Change in Periodontitis during Pregnancy and Risk of Preterm Birth and Low Birthweight (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 1.
Abstract
Aim
Determine if periodontitis progression during pregnancy is associated with adverse birth outcomes.
Materials and Methods
We used clinical data and birth outcomes from the OPT Study, which randomized women to receive periodontal treatment before 21 weeks gestation (N=413) or after delivery (410). Birth outcomes were available for 812 women and follow-up periodontal data for 722, including 75 whose pregnancies ended <37 weeks. Periodontitis progression was defined as ≥ 3mm loss of clinical attachment. Birth outcomes were compared between non-progressing and progressing groups using the log rank and t tests, separately in all women and in untreated controls.
Results
The distribution of gestational age at the end of pregnancy (P > 0.1) and mean birthweight (3295 versus 3184 grams, P = 0.11) did not differ significantly between women with and without disease progression. Gestational age and birthweight were not associated with change from baseline in percent of tooth sites with bleeding on probing or between those who did versus did not progress according to a published definition of disease progression (P > 0.05).
Conclusions
In these women with periodontitis and within this study’s limitations, disease progression was not associated with increased risk for delivering a preterm or low birthweight infant.
Clinical Relevance
Scientific Rationale
Maternal periodontitis and disease progression during pregnancy have been associated with elevated risk for preterm birth. We used data from a recent clinical trial to explore possible associations between progressive periodontitis and birth outcomes.
Principal Findings
The distribution of gestational age at delivery and mean birthweights did not differ significantly between women who experienced progressive periodontitis and those who did not.
Clinical Implication
While it is important to treat dental diseases, including periodontitis, during pregnancy, women whose periodontal condition worsens during pregnancy are not at elevated risk for adverse pregnancy outcomes.
Keywords: preterm birth, low birthweight, periodontal disease, pregnancy, disease progression
INTRODUCTION
Many studies have reported an association between maternal periodontal disease and risk for adverse pregnancy outcomes (see, e.g., Vergnes et al. 2007; Xiong et al. 2006). A recent meta-analyses concluded that women with periodontitis are approximately 2 to 3 times more likely than periodontally healthy women to deliver a preterm, low birthweight or preterm and low birthweight infant (Vettore et al. 2006). The nature and consistency of the association, however, continues to be debated (Michalowicz et al. 2007, Vettore et al. 2006, Vettore et al. 2008). Isolating the effects of periodontitis on birth outcomes is difficult because of the multifactorial nature of these outcomes (Goffinet 2005). Preterm birth and periodontitis also share several important risk factors such as low socioeconomic status, smoking and black race.
It is possible that the fetal-placental unit in women with progressive periodontitis is exposed to inflammatory mediators that precipitate preterm labor and delivery. For example, aggressive periodontitis has been associated with elevated levels of interleukin-6 in saliva (Aurer et al. 1999). When elevated in the serum or amniotic fluid, II-6 predicts preterm labor and delivery (Greci et al. 1998, von Minckwitz et al. 2000).
Periodontal disease progresses sporadically and episodically, and common clinical periodontal measures and indices (e.g., probing depth, clinical attachment loss) may not reflect current disease activity (Page et al. 1992). Furthermore, the rate of clinical disease progression is relatively low, even in untreated patients (Lindhe et al. 1989). Thus, relatively large numbers of women are needed study associations between disease progression during pregnancy and birth outcomes.
To date, only one research group has examined the association between progressive periodontitis and adverse pregnancy outcomes (Offenbacher et al. 2006, Riche et al. 2002). They reported that very preterm birth rates (< 32 weeks gestation) were significantly higher in women with progressive periodontitis when compared to those with stable or non-progressing disease (6.4% vs. 1.8%, P < 0.001) (Offenbacher et al. 2006). Preterm birth rates < 37 weeks did not differ significantly between these groups. The same researchers reported that the association between disease progression and risk for preterm birth before 37 weeks was significant in preeclamptic but not non-preeclamptic women (Riche et al. 2002).
Previously we reported that non-surgical treatment in pregnant women with periodontitis did not significantly alter rates of preterm birth, low birthweight or fetal growth restriction (Michalowicz et al. 2006). The present paper's analyses examine the relationship between progressive periodontitis and risk for preterm birth and low birthweight using data from the Obstetrics and Periodontal Therapy (OPT) Study.
METHODS
Details about the OPT Study and its obstetrical and clinical periodontal results have been reported elsewhere (Michalowicz et al. 2006). The OPT Study was a randomized, single-blind controlled trial designed to determine if non-surgical periodontal treatment alters the frequency and severity of preterm delivery in women with periodontitis. Women were recruited from obstetrics clinics in Minneapolis MN, Lexington KY, Jackson MS and New York NY that serve populations at elevated risk for preterm birth. Eligible women had periodontitis, defined as 4 or more teeth with probing depth at least 4 mm and clinical attachment loss at least 2 mm, and bleeding on probing for at least 35% of tooth sites. Women were ineligible if they had multiple fetuses, required antibiotic prophylaxis prior to dental treatment, had any medical condition that precluded elective dental treatment, or were likely to have less than 20 teeth remaining after treatment of moderate to severe caries, abscesses or other non-periodontal pathoses. Following baseline assessments between 13 weeks 0 days to 16 weeks 6 days gestation, women were randomly assigned to receive scaling and root planing before 21 weeks gestation (N = 413) or after delivery (410). Women also were seen for monthly visits, during which treatment women received tooth polishings and oral hygiene instruction and control women received brief examinations.
At baseline, all women were evaluated by a dentist for essential dental treatment needs. To eliminate oral sources of infection or pain during pregnancy, teeth with urgent or emergent care needs were treated with temporary or permanent restorations, endodontic therapy or extraction before 21 weeks gestation. Over half of women (58.7%) were judged to have essential dental care needs and nearly three-fourths of these (72.7%) completed recommended treatment (Michalowicz et al. 2008).
Periodontal assessment
Women received comprehensive periodontal examinations at baseline and again at 21 to 24 weeks and 29 to 32 weeks gestation. Using a manual probe, calibrated and blinded examiners measured probing depth (PD), gingival recession and bleeding on probing at 6 sites on all teeth excluding third molars. Clinical attachment loss (CAL) was calculated from the PD and recession measures. Bleeding on probing was scored as present or absent.
Rescue periodontal treatment
During the monthly follow-up visits, participants were monitored for oral adverse events including abscesses, exophytic soft tissue lesions and gingival hyperplasia. Periodontitis progression, defined in the study protocol as an increase in CAL from baseline of at least 3mm, was monitored at the follow-up periodontal examination visits.
All women with oral lesions or progressive periodontitis were offered treatment, which was not delayed until postpartum unless contraindicated because of advanced gestation. Abscesses and exophytic or hyperplastic lesions could be treated with scaling, root planing, soft tissue curettage, or by surgical excision. Therapists also had the discretion to monitor rather than treat certain lesions. Women with progressive periodontitis at fewer than 6 tooth sites received root planing at the affected teeth only. Control women with progressive periodontitis at 6 or more sites were offered full-mouth scaling and root planing. Treatment group participants with progressive disease at 6 or more tooth sites were referred to a consulting periodontist and could receive a second course of full-mouth scaling and root planing and/or systemic antibiotics, or subgingival irrigation with antimicrobial solutions.
Pregnancy Outcomes
Gestational age was determined at baseline using the woman’s last menstrual period information and ultrasound data as described elsewhere (Carey et al. 2000). Birthweight was abstracted from the child’s medical record by blinded and trained nurses. Gestational age at delivery was available for 812 women. Birth outcomes were not available for 6 treatment women (4 were lost to follow-up, 1 withdrew consent, 1 electively aborted the pregnancy) and 5 controls (3 were lost to follow-up, 1 withdrew consent, 1 electively aborted the pregnancy).
Change in periodontitis during pregnancy
We studied the relationship between change in periodontitis during pregnancy and adverse birth outcome using three definitions of change. First, we used our a priori definition of disease progression, which was any increase in CAL ≥ 3 mm. Women with and without ≥ 3 mm of CAL at any tooth site were termed, respectively, as having “progressive” or “non-progressive” disease. We also calculated the change from baseline in the percent of sites with BOP, then grouped participants into tertiles in terms of this change. Finally, we used a definition of progression previously used to explore the relationship between periodontitis progression and birth outcomes: “4 or more [tooth] sites with 2 mm or more of increasing probing depths at each site, with the postpartum probing depth being 4 mm or more” (Offenbacher et al. 2006). Because we did not examine women post-partum, we used the last examination data available for this classification.
Statistical analyses
We included subjects who had both follow-up periodontal data and birth outcomes. Time-to-event analyses used as the event time gestational age at end of pregnancy, where those lost to follow-up (n = 7), withdrawing consent (n = 2), and having elective abortions (n = 2) were censored at the last available follow-up visit or the elective abortion. Otherwise, gestational ages were censored at 37 weeks (259 days).
We compared birth outcomes between non-progressing and progressing groups using all women and controls only. We were required by the study’s data and safety monitoring board and the applicable institutional review boards to offer treatment to all women who were found to have progressive disease. In this sense, we observed in control women the natural history of periodontitis to the point of clinical disease progression, defined as an increase in CAL ≥ 3 mm.
In analyzing controls only, we compared groups with non-progressive and progressive periodontitis using time-to-event analyses and the log-rank test and Kaplan-Meier plots. When all treatment and control women were included, the time-to-event analyses used Cox regression including treatment group and the interaction of treatment group with progression status. Additional analyses examined the effects of additional or rescue treatment on birth outcomes. For these, we included in the models an indicator of receipt of rescue (for controls) or additional treatment (for treatment group women) before delivery and, as needed, the interaction between initial group assignment and the receipt of rescue treatment. Time-to-event analyses based on tertiles of change included the interaction of treatment group with tertile of change.
Simple comparisons of two groups used either a two-sample t-test (for continuous dependent variables like birthweight) or Pearson's chi-squared test (for categorical dependent variables like tertile of baseline percent bleeding on probing). Other analyses of continuous dependent variables used multiple linear regressions. Analyses were conducted using JMP (v. 4 and v. 7, SAS Institute Inc.).
In defining change from baseline to follow-up for clinical periodontal measures, we used the second post-randomization examination (29 to 32 weeks gestation) when it was available, and otherwise used the first post-randomization examination (21 to 24 weeks).
RESULTS
Summary of sample population
Follow-up periodontal data were available for 722 women (87.7% of randomized women), including 645 who experienced a live full-term birth, 69 a live preterm birth, and 6 a spontaneous abortion (pregnancy loss before 20 weeks) or preterm stillbirth (pregnancy loss between 20 weeks and 36 weeks 6 days). Two women had follow-up periodontal data but no birth outcome data. Women who experienced an event (spontaneous abortion or stillbirth or live preterm birth) before 29–32 weeks were not recalled for a post-partum periodontal examination. For these women, changes in periodontal status were determined using baseline and 21–24 week clinical data, if available. The rate of live preterm births, as a fraction of all live births, was lower in women who had follow-up periodontal data (69/714, or 9.7%) when compared to women who did not (13/79, or 16.5%).
Of those with follow-up periodontal data, 115 women (15.9%), including 60 controls, experienced progressive periodontitis, defined as an increase in CAL ≥ 3mm. [Note: Previously (Michalowicz et al. 2006), we reported progression based on adverse event reports made during the study, which were not always consistent with the study's periodontal measurements.] Forty-six women (6.4%), including 26 controls, lost at least 3 mm of clinical attachment at more than one tooth site. Overall though, only a small fraction of all tooth sites were affected (0.17% in the treatment group and 0.28% in the control group). Based on Offenbacher et al.’s definition (Offenbacher et al. 2006), 135 (18.7%) women had progressive disease, including 30/352 (8.5%) treatment women and 105/370 (28%) untreated controls.
Nine women (3 treatment, 6 control) experienced progressive disease at 6 or more tooth sites. Of these, one treatment group subject and 2 controls received full-mouth scaling and root planing, one control received full-mouth scaling and root planing plus systemic antibiotics, and one treatment group subject received systemic antibiotics alone. The others were treated after delivery or declined treatment. Of the 54 controls who lost clinical attachment at fewer than 6 sites, 25 were treated before delivery (22 received localized scaling and root planing at the affected teeth and 3 had the lesion excised or the affected tooth extracted).
A. Gestational age at the end of pregnancy
1. Disease progression defined as any CAL ≥ 3 mm
Overall, pregnancies ended before 37 weeks in 64/605 (10.6%) women with stable disease and in 11/115 (9.6%) women with progressive disease. Figure 1 depicts the distribution of gestational ages of pregnancies ending before 37 weeks in all women, by disease progression status. The curve for women with non-progressive disease (red line) lies above that for women with progressive disease (green line), indicating that latter group tended to have fewer preterm events and longer gestation. The difference, however, was not statistically significant (P = 0.31. Table 1). The distributions of gestational age did not differ between progressing and non-progressing groups in control women only (Table 1). Pregnancies ended before 37 weeks for 7/60 (11.7%) controls with progressive disease, only one of which occurred before 32 weeks. When considering all subjects, the provision of additional or rescue treatment did not significantly affect gestational age at delivery (P = 0.43 from Cox regression, P = 0.41 for the interaction between rescue treatment and group assignment in the same regression). For controls only, the provision of rescue treatment did not significantly affect gestational age at delivery (P = 0.32).
Figure 1.
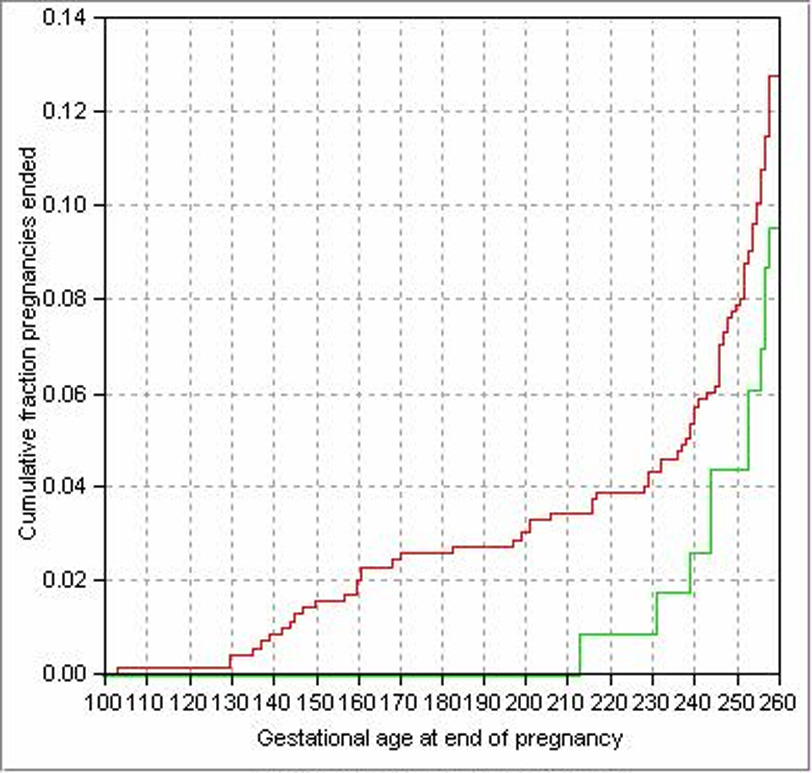
Distribution of gestational age at end of pregnancy in all women, by disease progression defined as an increase in attachment loss ≥ 3mm. The red and green lines show cumulative fraction of pregnancies ended for each gestational age; the red line is for women who had no progressing sites after baseline, the green line is for women who had at least one progressing site (P = 0.31).
Table 1.
P values from Cox Regressions (for analysis of all women) and log rank test (for controls only) comparing the distributions of gestational age at the end of pregnancy in women with and without changes from baseline in their periodontal condition
| All women | Controls only | |
|---|---|---|
| Periodontitis Progression | ||
| CAL ≥ 3mm (Yes/No) | 0.31 | 0.73 |
| Offenbacher et al. definition (Yes/No) | 0.54 | 0.33 |
| Change in % of Sites with BOP | ||
| Comparing among tertiles * | 0.06 | 0.43 |
2. Disease progression defined by Offenbacher et al. (Offenbacher et al. 2006)
The distribution of gestational ages at the end of pregnancy did not differ significantly between women with and without disease progression when considering all women (P = 0.54, Figure 2) or controls alone (P = 0.33). Again, the provision of additional or rescue treatment did not significantly affect gestational age at delivery in all subjects (P = 0.47) or in controls alone (P = 0.67).
Figure 2.
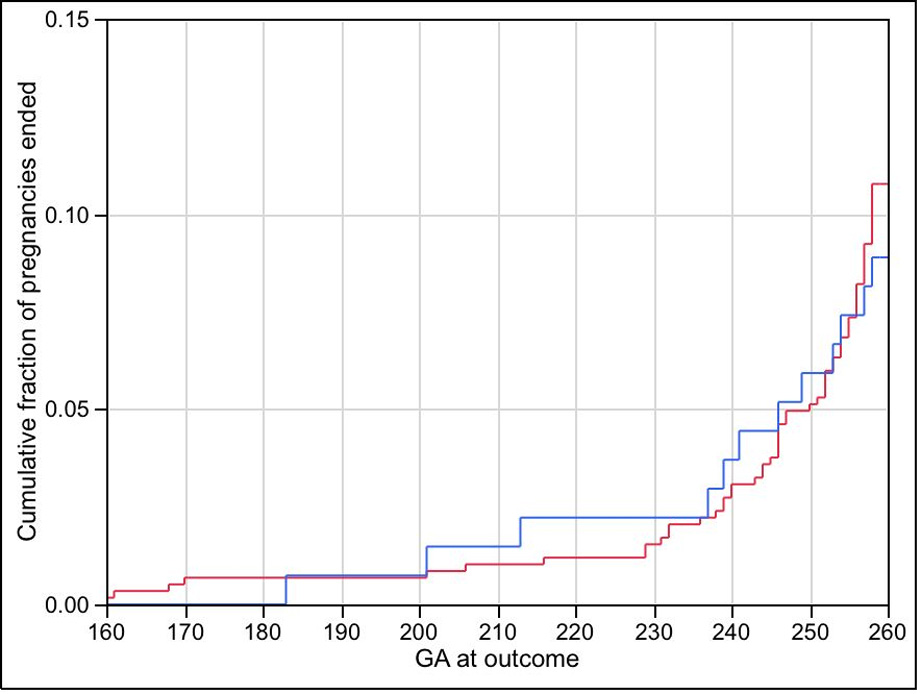
Gestational age at the end of pregnancy for all women, by disease progression according to Offenbacher et al. (2006). The red and blue lines show cumulative fraction of pregnancies ended for each gestational age; the red line is for women who did not progress, the blue line is for women who did progress (P = 0.54).
3. Change in disease status according to change from baseline in percent of sites bleeding on probing (BOP)
Women were grouped according to change in the percent of sites with BOP. The 33rd and 67th percentiles of the distribution of change (defining the boundaries between the 1st and 2nd and between the 2nd and 3rd tertiles) were −20.2 and −1.8 for all women and −7.1 and 0.7 for controls alone, where negative values indicate a reduction from baseline in BOP.
Considering all women, tertiles of change in percent BOP had a borderline significant association with gestational age at end of pregnancy (P = 0.06). Within each of the treatment groups (treatment or control), those in the lowest (best) tertile tended to have fewer preterm events and longer gestation than those in the highest (worst) tertile. Control women who experienced relatively large reductions in BOP had the most favorable birth outcomes whereas treatment group women who experienced the smallest improvements in BOP had the least favorable birth outcomes. These differences, however, were not statistically significant. Considering controls alone, gestational age at end of pregnancy did not differ significantly among the tertiles of change in BOP (P = 0.43, Table 1).
B. Birthweight
Table 2 lists average birth weights according to change in maternal periodontal status. Among all women, mean birthweight was higher, though not significantly, for women with progressive disease, defined as any CAL ≥ 3 mm, compared to those with non-progressing conditions (P = 0.11). The same trend was noted in the controls only (P = 0.11). Mean birthweight did not differ significantly between groups with and without disease progression as defined by Offenbacher et al. (2006) when considering all women (P = 0.87) or controls only (P = 0.17). Mean birthweight also did not differ significantly among groups according to tertiles of change in BOP, considering all women (P = 0.54) or controls only (P = 0.90, Table 2).
Table 2.
Mean birth weight (in grams, ± SEM), by change in maternal periodontal status
| All women | Controls only | |
|---|---|---|
| Disease progression defined as CAL >= 3mm | ||
| Yes* | 3295 ± 64 | 3319 ± 93 |
| No | 3184 ± 26 | 3154 ± 39 |
| Disease progression according to Offenbacher et al. (2006) | ||
| Yes† | 3236 ± 61 | 3320 ± 59 |
| No | 3247 ± 25 | 3226 ± 37 |
| Tertile of change in % of sites with BOP‡ | ||
| I | 3248 ± 54 | 3250 ± 55 |
| II | 3271 ± 38 | 3237 ± 55 |
| III | 3199 ± 53 | 3271 ± 53 |
C. Preeclampsia
Finally, we tested whether the relationship between these pregnancy outcomes and periodontal disease progression differed in preeclamptic and non preeclamptic women as suggested by others (Riche et al. 2002). Preeclampsia was defined as pregnancy-associated hypertension occurring 4 hours to 14 days after an episode of pregnancy-associated proteinuria in a woman with no previous hypertension or proteinuria; pregnancy associated hypertension in conjunction with pulmonary edema or thrombocytopenia (<100,000 platelets per cubic millimeter); or the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). For these analyses, we included all treatment and control women and did Cox regressions with independent variables preeclampsia (yes/no) and tertile of change in percent BOP and their interaction. Forty-six women with follow-up periodontal data were diagnosed with preeclampsia.
Preeclampsia was strongly associated with delivery before 37 weeks (relative hazard = 4.8, 95% CI 2.7 to 8.4) and low birthweight (adjusted average [± SE] in non-preeclamptic women = 3287 [± 26]; in preeclamptic women, 2868 [± 104]; P < 0.001). However, the interaction between preeclampsia and tertile of change in the percent of sites with BOP was not statistically significant (P > 0.1), indicating that preeclampsia did not affect the relationship (or lack thereof, in this case) between change in BOP and risk for preterm delivery or low birthweight.
DISCUSSION
The OPT Study was a randomized clinical trial designed to determine if non-surgical periodontal treatment of pregnant women improves preterm birth rates. The present study – a secondary data analyses of the OPT Study data – was conducted to explore the relationships between periodontal disease progression and birth outcomes. Neither maternal periodontal disease progression after 13 to 17 weeks of gestation, nor change in the percent of tooth sites with bleeding on probing was significantly associated with risk for preterm delivery or low birthweight. We found similar results when testing for associations separately in all study subjects and in untreated controls alone. This suggests that the relationship between change in periodontal status and birth outcomes is largely unaffected by non-surgical periodontal treatment. Our findings are consistent with an earlier study that found no significant association between progressive periodontitis and risk for preterm birth at less than 37 weeks (Offenbacher et al. 2006). In this earlier report, however, progressive disease was associated with very preterm birth risk (< 32 weeks). In contrast, we found no evidence that progressive disease increases a woman’s risk for very preterm delivery. Of the 115 women in the current study who experienced progressive disease, defined as CAL ≥ 3 mm, only one delivered before 32 weeks (Figure 1). Of the 135 women who met Offenbacher et al.’s (2006) criteria for disease progression, only three (2.2%) delivered before 32 weeks (Figure 2).
Our study has several limitations. First, we analyzed data from a clinical trial and not from a prospective cohort study. The trial was not designed to address the hypothesis of the current analyses. Notably, 413 women in this trial were randomized to receive treatment during their pregnancy, and 395 received this care. Because treatment may have confounded the relationship between disease progression and pregnancy outcomes, we analyzed all subjects and included in the Cox regression models treatment group assignment. We also analyzed the smaller group of untreated controls, only 3 of whom received full-mouth root planing before delivery. The consistency of findings between these groups provides some assurances that our findings are robust.
Another limitation of this current study is the number of women who were missing follow-up periodontal data. Although follow-up periodontal data was available for 87.7% of all OPT women, a disproportionate number of women with early “events” were missing these data. For example, while 90% (645/711) of full-term women and 85% (69/82) of live-preterm women had follow-up periodontal data, only 31% (6/19) of women who experienced a spontaneous abortion or stillbirth had follow-up data. As mentioned earlier, this deficiency was a result of the study protocol, which exited women from the trial once their pregnancy ended. Without post-baseline data, we could not determine periodontitis progression in these women. Thus, our findings regarding associations between disease progression and early pregnancy losses should be viewed with particular caution.
We also offered treatment to all women with progressive periodontitis. Because periodontal treatment is not contraindicated during pregnancy and because we asked participants not to seek dental care outside of the trial, we could not ethically withhold care from women with documented progressive periodontitis. Thus, any effect that progressive periodontitis may have on these preterm birth and low birthweight would have been mitigated by rescue treatment. In a sense, these control women “crossed over” into the treatment group, which may have limited our ability to detect associations between progressive disease and adverse pregnancy outcomes. However, when compared to women randomized to the treatment group, these control women received treatment only after their condition worsened and at a later point in their pregnancy. Thus, any effects that periodontal disease activity had on the fetal-placental unit would have been undisturbed up to the time of rescue treatment. This may be particularly relevant because increases in levels of inflammatory mediators in the periodontal tissues – which have been suggested to lie in the causal pathway between this periodontitis and adverse pregnancy outcomes – often precede the onset of clinical disease. For example, levels of prostaglandin E2 begin to increase in gingival crevicular fluid several months before disease is detected clinically (Offenbacher et al 1986, Preshaw et al 1999). Despite this, however, it is still possible that longer exposures to inflammatory mediators or bacteria associated with disease progression are necessary to adversely affect birth outcomes. Finally, repeated periodontal therapy may activate immune responses, which in turn affect pregnancy outcomes. One way this might occur is through increased TLR-4 expression on placental trophoblasts, which has been associated with pre-eclampsia, but not preterm labor (Kim et al 2005).
In addition, not all women with progressive disease received initial treatment or re-treatment prior to delivery. As mentioned, only 3 of 6 controls with generalized progressive disease were treated before delivery. Similarly, 25 of 54 controls with localized disease received any treatment before delivery. The remaining controls with progressive disease were treated after delivery or refused treatment. Furthermore, we found no evidence that rescue treatment (in controls) or additional treatment (in treatment group subjects) had a significant effect on gestational age at delivery (all P values > 0.3). Because of the relatively small number of untreated subjects with progressive disease, however, we had low statistical power to detect significant effects.
Finally, teeth that were deemed non-restorable were extracted prior to 21 weeks gestation in both treatment and control groups. We did not enroll women if we judged they would no longer meet the periodontal disease enrollment criteria following essential dental treatment, which included extractions. Nonetheless, tooth extraction is a form of periodontal intervention that was performed following randomization and for both groups. To the extent that this treatment improved a woman’s periodontal condition, it may have masked any effect of disease progression (and periodontal treatment) on preterm delivery or low birthweight. To further explore this issue, we compared changes in clinical measures between controls who had and did not have teeth extracted as part of essential dental treatment. One hundred eleven control women had at least one tooth extracted (63 had one, 32 had two). The change from baseline in the percent of tooth sites with BOP, however, did not differ significantly between women who did and did not have teeth extracted (−1.7 percentage points versus −1.5 percentage points, respectively; P = 0.94). The percentage of controls with progressive disease, defined as CAL >= 3mm, also did not differ significantly between these groups (15.3% versus 14.4%, P = 0.82). Thus, tooth extractions as part of essential dental care did not significantly alter a control woman’s risk for progressive disease. We cannot rule out the possibility, however, that extractions exerted some systemic effect that altered a woman’s risk for adverse pregnancy outcomes.
The relatively small number of women with preterm deliveries (75), progressive periodontitis (115 or 135, depending on the definition used), and preeclampsia (46) limited our power to detect slight associations among these outcomes. In general, women with progressive disease did not tend to have worse pregnancy outcomes than those without progressive disease. For example, the distribution curve of gestational ages at delivery for women with progressive disease lies below that for women without progressive disease (Figure 1), which indicates that the latter group tended to have more and earlier preterm deliveries than the former. In addition, mean birthweights tended to be higher, but not significantly so, in progressing compared to non-progressing women (Table 2). Thus, there is no reason to infer that our failure to find a positive association between disease progression and adverse pregnancy outcomes was because the sample lacked statistical power.
There also are several important differences between our current study and a previous one that reported an association between disease progression and risk for very preterm birth. Offenbacher et al. (2006) examined 1020 women early in the second trimester and again post-partum. In contrast, our follow-up examinations were conducted at 29 to 32 weeks gestation. It is possible that women who experienced progressive disease after the OPT Study’s final exam were at elevated risk for preterm birth or low birthweight infants. It is unlikely these events occurred before 32 weeks, however, because this is when we last examined women. Also, all women in our study had periodontitis at baseline. Periodontitis may be a risk factor for adverse pregnancy outcomes despite our previous finding that non-surgical treatment does not reduce this risk (Goldenberg et al. 2006). If true, OPT women would have been at elevated risk for these outcomes as a result of their existing disease, and our study may have lacked the statistical power to detect any small but additional increase in risk attributed to disease progression. Offenbacher et al. (2006) did not specify how many of their women with progressive disease were healthy at baseline. It is possible that very preterm birth is associated with disease progression only in previously healthy women. Finally, as mentioned earlier, we provided periodontal treatment to roughly half of these women before 21 weeks gestation. Offenbacher et al. used a prospective cohort study design that did not include treatment.
While most studies have found an association between periodontitis and risk for preterm birth and low birthweight (Xiong et al. 2006), our findings are consistent with several other reports (Davenport et al. 2002, Noack et al. 2005, Vettore et al. 2008). For example, Vettore et al. (2008) reported that women with the most extensive periodontal pocketing (PD ≥ 4 mm) were at significantly lower risk for delivering a low birthweight infant. Similarly, Davenport et al. (2002) found a significant inverse or negative relationship between mean pocket depth and risk for preterm birth and low birthweight. While most studies suggest that maternal periodontitis is associated with elevated risk for adverse pregnancy outcomes, large epidemiological studies and additional clinical trials are needed to further explore the nature of this association, which appears to be present in some but not all populations.
Acknowledgments
Supported by the National Institute of Dental and Craniofacial Research (UO1 DE014338).
Footnotes
The authors do not claim any conflicts of interest.
REFERENCES
- Aurer A, Aurer-Kozelj J, Stavljenic-Rukavina A, Kalenic S, Ivic-Kardum M, Haban V. Inflammatory mediators in saliva of patients with rapidly progressive periodontitis during war stress induced incidence increase. Collegium Antropologicum. 1999;23:117–124. [PubMed] [Google Scholar]
- Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, Heine RP, Nugent RP, Fischer ML, Leveno KJ, Wapner R, Varner M. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. national institute of child health and human development network of maternal-fetal medicine units. The New England Journal of Medicine. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- Davenport ES, Williams CE, Sterne JA, Murad S, Sivapathasundram V, Curtis MA. Maternal periodontal disease and preterm low birthweight: Case-control study. Journal of Dental Research. 2002;81:313–318. doi: 10.1177/154405910208100505. [DOI] [PubMed] [Google Scholar]
- Goffinet F. Primary predictors of preterm labour. BJOG : An International Journal of Obstetrics and Gynaecology. 2005;112 Suppl 1:38–47. doi: 10.1111/j.1471-0528.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF. Preterm birth and periodontal disease. The New England Journal of Medicine. 2006;355:1925–1927. doi: 10.1056/NEJMe068210. [DOI] [PubMed] [Google Scholar]
- Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. American Journal of Obstetrics and Gynecology. 1998;179:172–178. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, Gomez R, Mazor M, Saito S, Abrahams VM, Mor G. Toll-like receptor 4: a potential link between "danger signals," the innate immune system, and preeclampsia? American Journal of Obstetrics and Gynecology. 2005;193:921–927. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky SS. Periodontal loser sites in untreated adult subjects. Journal of Clinical Periodontology. 1989;16:671–678. doi: 10.1111/j.1600-051x.1989.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matseoane S, Curran AL, Deinard AS, Jr, Rogers TB. The safety of dental treatment in pregnant women. Journal of the American Dental Association. 2008;139:685–695. doi: 10.14219/jada.archive.2008.0250. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Durand R. Maternal periodontal disease and spontaneous preterm birth. Periodontology 2000. 2007;44:103–112. doi: 10.1111/j.1600-0757.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matseoane S, Tschida PA OPT Study. Treatment of periodontal disease and the risk of preterm birth. The New England Journal of Medicine. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- Noack B, Klingenberg J, Weigelt J, Hoffmann T. Periodontal status and preterm low birth weight: A case control study. Journal of Periodontal Research. 2005;40:339–345. doi: 10.1111/j.1600-0765.2005.00808.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, Mauriello SM, Moss KL, Beck JD. Progressive periodontal disease and risk of very preterm delivery. Obstetrics and Gynecology. 2006;107:29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. Journal of Periodontal Research. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Page RC, DeRouen TA. Design issues specific to studies of periodontitis. Journal of Periodontal Research. 1992;27:395–404. doi: 10.1111/j.1600-0765.1992.tb01704.x. discussion 412-6. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Lauffart B, Zak E, Jeffcoat MK, Barton I, Heasman PA. Progression and treatment of chronic adult periodontitis. Journal of Periodontology. 1999;70:1209–1220. doi: 10.1902/jop.1999.70.10.1209. [DOI] [PubMed] [Google Scholar]
- Riche EL, Boggess KA, Lieff S, Murtha AP, Auten RL, Beck JD, Offenbacher S. Periodontal disease increases the risk of preterm delivery among preeclamptic women. Annals of Periodontology. 2002;7:95–101. doi: 10.1902/annals.2002.7.1.95. [DOI] [PubMed] [Google Scholar]
- Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: A meta-analysis. American Journal of Obstetrics and Gynecology. 2007;196:135.e1–135.e7. doi: 10.1016/j.ajog.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Vettore MV, Lamarca Gde A, Leao AT, Thomaz FB, Sheiham A, Leal Mdo C. Periodontal infection and adverse pregnancy outcomes: A systematic review of epidemiological studies. Cadernos De Saude Publica / Ministerio Da Saude, Fundacao Oswaldo Cruz, Escola Nacional De Saude Publica. 2006;22:2041–2053. doi: 10.1590/s0102-311x2006001000010. [DOI] [PubMed] [Google Scholar]
- Vettore MV, Leal M, Leao AT, da Silva AM, Lamarca GA, Sheiham A. The relationship between periodontitis and preterm low birthweight. Journal of Dental Research. 2008;87:73–78. doi: 10.1177/154405910808700113. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Grischke EM, Schwab S, Hettinger S, Loibl S, Aulmann M, Kaufmann M. Predictive value of serum interleukin-6 and -8 levels in preterm labor or rupture of the membranes. Acta Obstetricia Et Gynecologica Scandinavica. 2000;79:667–672. [PubMed] [Google Scholar]
- Xiong X, Buekens P, Fraser W, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: A systematic review. BJOG : An International Journal of Obstetrics and Gynaecology. 2006;113:135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]