The Natural History of the Non-Nephrotic Membranous Nephropathy Patient (original) (raw)
Abstract
Background and objectives: Although early studies suggest that patients with idiopathic membranous nephropathy (MGN) and subnephrotic range proteinuria overall do well, these studies were small and follow-up was short or difficult to discern.
Design, setting, participants, & measurements: Three hundred ninety-five cases of idiopathic MGN with at least 12 mo of follow-up from the Toronto Glomerulonephritis Registry were reviewed to determine the outcome of the subgroup of patients that presented with subnephrotic range proteinuria. Onset and follow-up data included mean arterial pressure (MAP) and creatinine clearance (CrCl) as determined by the Cockcroft-Gault equation. Outcome variables included the rate of progression (slope of CrCl), 50% reduction in initial CrCl, and end-stage renal disease (ESRD).
Results: One hundred eight (27% of the total) patients presented with subnephrotic proteinuria and almost 40% (42 of 108) of this subgroup remained subnephrotic. Their long-term slope was −0.93 ml/min/yr. In contrast, those who subsequently developed nephrotic range proteinuria had a progression rate almost four times faster (−3.52 ml/min/yr). The majority who developed nephrotic syndrome did so within the first year of follow-up. The only distinguishing baseline feature between the two groups was a higher level of urine protein in the group that subsequently developed nephrotic syndrome (1.98 [0.3 to 3.4] versus 2.43 [0.5 to 3.4] g/d).
Conclusions: Patients with MGN and sustained subnephrotic range proteinuria have an excellent prognosis. Conservative management with close monitoring is recommended given the difficulty predicting which patients will develop nephrotic range proteinuria and then progress more rapidly.
Idiopathic membranous nephropathy (MGN) remains the most common cause of adult onset nephrotic syndrome. The natural history of the disease is stated to ascribe to the rule of thirds, wherein approximately one-third of the affected have a complete and spontaneous remission of their proteinuria, one-third enter a partial remission with subnephrotic range proteinuria, and a final third remain nephrotic and progress to end-stage renal disease (ESRD) (1–4). Studies utilizing multivariate analysis techniques have identified clinical predictors of poor renal survival. These include older age, male gender, and elevated serum creatinine at the time of diagnosis as well as the severity of proteinuria at the time of disease onset and during follow-up (5–10). However, despite these known predictors, the long-term outcome is variable with the 10 yr renal survival ranging from 60 to 80% (1–4). Thus, the optimal strategy for the management of patients with MGN has remained unclear with varying opinions emerging in the literature (11–13).
This variation in opinion extends to the MGN patient with subnephrotic range proteinuria. Although there exists data to suggest these patients overall do well, previous natural history studies included only small numbers of this subset of patients, often with limited follow-up or inadequate details with respect to their clinical course. In particular, the time course for evolution to nephrotic range proteinuria was rarely defined (2,4,6,14–17). The largest natural history study was published in 1979 and included 116 untreated patients with MGN, of which 28 (24.2%) presented with subnephrotic range proteinuria. However, the outcome for this subgroup of patients was not described, and almost 25% of the patients were followed for less than 1 yr (2). In other studies, between 15 and 46% of patients presented with subnephrotic range proteinuria (6,14,17). In the largest of these reports, 19% entered a complete remission, 21% had persistent subnephrotic range proteinuria, and only 6% progressed to nephrotic syndrome (17). This study, along with smaller studies that included a total of only 42 patients presenting with subnephrotic range proteinuria, noted an excellent renal survival as long as proteinuria did not progress (4,14,15). Progression to nephrotic range proteinuria was noted to be rare, occurring in only four of 42 patients (10%) (4,15,16). In addition, the time course for the evolution to nephrotic range proteinuria could not be determined in these early studies, and all were published before the development of the classes of drugs that block the renin angiotensin system (RAS), which could potentially alter disease progression.
We describe the long-term outcome of the largest cohort to date of patients with idiopathic MGN who present with subnephrotic proteinuria. This cohort was followed prospectively, allowing for the long-term assessment of these patients, including those that evolved to nephrotic range proteinuria, and compares these patients to the classic MGN patient who presents with nephrotic syndrome. Based on these data, a management strategy is suggested for this cohort.
Materials and Methods
Study Population
The Toronto Glomerulonephritis Registry was initiated in 1974 and includes all cases of biopsy-proven glomerulonephritis from the Toronto area. We identified 395 patients with idiopathic MGN who had at least 12 mo of follow-up. One hundred eight patients were identified at the time of biopsy (or presentation) to have subnephrotic proteinuria, <3.5 g/d. Data were prospectively collected at 6 to 12 mo intervals and compiled on standardized forms that included assessments of the patient's clinical status, medications, and laboratory parameters.
Data Collected
Demographic variables included age at biopsy, race, and gender. Initial and follow-up values included assessment of BP, serum creatinine, albumin, and total cholesterol as well as 24 h urine for total protein and creatinine. Also recorded was exposure to immunosuppressive agents and BP medications including the angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blocker (ARB) classes of antihypertensive medications. Kidney biopsy data included the degree of interstitial fibrosis, tubular atrophy, vascular sclerosis, and the presence or absence of glomerular sclerotic lesions on light microscopy, as well as the stage of MGN on electron microscopy.
Definitions
Creatinine clearance (CrCl) was adjusted for age, gender, and weight using the Cockcroft-Gault equation. An average mean arterial pressure (MAP) and total urine protein were determined for each 6-mo period of follow-up. Thus, the reported follow-up MAP and total urine protein is the average of the 6-mo means. Nephrotic range proteinuria was defined as a 24 h urine total protein value ≥3.5 g/d. A complete remission was defined as a urine protein ≤0.3 g/d in a previously nephrotic patient. A partial remission was defined as a 50% reduction in urine protein and to a level of proteinuria <3.5 g/d. Exposure to immunosuppressive agents and BP medications are reported as intent to treat regardless of the duration of exposure. However, a minimum dose of 10 mg of prednisone, 1.5 mg/kg of cyclosporine or azathioprine, 1 mg/kg of cyclophosphamide, or 0.15 mg/kg of chlorambucil for a duration of at least 1 mo was necessary to satisfy the criteria for immunosuppressive therapy, whereas therapy with ACEi or ARB is presented as any exposure irrespective of dose and time prescribed. Renal failure was defined as a CrCl <15 ml/min, initiation of dialysis or a renal transplantation. Slope refers to the loss of kidney function in ml/min/yr determined by plotting the calculated CrCl values against observation time and includes all measurements from the time of presentation. Treatment eras in relation to ACEi/ARB therapy and immunosuppression were defined by the time of biopsy, i.e., before 1980 (Era 1), from 1980 to 1990 (Era 2), and after 1990 (Era 3). Grading of the renal biopsy tissue was completed independently by a renal pathologist and two of the study authors (ST, DC) who were blinded to the clinical data, as described previously (18).
Statistical Analyses
Continuous variables were assessed for normality both visually (normal probability plot) and by inferential statistics (Shapiro-Wilk W and Kolmogorov-Smirnov tests). Normally distributed variables were expressed as a mean ± SD, and nonparametric variables were expressed as a median and range. For continuous variables, either a t test or the Wilcoxon Rank-Sum test was utilized to assess for significant differences among groups where appropriate. Categorical variables were expressed as a percentage and compared using the chi-square test. The rate of renal function decline or the slope was determined by simple regression. Renal survival was compared by Kaplan-Meier curves and the log-rank test.
Results
Three hundred ninety-five patients with idiopathic MGN with at least 12 mo of follow-up were included. The majority of the cohort was male (66%) and Caucasian (79%). The average age of onset for the entire cohort was 48 ± 16 yr. The majority of the cohort, 287 patients, presented with nephrotic range proteinuria and preserved kidney function with a median proteinuria of 7.4 (3.6 to 31.3) g/d and an average CrCl of 85 ± 35 ml/min. The outcome of this cohort has been previously published (8). Our focus was the 108 patients (27%) who presented with subnephrotic range proteinuria. Sixty-six of these patients subsequently developed nephrotic range proteinuria, but 42 patients, representing 39% of this cohort and 11% of the total MGN population, remained with low-level, subnephrotic range proteinuria through the observation period. The 42 patients who never reached nephrotic levels were followed over a median observation period of 55 mo (group 1). The 66 patients, who presented with subnephrotic range proteinuria, but who developed nephrotic range proteinuria, were monitored over a median follow-up period of 82 mo (group 2). The outcomes of these groups are compared with the 287 MGN patients who presented with nephrotic range proteinuria.
The baseline characteristics, follow-up and outcomes for the never nephrotic (group 1) and the nephrotic post presentation group (group 2) are summarized in Table 1. There were no distinguishing baseline features between the two groups, with the exception of a statistically significant higher level of baseline proteinuria in the group that subsequently developed nephrotic syndrome with a median of 2.43 g/d compared with a median of 1.98 g/d in the never nephrotic group. Baseline creatinine clearance as well as baseline and follow-up MAP were not significantly different nor were the mean number of BP medications. The number of months wherein the patient was considered to have been on a maximal dose of either ACEi or ARB ranged from 0 to 75 mo in the never nephrotic group (group 1) and 0 to 81 mo in the nephrotic postpresentation group (group 2), with approximately twice as many patients receiving RAS blockade in group 2. With respect to immunosuppression, approximately half of the patients that had nephrotic range proteinuria at some time during the course of their illness received immunosuppression compared with 19% in the never nephrotic group. Immunosuppression utilized in the never nephrotic group included prednisone in six patients, azathioprine in two patients, and alkylating agents in three patients. Three of these patients received more than one form of immunosuppression, either prednisone in combination with azathioprine or an alkylating agent. In the group that later developed nephrotic syndrome (group 2), 30 patients had a least 1 mo of exposure to prednisone, one patient was treated with azathioprine, nine with alkylating agents, and six with cyclosporine. Again, combinations of immunosuppressive therapy were utilized in eight of these patients. Almost all were treated following conversion from subnephrotic to nephrotic range proteinuria (only three patients received prednisone while subnephrotic).
Table 1.
Baseline characteristics, follow-up, and outcome data for the never nephrotic (group 1) and nephrotic post presentation (group 2) patients
| Never Nephrotic | Nephrotic Post Presentation | |
|---|---|---|
| Number | 42 | 66 |
| Follow-up (months) | 55 (12 to 334) | 82 (13 to 284) |
| Baseline | – | – |
| female (%) | 55 | 41 |
| age (years) | 43 ± 18 | 46 ± 14 |
| MAP (mmHg) | 96 ± 15 | 99 ± 12 |
| CrCl (ml/min) | 84 ± 34 | 87 ± 30 |
| protein (g/day) | 1.98 (0.3 to 3.4) | 2.43 (0.5 to 3.4)a |
| serum albumin (g/L) | 33 ± 7 | 30 ± 6 |
| Follow-Up | – | – |
| proteinuria (g/day) | 1.1 (0.3 to 3.4) | 3.2 (0.6 to 12.3)a |
| complete remission (%) | NA | 32 |
| partial remission (%) | NA | 39 |
| MAP (mmHg) | 94 ± 11 | 98 ± 8 |
| number BP Meds | 0 (0 to 2.75) | 0.4 (0 to 2.9) |
| ACEI/ARB (%) | 24 | 42a |
| immunosupression (%) | 19 | 56a |
| Outcome | – | – |
| slope (ml/min/yr) | −0.93 ± 4.26 | −3.52 ± 7.47a |
| Δ50% CrCl (%) | 5 | 12 |
| renal failure (%) | 2 | 3 |
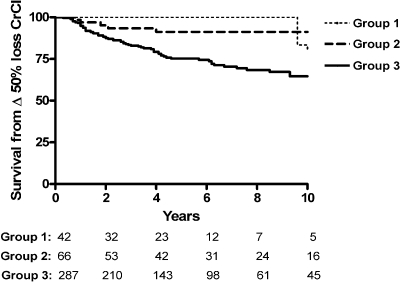
The slope or rate of loss of renal function was significantly lower in group 1 (−0.93 ± 4.26 ml/min/yr) compared with the group that subsequently developed nephrotic range proteinuria (−3.52 ± 7.47 ml/min/yr). Only 5%, or two of 42 patients, lost in excess of 50% of their kidney function over 10 yr of follow-up (Figure 1), with only one of these patients progressing to ESRD over a period of 154 mo. This patient presented with a low CrCl (34 ml/min). Thus, his rate of progression to ESRD (slope −0.72 ml/min/yr) was within the range of the mean for this group. The other patient displayed a more aggressive course, with a normal baseline CrCl, but uncontrolled hypertension at presentation and throughout the course of follow-up. His rate of loss of renal function was −5.88 ml/min/yr and not compatible with this MGN disease group. Presumably, severe uncontrolled hypertension factored prominently in his accelerated rate of progression.
Figure 1.
Survival from 50% loss in kidney function over 10 yr comparing the never nephrotic group (group 1), nephrotic post presentation (group 2), and nephrotic at presentation (group 3); P = 0.0001.
In contrast, despite presenting with subnephrotic range proteinuria, the rate of progression in the group who subsequently developed nephrotic range proteinuria was not significantly different from the classic nephrotic syndrome at presentation (−3.52 ± 7.47 and −4.76 ± 9.85 ml/min/yr, respectively, P = ns). A greater proportion of group 2 patients lost in excess of 50% of their kidney function over 10 yr of follow-up (Figure 1) including two patients who progressed to ESRD. The first patient presented with normal kidney function (baseline CrCl was 99 ml/min) and 1.4 g/d proteinuria, but within one month had >5 g/d and progressed to ESRD over a period of 28 mo (slope −32.4 ml/min/yr). The second patient presented with 3.3 g/d, but became nephrotic with over 10 g/d of proteinuria within 4 mo and progressed to ESRD over 39 mo (slope −33.6 ml/min/yr). Neither patient had sclerosis, fibrosis, or tubular atrophy on their initial biopsy. In those patients who presented with nephrotic range proteinuria, 42 of 287 (15%) progressed to ESRD over a similar observation period.
Biopsy features of patients that never developed nephrotic range proteinuria are presented in Table 2. The vast majority of the patients did not have significant interstitial fibrosis, tubular atrophy, or vascular arteriosclerosis. The percent of global or partial glomerulosclerosis in this group was not significantly different from the percentages noted in the group who subsequently developed nephrotic range proteinuria. By electron microscopy, the majority of the patients were classified as either stage 2 (35%) or 3 (38%) membranous nephropathy (stage 1, 9%; stage 4, 15%). There was no indication of either a changing incidence or a change in biopsy practice in patients presenting with subnephrotic range proteinuria over time, since these patients accounted for 27% of the renal biopsies performed in Eras 1 and 2, and 29% of the renal biopsies performed in Era 3.
Table 2.
Presentation biopsy findings in patients with persistent subnephrotic proteinuria (group 1)
| Parameter | SCORE (%)a | |||
|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |
| Interstitial Disease | 49% | 37% | 5% | 7% |
| Tubular Atrophy | 49% | 39% | 2% | 7% |
| Vascular Sclerosis | 64% | 17% | 11% | 6% |
As might be expected, utilization of conservative therapy including ACEi and ARB increased over time with only 16% of the entire cohort receiving these therapies in the first era compared with 77% by Era 3 (P < 0.001). Examination of the entire cohort revealed significantly higher rates of immunosuppression use over time, with 47% of patients receiving some form of immunosuppression during Era 1, increasing to 61% by Era 3 (P < 0.001). Furthermore, the use of cytotoxic-based regimens increased over time while the use of prednisone alone decreased. Specifically, the never nephrotic group (group 1) did not show a statistically significant decrease or increase over time with respect to immunosuppression, but did reveal a significantly increased utilization of conservative therapy over time, with 7% of patients receiving ACEi/ARB during Era 1, 15% during Era 2, and 75% during Era 3 (P < 0.001). However, despite the increasing use of ACEi and/or ARBs, the percentage of patients who presented with subnephrotic range proteinuria progressing to nephrotic range proteinuria did not change significantly from Era 1 to 3.
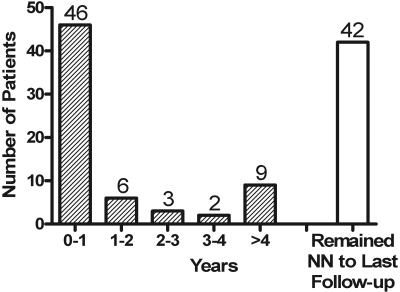
Figure 2 depicts the time course for the 66 patients who progressed from subnephrotic to nephotic range proteinuria. The majority, 70% (46 of 66), did so within the first year of follow-up with fewer and fewer progressing to that level of proteinuria over the subsequent 4-yr observation period, with only six of 66 (9%) progressing in year two, three of 66 (5%) progressing in year three, and two of 66 (3%) progressing in year four. Another nine patients (14%) did progress beyond 4 yr of follow-up, but 42 of 108 patients never progressed to nephrotic range proteinuria. The observation period in the later group spanned 6 to 20 yr. In the group that reached nephrotic range proteinuria, subsequent evaluation revealed 21 patients had a complete remission and 26 patients had a partial remission. The proportions of patients who entered a remission paralleled the history of those patients who were nephrotic at presentation.
Figure 2.
Time (years) for progression from non-nephrotic to nephrotic range proteinuria.
Discussion
As the most common cause of adult onset nephrotic syndrome, idiopathic MGN is most typically described as presenting with nephrotic range proteinuria (1,4). In our study, however, over one quarter (27%) of patients with idiopathic MGN presented with subnephrotic range proteinuria with almost 40% of these patients (11% of the total MGN patient population) never increasing beyond this level despite many years of observation. This higher than expected percentage of never nephrotic MGN is not likely to reflect changes in local biopsy policy since the percentage of patients in this subgroup did not change over the different eras of observation. Furthermore, the percentage is very similar to that reported by Noel et al. in his 1979 natural history study of 116 untreated patients wherein 24.2% presented with subnephrotic range proteinuria (2). On the contrary, one could postulate that this group is actually underrepresented and may be significantly larger than we observed, given that nephrologists may limit biopsies to patients with indicators of more active disease, including impaired or progressive renal dysfunction, nephrotic range proteinuria, and/or an active urine sediment, none of which was seen in this cohort (19–21).
Overall, these patients with idiopathic MGN and persistent low-grade proteinuria have a very favorable long-term prognosis. Presenting identifying characteristics (versus group 2) are few with the exception of a lower median urine protein at presentation. Although statistically significant, it is difficult to reconcile a less than 500 mg median difference between those who maintain low level proteinuria and those who convert to nephrotic range proteinuria as a clinically relevant useful predictive tool.
Female patients represented in excess of 50% of the never nephrotic group. Although this is consistent with the literature, suggesting males have a worse renal survival (5,7), it is important to note that despite a lower percentage of males within this group, their rate of progression was not significantly different from the females. This is consistent with a recent analysis from our group, wherein we demonstrated that progression (slope of CrCl) is not dependent on sex in MGN except when urine protein persistently exceeds 8 g/d (22).
Contrary to the findings from previous studies, wherein age and initial creatinine clearance predicted an adverse outcome in multivariate analysis (5,10), no significant differences between the groups were noted in these parameters at presentation. There were also no distinguishing pathologic features. This variance from the literature is at least in part explained by our recent analysis of patients with nephrotic range proteinuria and MGN. We demonstrated that pre-existing patient factors, including age and BP, resulted in a lower creatinine clearance at presentation. This in turn was associated with histologic findings suggestive of underlying chronic damage including increased tubulointerstitial disease, vascular sclerosis, and secondary focal and segmental glomerulosclerosis (FSGS) lesions (18). These findings translated into reduced survival rates because the typically older, hypertensive MGN patient started lower on the survival curve (lower CrCl at presentation), not necessarily because the patient had more aggressive disease. Thus, in addition to assessing renal survival, our recent studies have focused on the rate of deterioration in renal function as measured by the slope of creatinine clearance (8,18).
Our data confirm the dominance of the level of persisting proteinuria on progression. In group 1, the rate of deterioration was negligible (−0.08 ± 0.35 ml/min/mo) and indistinguishable from our earlier data in MGN patients who have sustained a complete remission (−0.12 ± 0.40 ml/min/mo) (8). The solitary patient in our cohort of 42 patients with persistent low-grade proteinuria who did progress to ESRD did so over a period of almost 13 yr and his rate of deterioration was the same as the rest of the cohort. In this case, his progression to ESRD was more clearly related to a low presenting creatinine clearance (34 ml/min) rather than an unusual rate of disease progression. His biopsy revealed evidence of chronic damage including interstitial fibrosis, tubular atrophy, and vascular damage confirming underlying chronic damage. It is possible that his membranous nephropathy was present for years before diagnosis or that he had significant chronic kidney injury from a cause other than his membranous nephropathy. The importance of urine protein is also clearly seen in the patients initially in group 1 who developed nephrotic range proteinuria. Their rate of progression subsequently increased significantly and did not differ appreciably from those who initially presented with nephrotic syndrome.
As expected, our era comparison did reveal that conservative therapy with ACEI or ARB is being utilized more over time for the management of proteinuria. Although obviously not a prospective randomized trial, there was no indication that the introduction of ACEI or ARB therapy has altered the incidence or the percentage of patients who progress to nephrotic range proteinuria in those with MGN presenting with low-level proteinuria. Given the very favorable outcome in the group of patients with persistently subnephrotic range proteinuria, there appears to be no indication for immunosuppressive therapies as the risk is likely to outweigh any benefit. The small, but steady, percentage that continued to be exposed to immunosuppression over the observation period would suggest that this message needs to be emphasized. Again, although not proven by this study, conservative therapy including treatment of hypertension to lower BP targets (<130/80 mmHg) along with smoking cessation and treatment of hypercholesterolemia to LDL targets <2 mmol/L would seem prudent given the established risk factor of proteinuria in cardiovascular disease (23–25).
Finally, close follow-up of these patients remains important, as a significant proportion of the patients who present with lower levels of proteinuria will evolve to nephrotic range proteinuria and then follow a course similar to the classic nephrotic-at-presentation group. Although the majority that do evolve to this state do so within one year of biopsy (70%) (Figure 1), the remaining 30% evolved over a much longer time frame. Since after conversion their progression parallels those presenting with nephrotic syndrome, all these patients require the same careful approach with respect to subsequent monitoring and therapy.
In summary, a significant percentage of MGN patients present with subnephrotic range proteinuria. A significant proportion will maintain this low level persistently over time and will rarely if ever progress due to their MGN compared with those who present with or develop nephrotic range proteinuria. Observation and conservative management is all that is required in the care of these patients, and immunosuppression with its inherent complications perhaps is best avoided. Despite the introduction of new BP targets and anti-proteinuric agents (RAS blockade), the incidence or progression rate of this cohort does not appear to have been altered. Careful ongoing follow-up is warranted because those that do convert to higher grades of proteinuria have the same outcome as the nephrotic MGN patient.
Disclosures
None.
Acknowledgments
This work was supported by funding from the Canadian Institute of Health Research (CIHR) – N New Emerging Team “Genes, Gender, and Glomerular-based Diseases.” Dr. MA Hladunewich's salary was supported by the Bayer/Canadian Hypertension Society CIHR Clinical Scholarship Research Award. We thank the glomerulonephritis registrars Naomi Ryan and Paul Ling for help in collection and management of data and the following nephrologists for contributing their patients and support to the registry: Drs Shelley Albert, Joanne Bargman, Murray Berall, William Berry, Jason Bornstein, George Buldo, Carl Joseph Cardella, Chris Chan, Stephen Chow, Edward Herman Cole, Sandra Donnelly, Ivan Otto Elkan, Samuel Stanley Alexander Fenton, Marc Berel Goldstein, Richard Goluch, Gavril Hercz, Marion Ruth Hockley, Vanita Jassal, Kamel Kamel, Amrit Kang, Stavros Y Karanicolas, Doh Kim, Leo Lam, Alexander Gordon Logan, Charmaine Elizabeth Lok, Philip McFarlane, Arifie Manuel, Hitesh Mehta, David Mendelssohn, Judith Ann Miller, David Naimark, Bharat Nathoo, Paul Sun-Ying Ng, Matthew Oliver, Dimitrios George Oreopoulos, Sanjaya Pandeya, Roopa Prasad, Andreas Pierattos, Vasillios Poulopoulos, York Pei, Bajinder Reen, Robert Murray Richardson, Janet Roscoe, Douglas Ryan, Jasdip Sachdeva, Carl Shaffiet-Ali Saiphoo, Daniel Sapir, Joanna Sasal, James William Scholey, Martin Schreiber, Melvin Silverman, Andrew Steele, Esther Szaky, Paul Tam, Robert Ting, Sheldon Tobe, Donald Steward Thompson, Arturo Wadgymar, Leonard Warner, Charles Wei, Catharine Whiteside, Gordon Wong, George Wu, and Jeffery Zaltzman, and participating pathologists Timothy Feltis, Andrew M Herzenberg, Serge Jothy, Ginette Lajoie, Christopher Sherman, Linda Sugar, and Joan Sweet. This study was supported in part by a grant from the Canadian Institutes of Health Research, Net Grant on Genes, Gender and Glomerulonephritis (no. 452773L). This work was presented in part as a poster at the ASN in 2003 (Renal Week; San Diego, CA).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.MacTier R, Boulton Jones JM, Payton CD, McLay A: The natural history of membranous nephropathy in the West of Scotland. Q J Med 60: 793–802, 1986 [PubMed] [Google Scholar]
- 2.Noel LH, Zanetti M, Droz D, Barbanel C: Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med 66: 82–90, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Zucchelli P, Ponticelli C, Cagnoli L, Passerini P: Long-term outcome of idiopathic membranous nephropathy with nephrotic syndrome. Nephrol Dial Transplant 2: 73–78, 1987 [PubMed] [Google Scholar]
- 4.Davison AM, Cameron JS, Kerr DN, Ogg CS, Wilkinson RW: The natural history of renal function in untreated idiopathic membranous glomerulonephritis in adults. Clin Nephrol 22: 61–67, 1984 [PubMed] [Google Scholar]
- 5.Shiiki H, Saito T, Nishitani Y, Mitarai T, Yorioka N, Yoshimura A, Yokoyama H, Nishi S, Tomino Y, Kurokawa K, Sakai H: Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int 65: 1400–1407, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Marx BE, Marx M: Prediction in idiopathic membranous nephropathy. Kidney Int 56: 666–673, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Tu WH, Petitti DB, Biava CG, Tulunay O, Hopper J, Jr.: Membranous nephropathy: Predictors of terminal renal failure. Nephron 36: 118–124, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC: Idiopathic membranous nephropathy: Definition and relevance of a partial remission. Kidney Int 66: 1199–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Pei Y, Cattran D, Greenwood C: Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int 42: 960–966, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 12.du Buf-Vereijken PW, Feith GW, Hollander D, Gerlag PG, Wirtz JJ, Noordzij TC, Wetzels JF: Restrictive use of immunosuppressive treatment in patients with idiopathic membranous nephropathy: High renal survival in a large patient cohort. Qjm 97: 353–360, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Torres A, Dominguez-Gil B, Carreno A, Hernandez E, Morales E, Segura J, Gonzalez E, Praga M: Conservative versus immunosuppressive treatment of patients with idiopathic membranous nephropathy. Kidney Int 61: 219–227, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Honkanen E, Tornroth T, Gronhagen-Riska C, Sankila R: Long-term survival in idiopathic membranous glomerulonephritis: Can the course be clinically predicted? Clin Nephrol 41: 127–134, 1994 [PubMed] [Google Scholar]
- 15.Erwin DT, Donadio JV, Jr., Holley KE: The clinical course of idiopathic membranous nephropathy. Mayo Clin Proc 48: 697–712, 1973 [PubMed] [Google Scholar]
- 16.Donadio JV, Jr., Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M, Ilstrup DM, Chu CP: Idiopathic membranous nephropathy: The natural history of untreated patients. Kidney Int 33: 708–715, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Murphy BF, Fairley KF, Kincaid-Smith PS: Idiopathic membranous glomerulonephritis: Long-term follow-up in 139 cases. Clin Nephrol 30: 175–181, 1988 [PubMed] [Google Scholar]
- 18.Troyanov S, Roasio L, Pandes M, Herzenberg AM, Cattran DC: Renal pathology in idiopathic membranous nephropathy: A new perspective. Kidney Int 69: 1641–1648, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP: The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Covic A, Schiller A, Volovat C, Gluhovschi G, Gusbeth-Tatomir P, Petrica L, Caruntu ID, Bozdog G, Velciov S, Trandafirescu V, Bob F, Gluhovschi C: Epidemiology of renal disease in Romania: A 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 21: 419–424, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rivera F, Lopez-Gomez JM, Perez-Garcia R: Clinicopathologic correlations of renal pathology in Spain. Kidney Int 66: 898–904, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW: The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant 23: 2247–2253, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, Kanashiki M, Saito Y, Ota H, Nose T: The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 69: 1264–1271, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M: Proteinuria and metabolic syndrome as predictors of cardiovascular death in non-diabetic and type 2 diabetic men and women. Diabetologia 49: 56–65, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Tanihara S, Hayakawa T, Oki I, Nakamura Y, Sakata K, Okayama A, Fujita Y, Ueshima H: Proteinuria is a prognostic marker for cardiovascular mortality: NIPPON DATA 80, 1980–1999. J Epidemiol 15: 146–153, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]