protocols) recommended by the respective manufacturers: P-ERK1/2, ERK1/2, P-p38, p38, P-JNK, JNK, P-AKT, AKT, P-GSK-3β, GSK-3β, and IκB (Cell Signaling Technology); P-IκB (Santa Cruz Biotechnology, Inc.); Klotho (provided by M. Kuro-o); and α-tubulin (EMD). HRP-conjugated secondary antibodies (EMD) were used at a dilution of 1:5,000. Adjustments of total image size, brightness, and contrast were made using Photoshop CS (Adobe).
Multiplex ELISA
Signal transduction was assessed using the Beadlyte 8-plex Multi-Pathway Signaling kit (Millipore). Active caspase-3 beadmates (Upstate Biotechnology) were used to assess apoptosis. P-AKT, P-GSK-3β (Cell Signaling Technology), Cyclin D1, c-myc, and CYP27B1 (Santa Cruz Biotechnology, Inc.) antibodies were conjugated to various Bio-Plex carboxylated beads (with unique optical codes) using the Bio-Plex Amine Coupling kit (Bio-Rad Laboratories). α-Tubulin antibody (EMD) was also conjugated to Bio-Plex carboxylated beads to be used as an internal control. Samples were run on a Luminex 200 multiplex testing system using the Universal Cell Signaling Assay kit and corresponding protocol (Millipore). Experimental values were divided by the α-tubulin control values to provide normalized data.
Flow cytometry
PTEC and FHs74Int cells were stained in suspension for BrdU or TUNEL incorporation with the 5-Bromo-2′-deoxy-uridine Labeling and Detection kit I and In Situ Cell Death Detection kit, Fluorescein (Roche), respectively, using the protocols provided by the manufacturer (Roche). Flow cytometry was performed at the Harvard Medical School Department of Pathology flow cytometry core facility using a FACSDCalibur (BD Biosciences) cell sorter isolating 30,000 cells per sample.
Real-time quantitative PCR
Quantitative RT-PCR was performed to quantify the relative expression of 1α-hydroxylase mRNA in the kidney and intestine of 6-wk-old wild-type or Fgf-23−/− mice. Tissues were snap frozen in liquid nitrogen and total RNA was isolated by phenol chloroform extraction using Trizol Reagent. Subsequently, 1 μg of RNA was reverse transcribed using the QuantiTectR Reverse Transcription kit (Qiagen). RT-PCR was performed using a real-time PCR system (ABI 7300; Applied Biosystems) with the following conditions: initial denaturing at 95°C for 2 min, followed by 45 three-step cycles of denaturing at 95°C for 10 s, annealing at 55°C for 20 s, and extension at 68°C for 1 min. The following primers were used: 1a-hydroxylase forward, 5′-CAGATGTTTGCCTTTGCCC-3′ and reverse, 5′-TGGTTCCTCATCGCAGCTTC-3′; and m36B4 forward, 5′-AGATGCAGCAGATCCGCAT-3′ and reverse, 5′-GTTCTTGCCCATCAGCACC-3′. All samples were run in triplicate using SYBR green (Eppendorf) and compared with levels of endogenous m36B4 as an internal control.
Mice
Fgf-23−/−, 1α-hydroxylase−/−, and FGF-23 transgenic mice were generated as previously described (Dardenne et al., 2001; Larsson et al., 2004; Sitara et al., 2004). Fgf-23−/−/1α-hydroxylase−/− mice were created by crossbreeding FGF-23−/− with 1α-hydroxylase−/− mice (Razzaque et al., 2006). Standard PCR genotyping was performed using the following primers: Fgf-23, forward, 5′-GGATCCCCACCTCAGTTCTCA-3′ and reverse, 5′-TAGCCGTGTACAGGTGGGTCA-3′; 1α-hydroxylase forward, 5′-GCACCTGGCTCAGGTAGCTCTTC-3′ and reverse, 5′-GTCCCAGACAGAGACATCCGT-3′; and FGF-23 Transgenic forward, 5′-GGCAACATTTTTGGATCA-3′ and reverse, 5′-CCGGGGCTTCAGCACGTT-3′.
Immunohistochemistry
Immunohistochemistry was performed using Ki67 antibody (Dako) according to the manufacturer's guidelines with a 1:100 dilution. Nova Red (Vector Laboratories) POD substrate was used for Ki67 staining. TUNEL staining was conducted using the In Situ Cell Death Detection kit, Fluorescein and protocol. All sections (provided by T. Taguchi, Nagasaki University, Nagasaki, Japan) for Ki67 were counterstained with hematoxylin, whereas sections for TUNEL were counterstained with DAPI in fluorescent mounting medium (Vector Laboratories). Data were quantified by counting the number of Ki67- or TUNEL-positive nuclei per mm2. Images were acquired using a fluorescence microscope (80i; Nikon) at 25°C with 10 or 20× magnifications. Fluorescein filters were used to detect TUNEL. Images were captured using a charge-coupled device camera (Orca 100; Hamamatsu Photonics) and MetaMorph software (MDS Analytical Technologies). Adjustments of total image size, brightness, and contrast were made using Photoshop CS.
Statistics
One-way analysis of variance was performed and confirmed with two-tailed paired Student's t test using Prism 4 software (GraphPad Software, Inc.). P-values <0.05 were considered significant.
Online supplemental material
Fig. S1 shows phosphorylation targets of FGF-23–Klotho signaling by ELISA and control immunoblotting for Klotho expression. Fig. S2 shows dose-dependent effects of active vitamin D on apoptosis (TUNEL) of PTEC or FHs74Int cells by flow cytometry. Fig. S3 is a schematic diagram of FGF-23–Klotho signal transduction. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200803024/DC1.
Supplementary Material
[Supplemental Material Index]
Acknowledgments
We thank T. Taguchi for providing tissue sections, M. Azam for providing the Ras inhibitor and P. Wisniewski for conducting flow cytometry.
This work was supported by grants R01-073944 (to B. Lanske), R01-077276 (to M.S. Razzaque), and P01-AR048564 (to B.R. Olsen) from the National Institutes of Health.
Abbreviations used in this paper: ERK, extracellular signal–regulated kinase; IκB, inhibitor κB; PI3K, phosphoinositide-3 kinase; PTEC, proximal tubule epithelial cells.
References
- Bennasroune, A., A. Gardin, D. Aunis, G. Cremel, and P. Hubert. 2004. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit. Rev. Oncol. Hematol. 50:23–28. [DOI] [PubMed] [Google Scholar]
- Christakos, S., P. Dhawan, Q. Shen, X. Peng, B. Benn, and Y. Zhong. 2006. New insights into the mechanisms involved in the pleiotropic action of 1,25dihydroxyvitamin D3. Ann. N. Y. Acad. Sci. 1068:194–203. [DOI] [PubMed] [Google Scholar]
- Dardenne, O., J. Prudhomme, A. Arabian, F.H. Glorieux, and R. St-Arunaud. 2001. Targeted inactivation of the 25-hydroxyvitamin D3-1α-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 142:3135–3141. [DOI] [PubMed] [Google Scholar]
- Deeb, K.K., D.L. Trump, and C.S. Johnson. 2007. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nat. Rev. Cancer. 7:684–700. [DOI] [PubMed] [Google Scholar]
- Demay, M.B. 2006. Mechanism of vitamin D receptor action. Ann. N. Y. Acad. Sci. 1068:204–213. [DOI] [PubMed] [Google Scholar]
- Grant, W.B. 2006. Epidemiology of disease risks in relation to Vitamin D insufficiency. Prog. Biophys. Mol. Biol. 92:65–79. [DOI] [PubMed] [Google Scholar]
- Hayes, C.E. 2000. Vitamin D: a natural inhibitor of multiple sclerosis. Proc. Nutr. Soc. 59:531–535. [DOI] [PubMed] [Google Scholar]
- Holick, M.F. 2006. Vitamin D: its role in cancer prevention and treatment. Prog. Biophys. Mol. Biol. 92:49–59. [DOI] [PubMed] [Google Scholar]
- Johnson, C.S., P.A. Hershberger, and D.L. Trump. 2002. Vitamin D-related therapies in prostate cancer. Cancer Metastasis Rev. 21:147–158. [DOI] [PubMed] [Google Scholar]
- Johnson, C.S., J.R. Muindi, P.A. Hershberger, and D.L. Trump. 2006. The antitumor efficacy of calcitriol: preclinical studies. Anticancer Res. 26:2543–2549. [PubMed] [Google Scholar]
- Kuro-o, M., Y. Matsumura, H. Aizawa, H. Kawaguchi, T. Suga, T. Utsugi, Y. Ohyama, M. Kurabayashi, T. Kaname, E. Kume, et al. 1997. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 390:45–51. [DOI] [PubMed] [Google Scholar]
- Kurosu, H., Y. Ogawa, M. Miyoshi, M. Yamamoto, A. Nandi, K.P. Rosenblatt, M.G. Baum, S. Schiavi, M. Hu, O.W. Moe, and M. Kuro-o. 2006. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281:6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, T., R. Marsell, E. Schipani, C. Ohlsson, O. Ljunggren, H.S. Tenenhouse, H. Juppner, and K.B. Jonsson. 2004. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1 (I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 145:3087–3094. [DOI] [PubMed] [Google Scholar]
- Liu, S., W. Tang, J. Zhou, J.R. Stubbs, Q. Luo, M. Pi, and L.D. Quarles. 2006. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17:1305–1315. [DOI] [PubMed] [Google Scholar]
- Narvaez, C.J., G. Zinser, and J. Welsh. 2001. Functions of 1α,25-dihydroxyvitamin D3 in mammary gland: from normal development to breast cancer. Steroids. 66:301–308. [DOI] [PubMed] [Google Scholar]
- Peterlik, M., and H.S. Cross. 2006. Dysfunction of the vitamin D endocrine system as common cause for multiple malignant and other chronic diseases. Anticancer Res. 26:2581–2588. [PubMed] [Google Scholar]
- Razzaque, M.S., and B. Lanske. 2006. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 12:298–305. [DOI] [PubMed] [Google Scholar]
- Razzaque, M.S., R. St-Arnaud, T. Taguchi, and B. Lanske. 2005. FGF-23, vitamin D and calcification: the unholy triad. Nephrol. Dial. Transplant. 20:2032–2035. [DOI] [PubMed] [Google Scholar]
- Razzaque, M.S., D. Sitara, T. Taguchi, R. St-Arnaud, and B. Lanske. 2006. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D mediated process. FASEB J. 20:720–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, T., M. Kakitani, Y. Yamazaki, H. Hasegawa, Y. Takeuchi, T. Fujita, S. Fukumoto, K. Tomizuka, and T. Yamashita. 2004. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara, D., M.S. Razzaque, M. Hesse, S. Yoganathan, T. Taguchi, R.G. Erben, H. Juppner, and B. Lanske. 2004. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama, O., A. Imura, A. Iwano, J.N. Freund, B. Henrissat, T. Fujimori, and Y. Nabeshima. 2004. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J. Biol. Chem. 279:9777–9784. [DOI] [PubMed] [Google Scholar]
- Torres, P.U., D. Prie, V. Molina-Bletry, L. Beck, C. Silve, and G. Friedlander. 2007. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int. 71:730–737. [DOI] [PubMed] [Google Scholar]
- Tsujikawa, H., Y. Kurotaki, T. Fujimori, K. Fukuda, and Y. Nabeshima. 2003. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17:2393–2403. [DOI] [PubMed] [Google Scholar]
- Urakawa, I., Y. Yamazaki, T. Shimada, K. Iijima, H. Hasegawa, K. Okawa, T. Fujita, S. Fukumoto, and T. Yamashita. 2006. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 444:770–774.17086194 [Google Scholar]
- Yamashita, T. 2005. Structural and biochemical properties of fibroblast growth factor 23. Ther. Apher. Dial. 9:313–318. [DOI] [PubMed] [Google Scholar]
- Yu, X., and K.E. White. 2005. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 16:221–232. [DOI] [PubMed] [Google Scholar]
- Yu, X., O.A. Ibrahimi, R. Goetz, F. Zhang, S.I. Davis, H.J. Garringer, R.J. Linhardt, D.M. Ornitz, M. Mohammadi, and K.E. White. 2005. Analysis of the biochemical mechanism for the endocrine actions of the fibroblast growth factor-23. Endocrinology. 146:4647–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]