Cobalamin Deficiency in Elderly Patients: A Personal View (original) (raw)
Abstract
Cobalamin (vitamin B12) deficiency is particularly common in the elderly (>65 years of age) but is often unrecognized because its clinical manifestations are subtle; however, they are also potentially serious, particularly from a neuropsychiatric and hematological perspective. In the elderly, the main causes of cobalamin deficiency are pernicious anemia and food-cobalamin malabsorption. Food-cobalamin malabsorption syndrome is a disorder characterized by the inability to release cobalamin from food or its binding proteins. This syndrome is usually caused by atrophic gastritis, related or unrelated to Helicobacter pylori infection, and long-term ingestion of antacids and biguanides. Management of cobalamin deficiency with cobalamin injections is currently well documented but new routes of cobalamin administration (oral and nasal) are being studied, especially oral cobalamin therapy for food-cobalamin malabsorption.
1. Introduction
Cobalamin or vitamin B12 deficiency is common in elderly patients [1] but is often unrecognized or not investigated because the clinical manifestations of cobalamin deficiency are subtle. However, the complications of cobalamin deficiency, particularly the neuropsychiatric and hematological [1–4], are potentially serious and therefore require investigation in all patients who present with vitamin or nutritional deficiency. Classic disorders such as pernicious anemia are the cause of cobalamin deficiency in only a limited number of patients, especially elderly patients [4]. A more common problem is food-cobalamin malabsorption, a disorder characterized by the inability to release cobalamin from food or its binding proteins [4]. This review summarizes the current knowledge on cobalamin deficiency, with a particular focus on food-cobalamin malabsorption and oral cobalamin therapy.
2. Definition of Cobalamin Deficiency
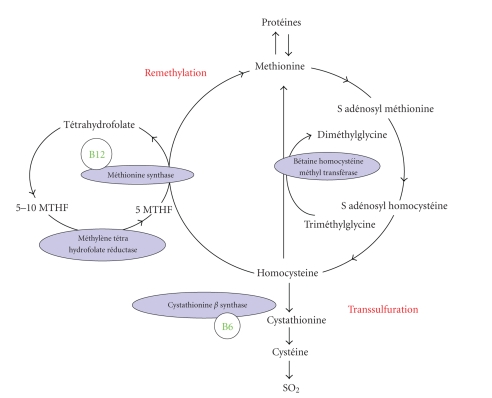
Literature of the last ten years has provided several definitions of cobalamin deficiency [5–7]. The definitions of cobalamin deficiency used in this review are shown in Table 1 [7, 8]. To date, cobalamin deficiency is often defined in terms of the serum concentration of cobalamin and of homocysteine and methyl malonic acid, two components of the cobalamin metabolic pathway, (Figure 1) but in clinical practice, no single test has emerged as the gold standard for diagnosis of cobalamin deficiency especially in elderly patients. Moreover, the major diagnostic challenge remains patients who develop subtle cobalamin deficiency, often without hematological abnormalities (usefulness of an early treatment to prevent irreversible neurological damages) [4]. In the future, new serum cobalamin assay kits (e.g., the holotranscobalamin assay kit) might perhaps replace older assay kits and should become the standard for testing [6, 9].
Table 1.
Definitions of cobalamin (vitamin B12) deficiency [5–7].
| (i) Serum cobalamin levels <150 pmol/L and clinical features and/or hematological anomalies related to cobalamin deficiency |
|---|
| (ii) Serum cobalamin levels <150 pmol/L (<200 pg/mL) on 2 separate occasions |
| (iii) Serum cobalamin levels <150 pmol/L and total serum homocysteine levels >13 _μ_mol/L or methylmalonic acid levels >0.4 _μ_mol/L (in the absence of renal failure and folate and vitamin B6 deficiencies) |
| (iv) Low serum holotranscobalamin levels <35 pmol/L |
Figure 1.
Cellular impact of cobalamin deficiency.
3. Epidemiology of Cobalamin Deficiency
Epidemiological studies show that in the general population of industrialized countries, cobalamin deficiency has a prevalence of around 2 to 20%, depending on the definition of cobalamin deficiency used [4, 9]. The Framingham study demonstrated a prevalence of 12% among elderly people living in the community [10]. Other studies focusing on elderly people, particularly those who are in institutions or who are sick and malnourished, have suggested a higher prevalence of at least 30% [11, 12]. Using the definition in Table 1(serum cobalamin levels <150 pmol/L [<200 pg/mL] on 2 separate occasions), we found that cobalamin deficiency had a prevalence of 5% in a group of patients followed or hospitalized in a tertiary reference hospital [8]. We also documented that around 4% of the anemia were related to a cobalamin deficiency in a population of 300 consecutive anemia hospitalized in our department (tertiary reference center) [8]. In the NHANES III study, 34% of all anemia in elderly patients is caused by folate, cobalamin, or iron deficiency, alone or in combination (nutritient-deficiency anemia) [8].
4. Cobalamin Metabolism and Function
Cobalamin metabolism is complex and is made up of many processes, defects in any one of which can lead to cobalamin deficiency [4, 13–15]. The different stages of cobalamin metabolism and corresponding causes of cobalamin deficiency are shown in Table 2. Once metabolized, cobalamin is a cofactor and coenzyme for many biochemical reactions, including DNA synthesis, methionine synthesis from homocysteine, and conversion of propionyl into succinyl coenzyme A from methyl malonate [4, 9]. In a clinical setting, cobalamin absorption is measured imperfectly by the Schilling test [4, 8]. A typical Western diet contributes 3–30 _μ_g of cobalamin per day [13, 15] toward the recommended dietary allowance of 2.4 _μ_g/day for adults and 2.6 to 2.8 _μ_g/day during pregnancy [16]. The 5–10 year delay between the onset of cobalamin deficiency and the development of clinical illness is directly attributable to hepatic stores of cobalamin (>1.5 mg) and the enterohepatic cycle [4, 13]. Between 1–5% of free cobalamin (or crystalline cobalamin) is absorbed along the entire intestine by passive diffusion. This absorption explains the mechanism underlying oral treatment of cobalamin deficiencies [17, 18].
Table 2.
Stages of cobalamin metabolism and corresponding causes of cobalamin deficiency [13, 15].
| Stages and factors involved in cobalamin metabolism | Causes of cobalamin deficiency |
|---|---|
| Ingestion of food | Strict vegetarianism (patients who are sick in institutions or in psychiatric hospitals) |
| Digestion, which involves haptocorrin, gastric secretions (HCl and pepsin), intrinsic factor, pancreatic and biliary secretions, and the enterohepatic cycle | Gastrectomy, pernicious anemia, and food-cobalamin malabsorption |
| Absorption, which brings into play intrinsic factor and cubilin | Ileal resection, malabsorption, pernicious anemia, and food-cobalamin malabsorption |
| Transportation by transcobalamins | Congenital deficiency in transcobalamin II |
| Intracellular metabolism by various intracellular enzymes | Congenital deficiency in various intracellular enzymes |
5. Classical Causes of Cobalamin Deficiency
In elderly patients, cobalamin deficiency is classically caused by pernicious anemia and food-cobalamin malabsorption [1, 11, 14]. The principal characteristics of pernicious anemia have been reported in detail in several reviews [19–21]. Diagnosis of pernicious anemia is based on the presence of (1) intrinsic factor antibodies in serum (specificity: >98%, sensibility: around 50%) and/or (2) autoimmune atrophic gastritis (presence of Helicobacter pylori infection in gastric biopsies is an exclusion factor) [15, 19]. Cobalamin deficiency caused by dietary deficiency or malabsorption is rare. Dietary causes of deficiency are limited to elderly people who are already malnourished. This mainly concerns elderly patients living in institutions or in psychiatric hospitals [4, 13]. Since the 1980s, the malabsorption of cobalamin has become rarer, owing mainly to the decreasing frequency of gastrectomy and surgical resection of the terminal small intestine [4, 14]. Several disorders commonly seen in gastroenterology practice might, however, be associated with cobalamin malabsorption. These include deficiency in the exocrine function of the pancreas after chronic pancreatitis (usually alcoholic), lymphomas or tuberculosis (of the intestine), Crohn's disease, Whipple's disease, and uncommonly celiac disease [11, 15].
6. Food-Cobalamin Malabsorption
First, well-described by Carmel in 1995 [22], the food-cobalamin malabsorption is a syndrome characterized by the inability to release cobalamin from food or intestinal transport proteins, particularly in the presence of hypochlorhydria, in which the absorption of “unbound” cobalamin is normal. As various studies have shown [14, 22, 23], this syndrome is defined by cobalamin deficiency in the presence of sufficient food-cobalamin intake and normal Schilling test results, which rules out malabsorption or pernicious anemia. The principal characteristics of this syndrome are listed in Table 3. In theory, indisputable evidence of food-cobalamin malabsorption comes from using a modified Schilling test, which uses radioactive cobalamin bound to animal proteins (e.g., salmon, trout) and reveals malabsorption when the results of a standard Schilling test are normal [4, 14, 23].
Table 3.
Food-cobalamin malabsorption syndrome [4, 14, 15].
| Criteria for food-cobalamin malabsorption | Associated conditions or agents |
|---|---|
| – Low-serum cobalamin (vitamin B12) levels | – Gastric disease: atrophic gastritis, type A atrophic gastritis, gastric disease associated with_Helicobacter pylori_ infection, partial gastrectomy, gastric by-pass, and vagotomy |
| – Normal results of Schilling test using free cyanocobalamin labeled with cobalt-58, or abnormal results of derived Schilling test‡ | – Pancreatic insufficiency: alcohol |
| – No anti-intrinsic factor antibodies | – Gastric or intestinal bacterial overgrowth: achlorhydria, tropical sprue, Ogylvie's syndrome, and HIV |
| – No dietary cobalamin deficiency | – Drugs: antacids (H2-receptor antagonists and proton-pump inhibitors) or biguanides (metformin) |
| – Alcohol abuse | |
| – Sjögren's syndrome, systemic sclerosis | |
| – Haptocorrine deficiency | |
| – Ageing or idiopathic |
Food-cobalamin malabsorption has been found to be the leading cause of cobalamin malabsorption, especially in elderly patients [4, 11, 22]. In our experience (300 patients with a documented cobalamin deficiency), food-cobalamin malabsorption accounts for about 60–70% of the cases of cobalamin deficiency in elderly patients, whereas pernicious anemia accounted for only 15–25% [14, 23]. Some authors have speculated about the reality and significance of cobalamin deficiency related to food-cobalamin malabsorption [4], because many patients have only mild clinical or hematological features. Several of our patients, however, [14] had significant features classically associated with pernicious anemia, including polyneuropathy, confusion, dementia, medullar-combined sclerosis, anemia, and a pancytopenia. Nevertheless, the partial nature of this form of malabsorption might produce a more slowly progressive depletion of cobalamin than does the more complete malabsorption engendered by disruption of intrinsic factor-mediated absorption. The slower progression of depletion probably explains why mild preclinical deficiency is associated with food-cobalamin malabsorption more often than with pernicious anemia [4, 14].
Food-cobalamin malabsorption is caused primarily by atrophic gastritis [14]. Achlorhydria hampers the extraction of cobalamin from protein food sources. Over 40% of patients older than 80 years of age have gastric atrophy that might or might not be related to Helicobacter pylori infection [11, 24]. Other factors that contribute to food-cobalamin malabsorption in elderly people include chronic carriage of H. pylori and intestinal microbial proliferation (in which case cobalamin deficiency can be corrected by antibiotic treatment) [24, 25]; long-term ingestion of antiacids, including H2-receptor antagonists and proton-pump inhibitors [26, 27], particularly among patients with Zollinger-Ellison syndrome [28, 29], and biguanides (metformin) [30–32]; chronic alcoholism; surgery or gastric reconstruction (e.g., bypass surgery for obesity); partial pancreatic exocrine failure [4, 14], and Sjögren's syndrome or systemic sclerosis [33] (Table 3). In a series of 92 elderly patients (mean age: 76 years) with food-cobalamin malabsorption [14], we have reported at least one of these associated conditions or agents in 60% of the patients. These conditions mainly include atrophic gastritis (±H. pylori infection) in 30% of the patients and long-term metformin or antacid intake in 20% of the elderly patients.
7. Clinical Manifestations of Cobalamin Deficiency
The primary clinical manifestations of cobalamin deficiency are described in Table 4. They are highly polymorphic and of varying severity ranging from common sensory neuropathy and isolated anomalies of macrocytosis and hypersegmentation of neutrophils, to severe disorders, including combined sclerosis of the spinal cord, hemolytic anemia, and even pancytopenia [2, 14, 34–36]. In the aforementioned series of 92 patients with food-cobalamin malabsorption [14], we have found at least one clinical feature or hematological abnormalities in, respectively, 70% and 76% of the patients. Cobalamin deficiency appears to be more common among patients who have a variety of chronic neurologic conditions such as dementia, Alzheimer's disease, stroke, Parkinson's disease, and depression, although it is unclear if these are causal relationships [4, 37]. In our own studies in which we administered cobalamin to patients with dementia, improvement was not observed [8, 14]. Other studies have had similar results [1, 2, 9]. At this time, a causal role of cobalamin in these conditions remains speculative.
Table 4.
Main clinical features of cobalamin deficiency [2, 4, 14, 15, 34–36].
| Hematological manifestations | Neuro-psychiatric manifestations | Digestive manifestations | Other manifestations |
|---|---|---|---|
| – Frequent: macrocytosis, hypersegmentation of the neutrophils, aregenerative macrocytary anemia, LDH and bilirubin elevation, medullary megaloblastosis “(blue spinal cord)” | – Frequent: polyneurites (especially sensitive ones), ataxia, Babinski's phenomenon | – Classic: Hunter's glossitis, jaundice, LDH and bilirubin elevation “(intramedullary destruction)” | – Under study: atrophy of the vaginal mucosa and chronic vaginal and urinary infections (especially mycosis), hypofertility and repeated miscarriages (connection with cobalamin deficiency under study), venous thromboembolic disease, angina (hyperhomocysteinemia), osteoporosis |
| – Rare: isolated thrombocytopenia and neutropenia, pancytopenia | – Classic: combined sclerosis of the spinal cord | – Debatable: abdominal pain, dyspepsia, nausea, vomiting, diarrhea, disturbances in intestinal functioning | |
| – Very rare: hemolytic anemia, thrombotic microangiopathy (presence of schistocytes) | – Rare: cerebellar syndromes affecting the cranial nerves including optic neuritis, optic atrophy, urinary, and/or fecal incontinence | – Rare: resistant and recurring mucocutaneous ulcers cobalamin deficiency | |
| – Under study: changes in the higher functions, even dementia, stroke and atherosclerosis (hyperhomocysteinemia), parkinsonian syndromes, depression, multiple sclerosis |
8. Classical Treatment of Cobalamin Deficiency
The classic treatment for cobalamin deficiency, particularly when the cause is not dietary deficiency, is parenteral administration—in most countries intramuscular injection—of this vitamin (in the form of cyanocobalamin and, more rarely, hydroxy or methyl cobalamin) [1, 17, 18, 34]. However, traditions concerning both dose and schedule of administration vary considerably. In France, the recommended practice is to build up the tissue stores of the vitamin quickly and correct serum cobalamin hypovitaminosis, particularly in the case of pernicious anemia. The treatment involves the administration of 1000 _μ_g of cyanocobalamin per day for 1 week, followed by 1000 _μ_g per week for 1 month, followed by 1000 _μ_g per month, normally for the rest of the patient's life [11, 19]. In USA and UK, dosages ranging from 100 to 1000 _μ_g per month [or every 2-3 months when hydroxocobalamin is given] are used during the rest of the patient's life [4, 17]. Hydroxocobalamin may have several advantages due to a better tissular retention and storage. Additionally, recent works concern oral cobalamin therapy through food fortification [3, 11].
9. Oral Cobalamin Therapy
Since cobalamin is absorbed by intrinsic factor-independent passive diffusion (1% of oral cobalamin), daily high-dose oral cyanocobalamin can induce and maintain remissions in patients with megaloblastic anemia [15]. In cases of cobalamin deficiency other than those caused by nutritional deficiency, alternative routes of cobalamin administration have been used: oral [17, 18, 38–44] and nasal [45, 46]. These other routes of administration have been proposed as a way of avoiding the discomfort, inconvenience, and cost of monthly injections. Our working group has developed an effective oral treatment of food-cobalamin malabsorption [40–43] and for pernicious anemia [47] using crystalline cobalamin (cyanocobalamin). Our principal studies of oral cobalamin treatment (open, not randomized studies) are described in Table 5 [40–43, 47]. These data confirm the previously reported efficacy of oral crystalline cyanocobalamin, especially in food-cobalamin therapy [18, 36, 38]. All of our patients who were treated orally corrected their cobalamin levels and at least two-thirds corrected their hematological abnormalities [40–43, 47]. Moreover, one-third of patients experienced a clinical improvement on oral treatment. In most cases of food-cobalamin malabsorption, “low” cobalamin doses (i.e., 125–1000 _μ_g of oral crystalline cyanocobalamin per day) were used. These data is in accordance with the results of the two prospective randomized-controlled studies comparing oral cobalamin with intramuscular cobalamin therapy [17, 39]. A systematic review of randomized-controlled trials by the Vitamin B12 Cochrane Group supports the efficacy of oral cobalamin therapy, with a dose between 1000 and 2000 _μ_g given initially daily and then weekly [48]. In this analysis, serum cobalamin levels increased significantly in patients receiving oral cobalamin and both groups of patients (receiving oral and intramuscular treatment) had neurological improvement. The Cochrane group concludes that daily oral therapy “may be as effective as intramuscular administration in obtaining short term haematological and neurological responses in cobalamin deficient patients” [48]. Nevertheless to our knowledge, the effect of oral cobalamin treatment in patients presenting severe neurological manifestations has not yet been adequately documented. Thus until this has been done parenteral cobalamin therapy is still to be recommended for such patients. In a randomized, parallel-group, double-blind, dose-finding trial, Eussen et al. showed that the lowest dose of oral cyanocobalamin required to normalize mild cobalamin deficiency is more than 200 times the recommended dietary allowance of approximately 3 _μ_g daily (i.e., >500 _μ_g per day) [49]. The procedure for oral cobalamin treatment has, however, not been completely validated yet in real life, particularly the long-term efficacy [50]. To date, as several authors suggest, oral cobalamin therapy remains one of “medicine's best kept secrets” [51]. Since loading doses of cobalamin far exceed physiologic requirements, clinical responses may result from pharmacologic effects on either cobalamin-related processes or on cellular functions completely unrelated to the known biochemical actions of cobalamin [52]. As a result, blood cobalamin, methylmalonic acid and homocysteine values often fail to predict whether or not a patient will respond to cobalamin therapy [53]. Nevertheless, the following can be proposed: ongoing supplementation until associated disorders are corrected (e.g., by halting the ingestion of the offending medication or exogenosis, or by treating H. pylori infection or pancreatic exocrine failure), lifelong administration or, when applicable, sequential administration [54].
Table 5.
Experience of oral cobalamin therapy for food-cobalamin malabsorption in the university hospital of Strasbourg, France.
| Study characteristics (number of patients) | Therapeutic modalities | Results | |
|---|---|---|---|
| Open prospective study of well-documented cobalamin deficiency related to food-cobalamin malabsorption (n = 10) | Oral crystalline cyanocobalamin: 650 _μ_g per day during at least 3 months | – Normalization of serum cobalamin levels in 80% of the patients | [41] |
| – Significant increase of hemoglobin (Hb) levels (mean of 1.9 g/dL) and decrease of mean erythrocyte cell volume (ECV) (mean of 7.8 fL) | |||
| – Improvement of clinical abnormalities in 20% of the patients | |||
| Open prospective study of low-cobalamin levels not related to pernicious anemia (n = 20) | Oral crystalline cyanocobalamin: between 1000 _μ_g per day during at least 1 week | – Normalization of serum cobalamin levels in 85% of the patients | [42] |
| – No adverse effect | |||
| Open prospective study of well-documented cobalamin deficiency related to food-cobalamin malabsorption (n = 30) | Oral crystalline cyanocobalamin: between 1000 and 250 _μ_g per day during 1 month | – Normalization of serum cobalamin levels in 87% of the patients | [40] |
| – Dose effect: effectiveness dose of cobalamin ≥500 _μ_g per day | |||
| – No adverse effect | |||
| Open prospective study of low-cobalamin levels not related to pernicious anemia (n = 30) | Oral crystalline cyanocobalamin: between 1000 and 125 _μ_g per day during at least 1 week | – Significant increase of Hb levels (mean of 0.6 g/dL) and decrease of ECV (mean of 3 fL); normalization of Hb levels and ECV in 54% and 100% of the patients, respectively | [43] |
| – Normalization of serum cobalamin levels in all patients with at least a dose of vitamin ≥250 _μ_g per day | |||
| – Dose effect: effectiveness dose of cobalamin ≥500 _μ_g per day | |||
| – No adverse effect | |||
| Open prospective study of low cobalamin levels related to pernicious anemia (n = 10) | Oral crystalline cyanocobalamin: 1000 _μ_g per day during at least 3 months | – Significant increase of serum cobalamin levels in 90% of the patients (mean of 117.4 pg/mL) | [47] |
| – Significant increase of Hb levels (mean of 2.45 g/dL) and decrease of ECV (mean of 10.4 fL) | |||
| – Improvement of clinical abnormalities in 30% of the patients |
Acknowledgments
The authors would like to thank Professor Marc Imler and Jean-Louis Schlienger who initiated this work. The research on cobalamin deficiency was supported by a Grant of the Fondation de France (Prix Robert et Jacqueline Zittoun 2004).
References
- 1.Matthews JH. 12 cobalamin and folate deficiency in the elderly. Baillière's Clinical Haematology. 1995;8(3):679–697. doi: 10.1016/s0950-3536(05)80226-4. [DOI] [PubMed] [Google Scholar]
- 2.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76(5):871–881. [PubMed] [Google Scholar]
- 3.Reynolds E. Vitamin B12, folic acid, and the nervous system. The Lancet Neurology. 2006;5(11):949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 4.Carmel R. Current concepts in cobalamin deficiency. Annual Review of Medicine. 2000;51:357–375. doi: 10.1146/annurev.med.51.1.357. [DOI] [PubMed] [Google Scholar]
- 5.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency. A guide for the primary care physician. Archives of Internal Medicine. 1999;159(12):1289–1298. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 6.Zittoun J, Zittoun R. Modern clinical testing strategies in cobalamin and folate deficiency. Seminars in Hematology. 1999;36(1):35–46. [PubMed] [Google Scholar]
- 7.Klee GG. Cobalamin and folate evaluation: measurements of methylmalonic acid and homocystein vs vitamin B12 and folate. Clinical Chemistry. 2000;46:1277–1283. [PubMed] [Google Scholar]
- 8.Andrès E, Federici L, Serraj K, Kaltenbach G. Update of nutritient-deficiency anemia in elderly patients. doi: 10.1016/j.ejim.2008.01.016. European Journal of Internal Medicine. In press. [DOI] [PubMed] [Google Scholar]
- 9.Markle HV, Greenway DC. Cobalamin. Critical Reviews in Clinical Laboratory Sciences. 1996;33(4):247–356. doi: 10.3109/10408369609081009. [DOI] [PubMed] [Google Scholar]
- 10.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. American Journal of Clinical Nutrition. 1994;60(1):2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 11.Hvas A-M, Nexo E. Diagnosis and treatment of vitamin B12 deficiency. An update. Haematologica. 2006;91(11):1506–1512. [PubMed] [Google Scholar]
- 12.van Asselt DZ, Blom HJ, Zuiderent R, et al. Clinical significance of low cobalamin levels in older hospital patients. The Netherlands Journal of Medicine. 2000;57(2):41–49. doi: 10.1016/s0300-2977(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 13.Nicolas JP, Guéant JL. Absorption, distribution et excrétion de la vitamine B12 . Annales de Gastroenterologie et d'Hepatologie. 1994;30(6):270–282. [PubMed] [Google Scholar]
- 14.Andrès E, Affenberger S, Vinzio S, et al. Food-cobalamin malabsorption in elderly patients: clinical manifestations and treatment. The American Journal of Medicine. 2005;118(10):1154–1159. doi: 10.1016/j.amjmed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Andrès E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. Canadian Medical Association Journal. 2004;171(3):251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC, USA: National Academies Press; 1998. [PubMed] [Google Scholar]
- 17.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92(4):1191–1198. [PubMed] [Google Scholar]
- 18.Lane LA, Rojas-Fernandez C. Treatment of vitamin B12-deficiency anemia: oral versus parenteral therapy. The Annals of Pharmacotherapy. 2002;36(7):1268–1272. doi: 10.1345/aph.1A122. [DOI] [PubMed] [Google Scholar]
- 19.Loukili NH, Noel E, Blaison G, et al. Données actuelles sur la maladie de Biermer. À propos d'une étude rétrospective de 49 observations. La Revue de Médecine Interne. 2004;25(8):556–561. doi: 10.1016/j.revmed.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Toh B-H, van Driel IR, Gleeson PA. Pernicious anemia. The New England Journal of Medicine. 1997;337(20):1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 21.Chan JCW, Liu HSY, Kho BCS, et al. Pernicious anemia in Chinese: a study of 181 patients in a Hong Kong hospital. Medicine. 2006;85(3):129–138. doi: 10.1097/01.md.0000224710.47263.70. [DOI] [PubMed] [Google Scholar]
- 22.Carmel R. 10 Malabsorption of food-cobalamin. Baillière's Clinical Haematology. 1995;8(3):639–655. doi: 10.1016/s0950-3536(05)80224-0. [DOI] [PubMed] [Google Scholar]
- 23.Andrès E, Noel E, Kaltenbach G. Carences en vitamine B12 avec test de Schilling normal ou syndrome de non-dissociation de la vitamine B12 de ses protéines porteuses chez le sujet âgé. Etude de 60 patients. La Revue de Médecine Interne. 2003;24(4):218–223. doi: 10.1016/s0248-8663(02)00016-4. [DOI] [PubMed] [Google Scholar]
- 24.Carmel R, Aurangzeb I, Qian D. Associations of food-cobalamin malabsorption with ethnic origin, age, Helicobacter pylori infection, and serum markers of gastritis. The American Journal of Gastroenterology. 2001;96(1):63–70. doi: 10.1111/j.1572-0241.2001.03453.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaptan K, Beyan C, Ural AU, et al. Helicobacter pylori—Is it a novel causative agent in vitamin B12 deficiency? Archives of Internal Medicine. 2000;160(9):1349–1353. doi: 10.1001/archinte.160.9.1349. [DOI] [PubMed] [Google Scholar]
- 26.Howden CW. Vitamin B12 levels during prolonged treatment with proton pump inhibitors. Journal of Clinical Gastroenterology. 2000;30(1):29–33. doi: 10.1097/00004836-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Andrès E, Noel E, Ben Abdelghani M. Vitamin B12 deficiency associated with chronic acid suppression therapy. The Annals of Pharmacotherapy. 2003;37(11):p. 1730. doi: 10.1345/aph.1D189. [DOI] [PubMed] [Google Scholar]
- 28.Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. The American Journal of Medicine. 1998;104(5):422–430. doi: 10.1016/s0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 29.Jensen RT. Consequences of long-term proton pump blockade: insights from studies of patients with gastrinomas. Basic & Clinical Pharmacology & Toxicology. 2006;98(1):4–19. doi: 10.1111/j.1742-7843.2006.pto_378.x. [DOI] [PubMed] [Google Scholar]
- 30.Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care. 2000;23(9):1227–1231. doi: 10.2337/diacare.23.9.1227. [DOI] [PubMed] [Google Scholar]
- 31.Andrès E, Noel E, Goichot B. Metformin-associated vitamin B12 deficiency. Archives of Internal Medicine. 2002;162(19):2251–2252. doi: 10.1001/archinte.162.19.2251-a. [DOI] [PubMed] [Google Scholar]
- 32.Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age and Ageing. 2006;35(2):200–201. doi: 10.1093/ageing/afj042. [DOI] [PubMed] [Google Scholar]
- 33.Andrès E, Goichot B, Perrin A-E, Vinzio S, Demangeat C, Schlienger J-L. Sjögren's syndrome: a potential new aetiology of mild cobalamin deficiency. Rheumatology. 2001;40(10):1196–1197. doi: 10.1093/rheumatology/40.10.1196. [DOI] [PubMed] [Google Scholar]
- 34.Dharmarajan TS, Adiga GU, Norkus EP. Vitamin B12 deficiency: recognizing subtle symptoms in older adults. Geriatrics. 2003;58(3):30–38. [PubMed] [Google Scholar]
- 35.Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clinical and Laboratory Haematology. 2006;28(1):50–56. doi: 10.1111/j.1365-2257.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 36.Carmel R, Sarrai M. Diagnosis and management of clinical and subclinical cobalamin deficiency: advances and controversies. Current Hematology Reports. 2006;5(1):23–33. doi: 10.1007/s11901-006-0019-7. [DOI] [PubMed] [Google Scholar]
- 37.Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. Journal of Nutrition, Health and Aging. 2002;6(4):254–260. [PubMed] [Google Scholar]
- 38.Elia M. Oral or parenteral therapy for B12 deficiency. The Lancet. 1998;352(9142):1721–1722. doi: 10.1016/S0140-6736(05)79821-4. [DOI] [PubMed] [Google Scholar]
- 39.Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca S, Senturk T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clinical Therapeutics. 2003;25(12):3124–3134. doi: 10.1016/s0149-2918(03)90096-8. [DOI] [PubMed] [Google Scholar]
- 40.Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clinical and Laboratory Haematology. 2003;25(3):161–166. doi: 10.1046/j.1365-2257.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- 41.Andrès E, Kurtz J-E, Perrin A-E, et al. Oral cobalamin therapy for the treatment of patients with food-cobalamin malabsorption. The American Journal of Medicine. 2001;111(2):126–129. doi: 10.1016/s0002-9343(01)00792-6. [DOI] [PubMed] [Google Scholar]
- 42.Kaltenbach G, Noblet-Dick M, Andrès E, et al. Réponse précoce au traitement oral par vitamine B12 chez des sujets âgés hypovitaminiques. Annales de Medecine Interne. 2003;154(2):91–95. [PubMed] [Google Scholar]
- 43.Andrès E, Kaltenbach G, Noblet-Dick M, et al. Hematological response to short-term oral cyanocobalamin therapy for the treatment of cobalamin deficiencies in elderly patients. Journal of Nutrition, Health and Aging. 2006;10(1):3–6. [PubMed] [Google Scholar]
- 44.Butler CC, Vidal-Alaball J, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Journal of Family Practice. 2006;23(3):279–285. doi: 10.1093/fampra/cml008. [DOI] [PubMed] [Google Scholar]
- 45.Slot WB, Merkus FW, Van Deventer SJ, Tytgat GN. Normalization of plasma vitamin B12 concentration by intranasal hydroxocobalamin in vitamin B12-deficient patients. Gastroenterology. 1997;113(2):430–433. doi: 10.1053/gast.1997.v113.pm9247460. [DOI] [PubMed] [Google Scholar]
- 46.van Asselt DZB, Merkus FW, Russel FGM, Hoefnagels WHL. Nasal absorption of hydroxocobalamin in healthy elderly adults. British Journal of Clinical Pharmacology. 1998;45(1):83–86. doi: 10.1046/j.1365-2125.1998.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrès E, Loukili NH, Noel E, et al. Effects of oral crystalline cyanocobalamin 1000 μg/d in the treatment of pernicious anemia: an open-label, prospective study in ten patients. Current Therapeutic Research. 2005;66(1):13–22. doi: 10.1016/j.curtheres.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidal-Alaball J, Butler CC, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database of Systematic Reviews. 2005;20(3) doi: 10.1002/14651858.CD004655.pub2. Article ID CD004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eussen SJPM, de Groot LCPGM, Clarke R, et al. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trial. Archives of Internal Medicine. 2005;165(10):1167–1172. doi: 10.1001/archinte.165.10.1167. [DOI] [PubMed] [Google Scholar]
- 50.Roth M, Orija I. Oral vitamin B12 therapy in vitamin B12 deficiency. The American Journal of Medicine. 2004;116(5):p. 358. doi: 10.1016/j.amjmed.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 51.Graham ID, Jette N, Tetroe J, Robinson N, Milne S, Mitchell SL. Oral cobalamin remains medicine's best kept secret. Archives of Gerontology and Geriatrics. 2007;44(1):49–59. doi: 10.1016/j.archger.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Solomon LR. Disorders of cobalamin (vitamin B12) metabolismml: emerging concepts in pathophysiology, diagnosis and treatment. Blood Reviews. 2007;21(3):113–130. doi: 10.1016/j.blre.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Solomon LR. Cobalamin-responsive disorders in the ambulatory care setting: unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood. 2005;105(3):978–985. doi: 10.1182/blood-2004-04-1641. [DOI] [PubMed] [Google Scholar]
- 54.Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. Journal of Family Practice. 2007;56(7):537–542. [PubMed] [Google Scholar]