Identification of a vesicular aspartate transporter (original) (raw)
Abstract
Aspartate is an excitatory amino acid that is costored with glutamate in synaptic vesicles of hippocampal neurons and synaptic-like microvesicles (SLMVs) of pinealocytes and is exocytosed and stimulates neighboring cells by binding to specific cell receptors. Although evidence increasingly supports the occurrence of aspartergic neurotransmission, this process is still debated because the mechanism for the vesicular storage of aspartate is unknown. Here, we show that sialin, a lysosomal H+/sialic acid cotransporter, is present in hippocampal synaptic vesicles and pineal SLMVs. RNA interference of sialin expression decreased exocytosis of aspartate and glutamate in pinealocytes. Proteoliposomes containing purified sialin actively accumulated aspartate and glutamate to a similar extent when inside positive membrane potential is imposed as the driving force. Sialin carrying a mutation found in people suffering from Salla disease (R39C) was completely devoid of aspartate and glutamate transport activity, although it retained appreciable H+/sialic acid cotransport activity. These results strongly suggest that sialin possesses dual physiological functions and acts as a vesicular aspartate/glutamate transporter. It is possible that people with Salla disease lose aspartergic (and also the associated glutamatergic) neurotransmission, and this could provide an explanation for why Salla disease causes severe neurological defects.
Keywords: Salla disease, sialin, vesicular excitatory amino acid transporter, vesicular glutamate transporter
A classical neurotransmitter is defined as a substrate that is stored in synaptic vesicles, secreted through exocytosis, and then stimulates the target cells upon binding to specific receptors. Among excitatory and inhibitory amino acids, glutamate, GABA, and glycine fulfill these criteria and are well established as neurotransmitters (1–4). Although aspartate has been known as a signaling molecule for a long time, this amino acid does not fulfill the above criteria (5–16). Aspartate is usually costored with glutamate in a population of synaptic vesicles in specific neurons such as the CA1 neuron of hippocampus and synaptic-like microvesicles (SLMVs) of pinealocytes (10, 12, 13). However, the transporter responsible for the vesicular accumulation of aspartate has not yet been identified. The vesicular glutamate transporter (VGLUT) is responsible for vesicular storage of glutamate but not aspartate because VGLUT does not recognize aspartate as a transport substrate (4). We hypothesized that, like other vesicular neurotransmitter transporters, the putative vesicular aspartate transporter is present in aspartate-containing secretory vesicles and takes up aspartate by using an electrochemical gradient of protons as the driving force.
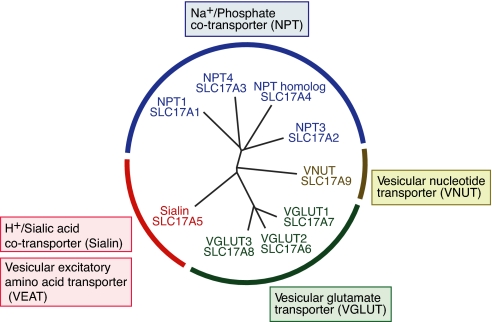
SLC17 is a type I phosphate transporter family consisting of nine genes that is divided into four distinct subfamilies: SLC17A1–4, Na+, and inorganic phosphate cotransporters (NPT); SLC17A5, a lysosomal H+/sialic acid cotransporter (sialin); SLC17A6–8, _VGLUT_s; and SLC17A9, a vesicular nucleotide transporter (VNUT) (Fig. 1) (4, 17). VGLUT and NPT1 are known to be bifunctional in nature and possess two distinct intramolecular transport machineries, i.e., Na+ and inorganic phosphate cotransport and membrane potential (Δψ)-dependent organic anion transport (18–20). Furthermore, biochemical studies on VGLUT and VNUT suggest involvement of a family-wide conserved transmembrane region as the substrate-binding site. Small spatial changes near the binding region may determine substrate specificity (17, 18). We suspected that a member of a SLC17 subgroup other than VGLUT was involved in the vesicular storage of aspartate. Sialin was the most appropriate candidate because this protein is highly expressed in nonlysosomal compartments of cells found in various regions of the central nervous system, some of which correspond to the sites of vesicular storage of aspartate (21, 22).
Fig. 1.
Phylogenetic tree of the SLC17 anion transporter family.
Here, we investigated this possibility, and we present evidence that sialin is responsible for vesicular storage for aspartate in neurons and pinealocytes. We also present an unexpected linkage between aspartergic neurotransmission and Salla disease, an autonomic neurological disorder.
Results and Discussion
Sialin Is a Constituent of Hippocampal Synaptic Vesicles.
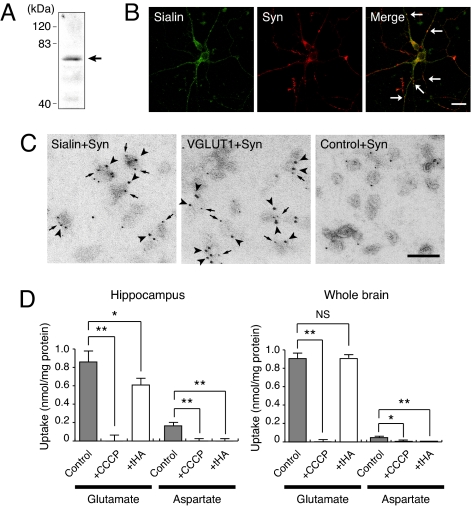
To test the working hypothesis that sialin acts as vesicular aspartate transporter, we first investigated whether sialin-containing synaptic vesicles were present in the hippocampus, and if yes, whether these synaptic vesicles can actively accumulate aspartate. We found that crude synaptic vesicles (P2 fraction) from hippocampus contain sialin (Fig. 2A). Immunohistochemistry with cultured hippocampal neurons indicated that sialin was approximately colocalized with synaptophysin, a marker of synaptic vesicles, in the processes (Fig. 2B, arrows). Immunoelectron microscopy indicated that a population of synaptophysin-containing vesicles possess sialin immunoreactivity (Fig. 2C). Quantitatively, 90% sialin–ImmunoGold particles were associated with 25% synaptophysin-containing vesicles. Upon the addition of ATP, synaptic vesicles from hippocampus accumulated aspartate in carbonyl cyanide 3-chlorophenylhydrazone (CCCP)-sensitive manner, whereas synaptic vesicles from whole brain did not (Fig. 2D). The ATP-dependent aspartate uptake in hippocampal synaptic vesicles was sensitive to d,l-_threo_-β-hydroxyaspartate (tHA), a false transmitter for vesicular aspartate pool (23). ATP-dependent glutamate uptake by hippocampal synaptic vesicles was also somewhat sensitive to tHA, but that of whole-brain synaptic vesicles was not (Fig. 2D). These results suggested that sialin is a constituent of hippocampal synaptic vesicles and that tHA-sensitive ATP-dependent aspartate/glutamate transporters other than VGLUT were present in synaptic vesicles of hippocampus.
Fig. 2.
ATP-dependent uptake of aspartate was detected in sialin-containing synaptic vesicle preparation from hippocampus. (A) Immunoblotting revealed the presence of sialin in the hippocampal P2 fraction. The position of sialin is marked by an arrow. (B) Sialin is partially colocalized with synaptophysin. Cultured hippocampal neurons were double immunostained with antibodies against sialin and synaptophysin. (Scale bar, 10 μm.) (C) Double-labeling immunoelectron microscopy demonstrated the association of sialin with synaptophysin-containing vesicles. Samples were treated with sets of anti-synaptophysin monoclonal antibodies (5-nm particles, arrows) and anti-sialin serum (10-nm particles, arrowheads), anti-synaptophysin monoclonal antibodies (5-nm particles, arrows) and anti-VGLUT1 serum (10-nm particles, arrowheads), or anti-synaptophysin monoclonal antibodies (5-nm particles) and control serum (10-nm particles). (Scale bar, 100 nm.) (D) ATP-dependent uptake of aspartate and glutamate at 5 min by P2 fractions isolated from hippocampus and whole brain. Concentrations of CCCP and tHA were 1 μM and 5 mM, respectively.
Sialin Is a Constituent of SLMVs.
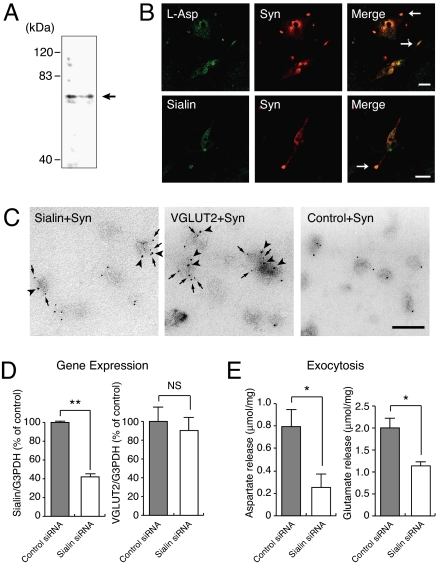
Subsequently, we investigated the expression and localization of sialin in rat pinealocytes because these cells are known to store both aspartate and glutamate in SLMVs and to secrete both amino acids through exocytosis upon depolarization (12). Sialin immunoreactivity was present within the punctated structure and approximately colocalized with synaptophysin, a marker of SLMVs, in cultured rat pinealocytes (Fig. 3 A and B). Aspartate immunoreactivity also colocalized with synaptophysin (Fig. 3B). Immunoelectron microscopy indicated that a major population of synaptophysin-containing vesicles possess sialin immunoreactivity (Fig. 3C): ≈90% total ImmunoGold particles were associated with 50% SLMVs. RNA interference (RNAi) using a probe directed against rat sialin in pinealocytes indicated selective loss of sialin expression, whereas VGLUT expression was not altered (Fig. 3D). Then, we measured the exocytosis of aspartate and glutamate from pinealocytes upon addition of KCl. We observed that RNAi treatment decreased exocytosis of aspartate and glutamate to 32% and 60% of the control level, respectively (Fig. 3E). Because sialin siRNA was incorporated into 70% pinealocytes under the condition, these results suggested that siRNA-incorporated pinealocytes have almost completely lost the ability of aspartate exocytosis. In a separate experiment, the siRNA-evoked suppression of aspartate exocytosis was partially recovered (50%) upon transduction of human sialin construct. Together, these results support the idea that sialin was a constituent of pineal SLMVs and was responsible for the vesicular storage of aspartate and glutamate.
Fig. 3.
Sialin was associated with pineal SLMVs and is involved in exocytosis of aspartate and glutamate. (A) Sialin was present in pineal SLMV fractions as revealed by immunoblotting. The position of sialin is marked by an arrow. (B) Sialin is colocalized with aspartate and synaptophysin. Cultured pinealocytes were double immunostained with antibodies against aspartate and synaptophysin (Upper) or sialin and synaptophysin (Lower). (Scale bar, 10 μm.) (C) Double-labeling immunoelectron microscopy demonstrated the association of sialin with synaptophysin. Samples were treated with sets of anti-synaptophysin monoclonal antibodies (5-nm particles, arrows) and anti-sialin serum (10-nm particles, arrowheads) or anti-synaptophysin monoclonal antibodies (5-nm particles, arrows) and anti-VGLUT2 serum (10-nm particles, arrowheads) or anti-synaptophysin monoclonal antibodies (5-nm particles) and control serum (10-nm particles). (Scale bar, 100 nm.) (D) RNAi directed against rat sialin decreased sialin expression without affecting the expression of VGLUT2. Quantitative analysis for sialin and VGLUT2 mRNA levels was performed by real-time PCR. (E) Exocytosis of aspartate and glutamate after 20-min incubation upon KCl stimulation from control siRNA-treated pinealocytes and sialin siRNA-treated pinealocytes.
Sialin-Mediated Vesicular Storage of Aspartate and Glutamate.
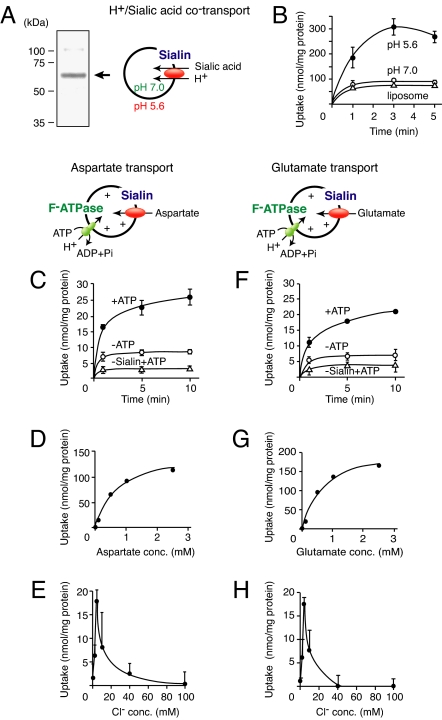
To demonstrate that sialin was a vesicular aspartate and glutamate transporter, mouse sialin was expressed in insect cells, purified, and reconstituted into liposomes (Fig. 4A). The purified sialin fraction contained a single major polypeptide with an apparent molecular mass of ≈60 kDa. We detected ΔpH-dependent sialic acid transport activity in proteoliposomes (Fig. 4 B and Table 1). The specific activity and the transport properties were comparable with those of purified lysosomal H+/sialic acid cotransporter reported (Fig. 4B and Table 1) (24). These results confirmed that the reconstituted sialin was a H+/sialic acid cotransporter. We then measured Δψ -dependent aspartate uptake in the same proteoliposome preparations (Fig. 4 C–E). Upon the addition of ATP, the proteolipsomes established Δψ (positive inside) through the active proton pumping of the bacterial F-ATPase (18). Aspartate was taken up against a concentration gradient in a time-dependent fashion (Fig. 4C). Only background level uptake was observed in liposomes lacking sialin. The ATP-dependent aspartate uptake exhibited dose dependence with _K_m and _V_max of 0.62 mM and 142 nmol/min per mg of protein, respectively (Fig. 4D). Aspartate transport was driven by Δψ (inside positive) but not ΔpH (Table 1).
Fig. 4.
Sialin is a vesicular aspartate/glutamate transporter. (A) Purified mouse sialin (10 μg of protein) was visualized by Coomassie brilliant blue staining after SDS/PAGE. The position of sialin is indicated by an arrow. (B) H+/sialic acid cotransport by the proteoliposomes was assayed by the pH jump method (acidic outside). (C–E) Sialin is a vesicular aspartate transporter. (C) Time course of ATP-dependent aspartate uptake by proteoliposomes containing reconstituted mouse sialin and bacterial F-ATPase. The reaction was started by adding radiolabeled aspartate to a final concentration of 100 μM in the presence (filled circles) or absence (open circles) of 2 mM ATP. (D) Dose dependence of aspartate uptake at 1 min. (E) Chloride ion dependence of aspartate uptake at 5 min was measured in buffer containing KCl at the indicated concentrations. (F–H) Sialin is a VGLUT. Radiolabeled glutamate was used instead of radiolabeled aspartate. (F) Time course. (G) Dose dependence. (H) Chloride ion dependence.
Table 1.
Uptake of aspartate, glutamate, or sialic acid by the sialin-containing proteoliposomes in the presence of various compounds
| Compound | Concentration, μM | Uptake, % of control | ||
|---|---|---|---|---|
| l-Aspartate | l-Glutamate | Sialic acid | ||
| Control | 100.0 ± 9.2 | 100.0 ± 9.3 | 100.0 ± 8.4 | |
| Valinomycin | 2 | 23.9 ± 5.5** | 16.5 ± 8.9** | 101.0 ± 8.7 |
| Nigericin | 2 | 77.8 ± 7.7* | 80.2 ± 16.8 | 17.3 ± 9.4** |
| Val + Nig | 2 | 0.0 ± 8.9** | 0.0 ± 9.0** | 13.0 ± 5.6** |
| CCCP | 1 | 6.2 ± 7.7** | 24.8 ± 4.1** | 15.6 ± 5.1** |
| Evans blue | 1 | 20.7 ± 3.7** | 14.6 ± 6.9** | 114.4 ± 3.3 |
| l-aspartate | 5,000 | 0.0 ±1.7** | 8.7 ± 10.0** | 109.9 ± 1.1 |
| d-Aspartate | 5,000 | 3.2 ± 3.3** | 6.9 ± 6.2** | 92.1 ± 6.6 |
| tHA | 5,000 | 0.0 ± 7.3** | 0.0 ± 0.8** | 84.2 ± 11.6 |
| l-glutamate | 5,000 | 1.3 ± 2.0** | 0.0 ± 4.5** | 98.0 ± 12.0 |
| Sialic acid | 5,000 | 57.5 ± 11.0* | 51.2 ± 10.8* | 9.8 ± 8.7** |
| l-lactate | 5,000 | 95.9 ± 10.9 | 97.0 ± 2.7 | 11.5 ± 8.0** |
| Succinate | 5,000 | 93.0 ± 3.0 | 84.7 ± 9.9 | 20.7 ± 8.4** |
Testing of a spectrum of possible _cis_-inhibitors indicated that aspartate uptake was insensitive to lactate, succinate, and sialic acid but sensitive to aspartate and tHA as opposed to that of H+/sialic acid cotransport (Table 1). Similar to that in VGLUT and VNUT (17, 18), aspartate transport obligatorily required chloride anions and was inhibited by Evans blue, whereas H+/sialic acid cotransport did not require chloride anions and was insensitive to Evans blue (Fig. 4E and Table 1). Furthermore, as expected from the results shown in Figs. 2 and 3, proteoliposomes containing sialin took up glutamate with properties similar to those of aspartate transport (Fig. 4 F–H and Table 1).
Thus, we concluded that sialin was a vesicular aspartate and glutamate transporter with properties similar to but distinct from those of VGLUTs. This function of sialin explained why RNAi decreased exocytosis of both aspartate and glutamate in pinealocytes (Fig. 3E) and why glutamate is usually colocalized with aspartate in aspartate-secreting cells (10, 12–15). It is noteworthy that other members of the SLC17 anion transporter family did not exhibit any aspartate/glutamate transport activity (Table 2).
Table 2.
Vesicular aspartate and glutamate transport activity is a special property of sialin
| Protein name | Gene name | Uptake, % of control | |
|---|---|---|---|
| Aspartate | Glutamate | ||
| Sialin | SLC17A5 | 100.0 ± 9.2 | 100.0 ± 9.3 |
| NPT1 | SLC17A1 | 3.9 ± 8.2 | 0.0 ± 5.1 |
| VGLUT2 | SLC17A6 | 2.7 ± 4.9 | 116.3 ± 3.1 |
| VGLUT3 | SLC17A8 | 0.0 ± 7.4 | 60.2 ± 11.2 |
| VNUT | SLC17A9 | 0.0 ± 0.7 | 1.1 ± 5.9 |
Effects of Mutations Causing Sialic Acid Storage Diseases.
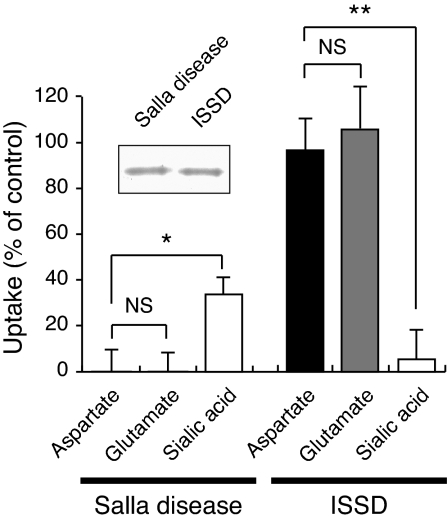
Mutations in the sialin gene are known to cause two distinct diseases, infantile sialic acid storage disease (ISSD), an early fatal disorder with many features characteristic of a lysosomal disorder and Salla disease, a neurological disorder in which the affected persons have a near-normal life expectancy (25–30). However, why different sialin mutations cause different diseases is less understood. A mutated form of sialin found in patients with ISSD was devoid of sialic acid transport activity, whereas the form of sialin commonly associated with Salla disease retained some sialic acid transport activity. These observations provided plausible explanations for the different pathogenicity of the two diseases (27, 31, 32). We found that the Δψ-dependent uptake of aspartate and glutamate of these two mutants was totally different. The mouse sialin mutant carrying the amino acid exchange R39C, a mutation that causes Salla disease in humans, was completely inactive in the Δψ-dependent uptake of aspartate and glutamate while retaining 34% of wild-type sialic acid cotransport activity (Fig. 5). In contrast, the Δψ-dependent uptake of aspartate and glutamate was fully active in the mouse H183R mutant, a mutation associated with ISSD in humans, whereas H+/sialic acid cotransport was completely inactive (Fig. 5). Thus, persons carrying sialin with an R39C mutation are expected to have impaired sialin-mediated aspartergic and glutamatergic neurotransmission; this at least partially explains why the central nervous system is so severely affected in Salla disease.
Fig. 5.
Transport activity of mutant sialins that are associated with Salla disease (R39C) and ISSD (H183R). (Inset) Coomassie brilliant blue-stained mutant protein (10 μg). Δψ-dependent uptake of aspartate and glutamate at 5 min and H+/sialic acid cotransport by proteoliposomes at 3 min is shown. Control activity corresponded to that of wild-type sialin (Fig. 4).
Sialin as a Vesicular Excitatory Amino Acid Transporter (VEAT).
The present work provides evidence that sialin acts as a VEAT. This means that sialin possesses dual physiological functions. When present in synaptic vesicle and SLMVs, sialin is responsible for vesicular storage and subsequent exocytosis of aspartate and glutamate. When present in lysosomes, it acts as an H+-coupled sialic acid exporter. Because all criteria for aspartate as a neurotransmitter are now satisfied, sialin should be a missing link in aspartergic neurotransmission and the sixth member of the vesicular neurotransmitter transporter family, which comprises vesicular monoamine transporter, vesicular acetylcholine transporter, vesicular inhibitory amino acid transporter, VGLUT, and VNUT. Because both glutamate and aspartate belong to excitatory amino acids, sialin should be named VEAT (Fig. 1). The present work predicts that glutamatergic neurotransmission is always accompanied by an aspartergic one. More extensive studies on the structure and function of sialin will help clarify the entire features of aspartergic and associated glutamatergic neurotransmission. For instance, sialin can be used as a marker for aspartergic neurons to explore the occurrence of aspartergic and associated glutamatergic neurotransmission in the central nervous system. The present work also pointed out the unexpected link between aspartergic/glutamatergic neurotransmission and Salla disease. This finding may facilitate the identification of potential molecular targets for the pharmacotherapy of this neurological disorder. Conversely, the studies on the pathogenesis of Salla disease can provide clues for exploring the physiology of aspartergic/glutamatergic neurotransmission.
Materials and Methods
Detailed materials and methods are provided in supporting information (SI) Methods.
cDNA.
cDNAs of mouse and human SLC17A5 (GenBank accession no. NM172773 and NM012434, respectively) were cloned by PCR.
Expression, Purification, and Reconstitution of Sialin.
Essentially the same procedure for purification and reconstitution of VGLUT was used as described in ref. 18. Wild-type and mutant mouse sialin were expressed in Sf9 cells, solubilized from the membrane with octyl glucoside, purified by affinity chromatography, and reconstituted into liposomes by dilution. Uptake of radiolabeled aspartate, glutamate, and sialic acid into proteoliposomes was assayed by the centrifuge column method as described in ref. 18.
RNAi Experiment.
Cells of the pineal glands from 3- to 4-week-old Wistar rats were isolated and cultured as described in ref. 12. After culturing for 4 days, pinealocytes were transfected with 25 nM AllStars negative control siRNA (Qiagen) (control siRNA) or rat sialin siRNA (target sequence: CACCAGAAACTCACAAGACAA). HiPerFect transfection reagent (Qiagen) was used for transfection of siRNA. Aspartate and glutamate release and mRNA levels of sialin and VGLUT2 were assayed 3 days later as described (12, 17). To rescue the RNAi effect, pinealocytes were cotransfected with 1 μg/ml pcDNA3.1/nV5-human sialin and 25 nM rat sialin siRNA.
Data Analysis.
All numerical values are shown as the mean ± SEM, n = 3–6. Statistical significance was determined by Student's t test. *, P < 0.05; **, P < 0.001.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Drs. M. Otsuka, S. Yatsushiro, A. Yamamoto for their contribution at the initial stage of this work, and Dr. S. Takamori (Tokyo Medical and Dental University) for providing cDNAs for VGLUT3. This work was supported in part by a Grant-in-Aid from the Japanese Ministry of Education, Science, Sport, and Culture and Ajinomoto Research Grant 3ARP (to Y.M.). N.E. and M.H. were supported by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: Spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- 2.Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: From bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 3.Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflügers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- 4.Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflügers Arch. 2004;447:629–635. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 6.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 7.Nadler JV, et al. Aspartate and glutamate as possible transmitters of excitatory hippocampal afferents. Nature. 1976;260:538–540. doi: 10.1038/260538a0. [DOI] [PubMed] [Google Scholar]
- 8.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 9.Yuzaki M, Forrest D, Curran T, Connor JA. Selective activation of calcium permeability by aspartate in Purkinje cells. Science. 1996;273:1112–1114. doi: 10.1126/science.273.5278.1112. [DOI] [PubMed] [Google Scholar]
- 10.Gundersen V, et al. Synaptic vesicular localization and exocytosis of l-aspartate in excitatory nerve terminals: A quantitative ImmunoGold analysis in rat hippocampus. J Neurosci. 1998;18:6059–6070. doi: 10.1523/JNEUROSCI.18-16-06059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patneau DK, Mayer ML. Structure–activity relationships for amino acid transmitter candidates acting at N-methyl-d-aspartate and quisqualate receptors. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatsushiro S, et al. l-Aspartate but not the d form is secreted through microvesicle-mediated exocytosis and is sequestered through Na+-dependent transporter in rat pinealocytes. J Neurochem. 1997;69:340–347. doi: 10.1046/j.1471-4159.1997.69010340.x. [DOI] [PubMed] [Google Scholar]
- 13.Fleck MW, Henze DA, Barrionuevo G, Palmer AM. Aspartate and glutamate mediate excitatory synaptic transmission in area CA1 of the hippocampus. J Neurosci. 1993;13:3944–3955. doi: 10.1523/JNEUROSCI.13-09-03944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford SE, Nadler JV. Aspartate release from rat hippocampal synaptosomes. Neuroscience. 2004;128:751–765. doi: 10.1016/j.neuroscience.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 15.Gundersen V, Holten AT, Storm-Mathisen J. GABAergic synapses in hippocampus exocytose aspartate on to NMDA receptors: Quantitative ImmunoGold evidence for cotransmission. Mol Cell Neurosci. 2004;26:156–165. doi: 10.1016/j.mcn.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Nadler JV. Reduced aspartate release from rat hippocampal synaptosomes loaded with clostridial toxin light chain by electroporation: Evidence for an exocytotic mechanism. Neurosci Lett. 2007;412:239–242. doi: 10.1016/j.neulet.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada K, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juge N, et al. Vesicular glutamate transporter contains two independent transport machineries. J Biol Chem. 2006;281:39499–39506. doi: 10.1074/jbc.M607670200. [DOI] [PubMed] [Google Scholar]
- 19.Uchino H, et al. p-Aminohippuric acid transport at renal apical membrane mediated by human inorganic phosphate transporter NPT1. Biochem Biophys Res Commun. 2000;270:254–259. doi: 10.1006/bbrc.2000.2407. [DOI] [PubMed] [Google Scholar]
- 20.Werner A, et al. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci USA. 1991;88:9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aula N, Kopra O, Jalanko A, Peltonen L. Sialin expression in the CNS implicates extralysosomal function in neurons. Neurobiol Dis. 2004;15:251–261. doi: 10.1016/j.nbd.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Yarovaya N, et al. Sialin, an anion transporter defective in sialic acid storage diseases, shows highly variable expression in adult mouse brain, and is developmentally regulated. Neurobiol Dis. 2005;19:351–365. doi: 10.1016/j.nbd.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Fleck MW, Barrionuevo G, Palmer AM. Release of d,l-threo-β-hydroxyaspartate as a false transmitter from excitatory amino acid-releasing nerve terminals. Neurochem Int. 2001;39:75–81. doi: 10.1016/s0197-0186(00)00111-x. [DOI] [PubMed] [Google Scholar]
- 24.Havelaar AC, et al. Purification of the lysosomal sialic acid transporter: Functional characteristics of a monocarboxylate transporter. J Biol Chem. 1998;273:34568–34574. doi: 10.1074/jbc.273.51.34568. [DOI] [PubMed] [Google Scholar]
- 25.Aula P, Gahl WA. Disorders of free sialic acid storage. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw–Hill; 2001. pp. 5109–5120. [Google Scholar]
- 26.Verheijen FW, et al. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat Genet. 1999;23:462–465. doi: 10.1038/70585. [DOI] [PubMed] [Google Scholar]
- 27.Aula N, et al. The spectrum of SLC17A5 gene mutations resulting in free sialic acid-storage diseases indicates some genotype–phenotype correlation. Am J Hum Genet. 2000;67:832–840. doi: 10.1086/303077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alajoki L, et al. Neurocognitive profiles in Salla disease. Dev Med Child Neurol. 2004;46:832–837. doi: 10.1017/s0012162204001458. [DOI] [PubMed] [Google Scholar]
- 29.Kleta R, et al. Clinical, biochemical, and molecular diagnosis of a free sialic acid storage disease patient of moderate severity. Mol Genet Metab. 2004;82:137–143. doi: 10.1016/j.ymgme.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Varho TT, et al. Phenotypic spectrum of Salla disease, a free sialic acid storage disorder. Pediatr Neurol. 2002;26:267–273. doi: 10.1016/s0887-8994(01)00406-4. [DOI] [PubMed] [Google Scholar]
- 31.Morin P, Sagne C, Gasnier B. Functional characterization of wild-type and mutant human sialin. EMBO J. 2004;23:4560–4570. doi: 10.1038/sj.emboj.7600464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wreden CC, Wlizla M, Reimer RJ. Varied mechanisms underlie the free sialic acid storage disorders. J Biol Chem. 2005;280:1408–1416. doi: 10.1074/jbc.M411295200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information