Robotic technology in urology (original) (raw)
Abstract
Urology has increasingly become a technology‐driven specialty. The advent of robotic surgical systems in the past 10 years has led to urologists becoming the world leaders in the use of such technology. In this paper, we review the history and current status of robotic technology in urology. From the earliest uses of robots for transurethral resection of the prostate, to robotic devices for manipulating laparoscopes and to the current crop of master–slave devices for robotic‐assisted laparoscopic surgery, the evolution of robotics in the urology operating theatre is presented. Future possibilities, including the prospects for nanotechnology in urology, are awaited.
Keywords: robotics, daVinci, prostatectomy, nanotechnology
Urology has emerged at the forefront of surgical robotics, spearheaded by the increasing use of the daVinci surgical system for robotic‐assisted prostatectomy. More than 400 daVinci robots are now in use worldwide, most of which are based in North America. Widespread media coverage has increased patient awareness of the possibilities that robotic surgery offers and has led to a market‐driven demand for robotic surgery for some procedures. The dramatic evolution of robotic surgery over the past 10 years is likely to be eclipsed by even greater advances over the next decade. We review the current status of robotic technology in urology.
History and background
The word “robot” was coined by Karel Capek in his play, Rossum's universal robots, in 1921.1 It is derived from the Czechoslovakian term “robota” meaning forced work. His original vision dealt with a world in which robots help humans with everyday tasks, but eventually turn on their masters and attempt world domination. This theme remains popular in fiction today (eg, the 2005 movie “I, Robot”) and has left the general public with an innate and exaggerated perception of what a robot may be capable of.
A surgical robot has been defined as “a computer‐controlled manipulator with artificial sensing that can be reprogrammed to move and position tools to carry out a range of surgical tasks”.2 Strictly speaking, the current popular surgical robots do not satisfy this definition, and some authors have suggested that the term “computer‐assisted surgery” more accurately describes the current generation of robotic devices.3 Whatever the conclusion of that pedantic debate, the term robotic is in popular use to describe the range of technology under discussion here.
In medicine, robots have been used for a variety of rudimentary tasks, such as the transport of pathology specimens from one area to another.4 However, the exciting potential of medical robotics lies in the areas of surgery and interventional medicine. The first applications of robotic technology in urology were developed by Guy's Hospital (London, UK) in association with Imperial College London (London, UK) in the late 1980s. The PROBOT used a robotic frame, guiding a rotating blade to complete a transurethral resection of the prostate.5 Initial studies on prostate‐shaped potatoes were followed up by clinical trials in patients, and demonstrated the safety and feasibility of the technology.
Current technology
AESOP
Several years passed before the next generation of robotic devices became available. Computer Motion (Berkeley, California, USA) first introduced the Automated Endoscopic System for Optimal Positioning (AESOP) in the mid‐1990s.6 This system uses voice (or pedal) control to direct the movements of a robotic arm. The arm usually holds a laparoscope, although it may alternatively hold a laparoscopic retractor. The surgeon has a preprogrammed voice card that allows the device to understand and respond to his or her commands. Laparoscopic images are steadier, with fewer camera changes and inadvertent instrument collisions than an inexperienced human assistant.7 It has proved very popular for procedures such as laparoscopic radical prostatectomy and laparoscopic pyeloplasty in which it is considered part of the standard set‐up in institutions where AESOP is available. It also allows “solo‐surgery” for certain procedures, obviating the need for an assistant.
EndoAssist
EndoAssist (Armstrong Healthcare, High Wycombe, UK) is a free‐standing laparoscopic camera manipulator, controlled by infrared signals from a headset worn by the surgeon. It was also introduced in the 1990s.8 It is considerably less expensive than AESOP but takes up more space in the operating room.
ZEUS
The ZEUS Robotic Surgical System was also first developed by Computer Motion. It represented the first of a new generation of “master–slave” surgical systems that allowed the surgeon to control laparoscopic instruments at a console remote from the operating table. It comprised the AESOP voice‐controlled laparoscope manipulator and two joysticks that controlled two robotic arms holding laparoscopic instruments. It was first used on humans in 1998. It made worldwide headlines in 2001 when the first transatlantic surgery on a human was reported.9 Surgeons in New York performed a laparoscopic cholecystectomy on a patient in Strasbourg without any complications.
The ZEUS system has now been phased out as a result of the merger of Computer Motion with Intuitive Surgical (Sunnyvale, California, USA) in 2003. Evidence had suggested that the increased degrees of freedom (df) and stereoscopic vision of the rival daVinci system rendered the ZEUS system inferior to the daVinci system.10
daVinci Surgical System
The daVinci Surgical System (Intuitive Surgical) has been responsible for the huge surge in the number of robotic procedures performed in the past 5 years. From about 1500 robotic procedures in 2000, more than 20 000 procedures were performed in 2004.11 Urology accounts for the largest single specialty increase, with over 8000 robotic prostatectomies alone performed in 2004. Robotic prostatectomy now accounts for over 10% of radical prostatectomies performed in the USA, a proportion that is increasing year on year. There are now >400 daVinci systems installed worldwide, but only five in the UK at this time, two of which are based in private hospitals.
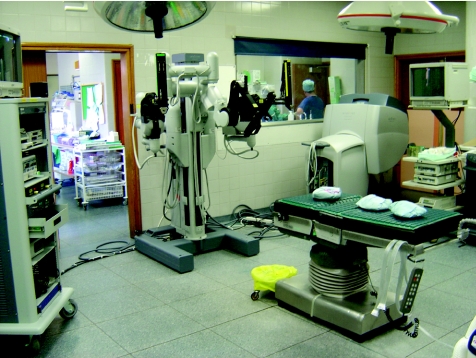
The daVinci is the most advanced master–slave system developed until now. The basic principle involves control of three (or four) robotic arms by a surgeon sitting at a console. The system has three components: (a) a surgeon console, (b) a patient‐side cart and (c) an image‐processing or insufflation stack (fig 1).
Figure 1 A daVinci robot at Guy's Hospital, London, UK.
Console: The surgeon controls the robot from a console placed away from the operating table. The three‐dimensional view from the endoscope is projected in the console at ×10 magnification. The surgeon's thumb and forefinger control the movements of the robotic arms. Foot pedals allow control of diathermy and other energy sources. Motion scaling enhances the elimination of tremor, allowing very smooth and precise movements.
Patient‐side cart: The robotic arms are mounted on this cart, one of which holds the high‐resolution three‐dimensional endoscope. Specialised EndoWrist (Intuitive Surgical, California, USA) instruments are mounted on the remaining arms.
Image‐processing/insufflation stack: The stack contains the camera‐control units for the three‐dimensional imaging system, image‐recording devices, a laparoscopic insufflator and a monitor allowing two‐dimensional vision for the assistants.
Although the three‐dimensional vision, enhanced magnification and motion scaling all make life a little easier for the operating surgeon, it is actually the EndoWrist technology that makes the daVinci such a remarkable piece of technology. Current laparoscopic instrumentation allows only 4 df. The EndoWrist instruments restore the 7 df that the human wrist normally enjoys, making complex laparoscopic procedures more facile.
Robotic procedures in urology
The endoscope‐manipulating robots mentioned above are in use for several urological procedures; however, it is during laparoscopic radical prostatectomy that they have become most popular. The combination of long laparoscopic procedure times, the need for a steady image and the somewhat awkward camera position for looking into the pelvis makes these robots particularly useful for this procedure. However, not all institutions can afford the initial outlay (around €100 000 for AESOP and €50 000 for EndoAssist), although there are minimal ongoing running costs.
However, it is the daVinci system that has heralded the increasing popularity of robotic procedures in urology. The list of procedures completed robotically is increasing steadily.
Robotic prostatectomy
By far the most common procedure performed robotically is radical prostatectomy. The forerunners in the development of this technique are the team from Detroit, who have described the Vattikutti Institute Prostatectomy technique. Since 2000, they have performed more than 2000 robotic prostatectomies at their one institute. They have refined a transperitoneal technique, which has produced very good oncological results with minimal morbidity. Describing their first 1100 cases, Menon et al12 reported operative times of 70–160 min and blood loss of 50–150 ml, with no blood transfusions and 95% of the patients being discharged within 24 h. Total continence was achieved in 96% of patients at 6 months. Of those patients who were potent preoperatively, 60% were having unassisted intercourse at 6 months. Since then, they have described an elegant method of dissecting the neurovascular bundle at the apex, the so‐called “Veil of Aphrodite”, which has produced potency rates approaching 97%.13 Comparing their robotic experience with their own laparoscopic radical prostatectomy experience, they observed considerably longer operating times and blood loss in the laparoscopic group.14 Perhaps most important of all, their positive margin rate was 9%, compared with 23% for their own open prostatectomy experience.15 However, the technique for establishing positive margins seemed to differ between the two groups.
A similar non‐randomised trial in California compared the results of 60 open prostatectomies with 60 robotic prostatectomies.16 Operative times were similar (214 v 231 min). Positive margin rates were lower in the robotic group (16.7% v 20%), as were blood loss rates (103 v 418 ml).
No randomised control trial has compared robotic prostatectomy with laparoscopic or even open surgery. Recruitment to such a trial does not seem to be possible.
Even though we await the longer‐term oncological outcome of robotic prostatectomy, we should expect the numbers undergoing this procedure to increase as the availability of the technology increases.
Robotic cystectomy
The initial robotic radical cystectomy and Hautmann neobladder was reported in 2003.17 The operative time was 8.5 h, with a blood loss of 200 ml. Menon's group have collaborated with the Mansoura group and reported on robotic nerve‐sparing radical cystoprostatectomy in 17 patients, some of whom underwent neobladder formation.18 The neobladder was anastomosed robotically after extracorporeal neobladder formation. The mean blood loss was <150 ml. Guy's Hospital has adopted its own version of the Vattikutti robotic cystectomy and the Eastbourne laparoscopic cystectomy, with promising early results.19 The advantages of decreased blood loss, improved operative vision, reduced hospital stay and postoperative pain need to be balanced against the increased technical difficulty, longer operative times and unproved long‐term oncological outcomes when compared with open radical cystectomy.20 A larger series and longer‐term outcome data are awaited.
Robotic pyeloplasty
Laparoscopic dismembered pyeloplasty remains a challenging procedure with prolonged operating times, even in the larger series.21 The increased dexterity and precision afforded by the daVinci system would seem to make robotic pyeloplasty an attractive option. The procedure has been reported in adult and paediatric populations, the paediatric populations using 5 mm robotic ports. Success rates equal those of laparoscopic and open pyeloplasty.22 Gettman et al23 reported on robotic dismembered pyeloplasty in nine patients, with a mean operative time of 138 min and no intraoperative complications. In a non‐randomised comparison, they also reported shorter operative and, as we might expect, anastomotic times compared with pure laparoscopic pyeloplasty.24 The total operative and suturing times were 140 and 70 min compared with 235 and 120 min for robotic and laparoscopic pyeloplasty, respectively.
The largest reported series (n = 50) describes operative times of 122 min, with anastomotic times averaging only 20 min.25
Other procedures
Most laparoscopic urological procedures have now been reported using daVinci robotic assistance. These include radical and simple nephrectomy, live‐donor nephrectomy, adrenalectomy, sural nerve grafting, ureteric reimplantation, colposuspension and even renal transplant.11 However, the perceived advantage of robotic over conventional laparoscopic procedures is likely to be more pronounced in those procedures that require complex reconstructive techniques, rather than more simple ablative surgery. We will probably not see a large series of robotic nephrectomies, for example, as there is not likely to be any marked advantage over conventional laparoscopic techniques.
Advantages of robotic technology
A debate exists regarding the benefits of robotic‐assisted procedures over conventional laparoscopic procedures. Although robotic procedures achieve all the associated benefits of laparoscopic over open surgery (decreased blood loss, shorter hospital stay and faster return to activity), the question remains of whether robotic surgery has any real advantages over laparoscopic surgery (table 1).
Table 1 Advantages and disadvantages of robotic surgery.
| Advantages | Three‐dimensional visualisation |
|---|---|
| Enhanced degrees of freedom | |
| No fulcrum effect | |
| Motion scaling | |
| Elimination of tremor | |
| Reduced fatigue | |
| Ergonomic positioning | |
| Disadvantages | Expensive capital and running costs |
| No tactile feedback | |
| Reduced trainee experience | |
| Set‐up times lengthy |
Some laparoscopists argue that robotic procedures are an industry‐driven phenomenon and that proper scientific evaluation should be undertaken before their role in urological surgery is clarified. In considering the potential benefits of the technology described above, we must carefully weigh the advantages against the considerable cost, in addition to other disadvantages of systems such as daVinci.
The most striking difference we notice when transitioning from laparoscopic to robotic surgery is the intuitive nature of the instrument movements. Laparoscopic surgery suffers from a fulcrum effect, in which the instrument tips move in the direction opposite to the hand of the surgeon. The daVinci robotic instruments cancel this effect, so that the instrument tips in the body move in the same direction as the surgeon's hands in the console. The significance of this is not clear, although it may allow those without laparoscopic skills to transition more smoothly to minimally invasive surgery. The Detroit group suggests that the learning curve is shorter for robotic than for laparoscopic surgery.15 Ahlering et al16 have also shown the feasibility of moving directly from open to robotic surgery without negotiating the laparoscopic learning curve.
The additional df offered by the EndoWrist instruments can offer advantages when performing complex reconstructive surgery such as laparoscopic pyeloplasty. However, we may argue that a well‐trained laparoscopist who is prepared to spend time practising suturing in a laparoscopic trainer may not perceive such advantages so readily.3 Three‐dimensional vision is also advantageous, although similar systems are also available for conventional laparoscopic procedures. Motion scaling and the elimination of tremor certainly allow smooth and precise movements. Movements may be scaled so that large movements at the console may be translated into micromotions at the operative site.26 It is presumed that the combination of these factors facilitates greater dexterity and vision, leading to greater accuracy and reduction in blood loss and surgical complications.6 It is also presumed that the surgeon enjoys a more comfortable operating position doing robotic surgery than conventional laparoscopic surgery. Studies are under way to evaluate the precise ergonomic relationship between man and machine.
Disadvantages
The most striking drawback of the daVinci system is the cost. The capital expenditure is well over €1 million, with annual running costs approximating €100 000 per annum. However, the proliferation of these machines in the private healthcare system suggests that there is a health economy case in favour of this outlay.
A more worrying surgical disadvantage is the lack of tactile feedback with this technology. Haptic technology is the focus of several scientific and engineering institutes, and we may find tactile feedback introduced into future generations of master–slave systems. It is also fair to say that the introduction of virtual reality simulators into surgical training may reduce the reliance that future surgeons may place on tactile feedback. Indeed virtual reality trainers may perfectly complement the technical skills necessary for robotic surgery.27
The addition of a fourth arm to the daVinci system reduces the need for a patient‐side assistant for some procedures. Although this may have financial benefits, it reduces the worthy laparoscopic experience a trainee can experience as the patient‐side operator during robotic procedures.
Set‐up times for robotic procedures are quite lengthy initially. The key to reducing this is to have a large and well‐trained multidisciplinary team. The current generation of daVinci is quite cumbersome, and it can often prove challenging to incorporate the system into the operating room layout. Components sometimes need to be stored elsewhere when not in use.
Future directions
Robotic surgical technology is in its infancy. There have not been any great advances in robotic technology for urologists in the past 5 years. Although robots have been appearing in other minimally invasive technologies, such as the Abltherm HIFU machine (Edap, France), there have not been any headline‐grabbing new robots in the recent past.
Although the daVinci system has certainly offered a credible addition to our minimally invasive arsenal, it is based on technology that was developed in the latter part of the last century. Indeed we could argue that the lack of any commercial competitor to the daVinci has somewhat stifled development, as the company seeks to recoup its costs. There have been several small modifications to the system, including the addition of a fourth arm, and this month Intuitive Surgical has launched daVinci S, an upgrade that offers faster set‐up and instrument changes, multiquadrant access and interactive video displays. However, we await the next generation of daVinci, which promises a smaller, more manageable console and cart, with the possibility of haptic technology to allow tactile feedback. We also eagerly await the development of new EndoWrist instruments and additional energy sources for use in the robotic operative discipline.
So what are the next possibilities for robotic surgery? The integration of preoperative imagery (computed tomography and magnetic resonance imaging) and intraoperative video to guide a surgeon's dissection is an exciting one.28 Such data, in combination with virtual reality simulators, could also allow the rehearsal of complex procedures in advance.
Some authors believe that robotic surgery can be extended into the realm of advanced diagnostic testing with the development and integration of ultrasonography, near infrared and confocal microscopy equipment.29
However, we must have a more imaginative outlook to appreciate the true “next generation” of surgical robots, the nanorobots.
Nanotechnology
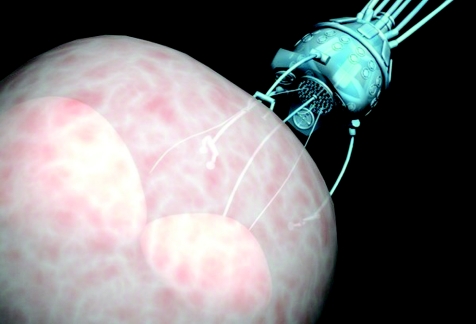
Nanotechnology refers to automated mechanical devices whose dimensions are measured in microns. The ongoing developments of molecular‐scale electronics, sensors and motors are expected to make it possible to produce microscopic robots with dimensions comparable to bacteria. Such technology could be used to deliver chemotherapy or targeted‐gene therapy into end organs or cells in remote parts of the body (fig 2). Such delivery systems could integrate very well with the development of vaccines and small‐molecule agents for renal cancer, for example.
Figure 2 Nanorobot performing cell surgery (source: Nanotechnology News Network).
Although developing nanoscale robots presents difficult fabrication and control challenges, considerable advances have already been made. The world's smallest untethered, controllable robot was recently disclosed, measuring 250×100 μm (http://www.dartmouth.edu/˜news/releases/2005/09/14.html). This robot is about the width of a human hair and half the length of a full stop. The key to miniaturising nanorobots even further is the further downscaling of integrated circuit processors. The integrated circuit industry believes that it will have reduced the size of today's processors by a factor of 25 within 10 years, leading to the development of nanorobots measuring just 50 μm, small enough to get into the smallest vessels in the body. The nanorobot pioneer, Dr Adriano Calvacanti, believes that nanorobots will be used in humans within a decade,30 although the potential uses in urology have been identified by others.31
Conclusions
Urology is an ancient specialty and urologists still enjoy using ancient surgical instruments (eg Clutton's Sounds, Aesculap, Tuttléngen, Germany) for urethral calibration), which have changed little in hundreds of years of practice. However, urology is also very much a technology‐driven specialty. The nature of endoscopic and laparoscopic surgery, which dominates urological practice, lends itself very favourably to highly advanced technology. It is not surprising that urologists have been quick to exploit the potential of the current generation of surgical robots. However, we should also be vigorous in the scientific evaluation of these new technologies.
These are exciting times. We are still very early on in the robot revolution and much more exciting developments are yet to come. The daVinci robot is an interesting start, but we suspect that the worlds of science and engineering have much more to offer.
A short video entitled Robotic Technology in Urology accompanies this paper and is available online at http://www.pmj.bmjjournals.com/supplemental
Acknowledgements
We thank the Charitable Trust of Guy's and St Thomas' Hospital, London, UK.
Competing interests: None.
Abbreviations
AESOP - Automated Endoscopic System for Optimal Positioning
References
- 1.Shah J, Mackay S, Rockall T.et al ‘Urobotics': robots in urology. BJU Int 200188313–320. [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta P, Jones A, Gill I S. Robotic urologic surgery: a perspective. BJU Int 20059520–23. [DOI] [PubMed] [Google Scholar]
- 3.Guillonneau B. What robotics in urology? A current point of view. Eur Urol 200343103–105. [DOI] [PubMed] [Google Scholar]
- 4.Challacombe B, Khan S, Murphy D.et al The history of robotics in urology. World J Urol 200624120. [DOI] [PubMed] [Google Scholar]
- 5.Harris S J, Arambula‐Cosio F, Mei Q.et al The Probot‐an active robot for prostate resection. Proc Inst Mech Eng [H] 1997211317–325. [DOI] [PubMed] [Google Scholar]
- 6.Nedas T G, Challacombe B J, Dasgupta P. Robotics in urology: an update. Int J Medi Robot Comput Assist Surg 2005113–18. [DOI] [PubMed] [Google Scholar]
- 7.Kavoussi L R, Moore R G, Adams J B.et al Comparison of robotic versus laparoscopic camera control. J Urol 19951542134–2136. [PubMed] [Google Scholar]
- 8.Finlay P A. Clinical experience with a goniometric head‐controlled laparoscopic manipulator. Proceedings of the IARP Workshop on Medical Robotics, Vienna, October 1996
- 9.Marescaux J, Leroy J, Gagner M.et al Transatlantic robot‐assisted telesurgery. Nature 2001413379–380. [DOI] [PubMed] [Google Scholar]
- 10.Sung G T, Gill I S. Robotic laparoscopic surgery: a comparison of the da Vinci and Zeus systems. J Urol 200158893–898. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Hemal A K. Emerging role of robotics in urology. J Min Access Surg 20051202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon M, Tewari A, Peabody J O.et al Vattikutti Institute Prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: experience of over 1100 cases. Urol Clin North Am 200431701–717. [DOI] [PubMed] [Google Scholar]
- 13.Menon M, Kaul S, Bhandari A.et al Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes following conventional nerve sparing and prostatic fascia sparing techniques. J Urol 2005174269–272. [DOI] [PubMed] [Google Scholar]
- 14.Tewari A, Srivasatava A, Menon M. Members of the VIP Team. A prospective comparison of radical retropubic and robot‐assisted prostatectomy: experience in one institution, BJU Int 200392205–210. [DOI] [PubMed] [Google Scholar]
- 15.Menon M. Robotic radical retropubic prostatectomy. BJU Int 200391175–176. [DOI] [PubMed] [Google Scholar]
- 16.Ahlering T E, Woo D, Eichel L.et al Robot‐assisted versus open radical prostatectomy: a comparison of one surgeon's outcomes. Urology 200463819–822. [DOI] [PubMed] [Google Scholar]
- 17.Beecken W D, Wolfram M, Engl T.et al Robot assisted laparoscopic radical cystectomy and intra‐abdominal formation of an orthotopic ileal neobladder. Eur Urol 200344337–339. [DOI] [PubMed] [Google Scholar]
- 18.Menon M, Hemal A K, Tewari A.et al Nerve‐sparing robot‐assisted radical cystoprostatectomy and urinary diversion. BJU Int 200392232–236. [DOI] [PubMed] [Google Scholar]
- 19.Dasgupta P, Hemal A, Rose K. Guy's and St. Thomas' Robotics Group. Robotic urology in the UK: establishing a programme and emerging role, BJU Int 200595723–724. [DOI] [PubMed] [Google Scholar]
- 20.Challacombe B J, Rose K, Dasgupta P. Laparoscopic radical and partial cystectomy. J Min Access Surg 20051188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy D, Challacombe B, Rane A. Reconstructive laparoscopic urology. J Min Access Surg 20051181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose K, Khan S, Dasgupta P. The current status of robotic and laparoscopic pyeloplasty. Int J Clin Pract 2006606–8. [DOI] [PubMed] [Google Scholar]
- 23.Gettman M T, Neururer R, Bartsch G. Anderson‐Hynes dismembered pyeloplasty performed using the da Vinci robotic system. Urology 200260509–513. [DOI] [PubMed] [Google Scholar]
- 24.Gettman M T, Peschel R, Neururer R.et al A comparison of laparoscopic pyeloplasty performed with the da Vinci surgical system versus standard laparoscopic techniques: initial clinical results. Eur Urol 200221133–138. [DOI] [PubMed] [Google Scholar]
- 25.Patel V. Robotic‐assisted laparoscopic dismembered pyeloplasty. Urology 20056645–49. [DOI] [PubMed] [Google Scholar]
- 26.Kim V B, Chapman W H, Albrecht R J. Early experience with telemanipulative robot‐assisted laparoscopic cholecystectomy using da Vinci. Surg Laparosc Endosc Percutan Tech 20021234–40. [DOI] [PubMed] [Google Scholar]
- 27.Nedas T G, Challacombe B, Dasgupta P. Virtual reality in urology. BJU Int 200494255–257. [DOI] [PubMed] [Google Scholar]
- 28.Satava R M, Bowersox J C, Mack M. Robotic surgery: state of the art and future trends. Contemp Surg 200157489–499. [Google Scholar]
- 29.Prasad S M, Ducko C T, Stephenson E R. Prospective clinical trial of robotically‐assisted endoscopic coronary grafting with 1 year follow‐up. Ann Surg 2001233725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalcanti A. Robots in surgery. Plenary Lecture, Euro Nano Forum 2005, Nanotechnology and the health of the EU citizen in 2020. Edinburgh, Scotland, UK, September 2005
- 31.Shergill I, Rao A, Arya M.et al Nanotechnology: potential applications in urology. BJU Int 200697219. [DOI] [PubMed] [Google Scholar]