BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models (original) (raw)
. Author manuscript; available in PMC: 2009 Sep 22.
Published in final edited form as: Oncogene. 2008 Apr 14;27(34):4702–4711. doi: 10.1038/onc.2008.109
Abstract
Genetic alterations in the kinase domain of the epidermal growth factor receptor (EGFR) in non-small cell lung cancer (NSCLC) patients are associated with sensitivity to treatment with small molecule tyrosine kinase inhibitors. Although first-generation reversible, ATP-competitive inhibitors showed encouraging clinical responses in lung adenocarcinoma tumors harboring such EGFR mutations, almost all patients developed resistance to these inhibitors over time. Such resistance to first-generation EGFR inhibitors was frequently linked to an acquired T790M point mutation in the kinase domain of EGFR, or upregulation of signaling pathways downstream of HER3. Overcoming these mechanisms of resistance, as well as primary resistance to reversible EGFR inhibitors driven by a subset of EGFR mutations, will be necessary for development of an effective targeted therapy regimen. Here, we show that BIBW2992, an anilino-quinazoline designed to irreversibly bind EGFR and HER2, potently suppresses the kinase activity of wild-type and activated EGFR and HER2 mutants, including erlotinib-resistant isoforms. Consistent with this activity, BIBW2992 suppresses transformation in isogenic cell-based assays, inhibits survival of cancer cell lines and induces tumor regression in xenograft and transgenic lung cancer models, with superior activity over erlotinib. These findings encourage further testing of BIBW2992 in lung cancer patients harboring EGFR or HER2 oncogenes.
Keywords: EGFR, HER2, lung cancer, BIBW2992, therapeutics
Introduction
Lung cancer continues to be the leading cause of cancer-related mortality, demonstrating the limited efficacy of traditional cytotoxic chemotherapy in patients with this disease (Bedano and Hanna, 2006; Jemal et al., 2006). The development of new and effective therapies for patients with advanced lung cancer remains a major health imperative, and targeted therapies may offer well-tolerated disease-modifying treatment options in patient populations defined by relevant oncogene mutation status. Members of the ERBB receptor tyrosine kinase family, including epidermal growth factor receptor (EGFR), HER2, HER3 and HER4, present an attractive option for targeted therapy in patients with NSCLC, due to observed patterns of oncogenic mutation of EGFR and HER2 (Lynch et al., 2004; Paez et al., 2004; Pao et al., 2004; Stephens et al., 2004; Shigematsu et al., 2005; Shimamura et al., 2006; Wang et al., 2006).
First-generation small molecule EGFR tyrosine kinase inhibitors, such as gefitinib and erlotinib, were shown to be effective against lung tumor cells harboring mutations in the kinase domain of EGFR, most commonly small in-frame deletions in exon 19 or the L858R missense mutation in exon 21 (Janne et al., 2005). However, despite the initial response, patients almost invariably develop resistance to these inhibitors and relapse after several months (Riely et al., 2006). About half of cases with acquired resistance to first-generation EGFR inhibitors can be accounted for by a secondary mutation, T790M, in exon 20 of the EGFR kinase domain (Kobayashi et al., 2005a; Pao et al., 2005a). T790M EGFR exhibits elevated enzymatic and transforming activity, both alone and in combination with primary alterations in exon 19 or 21 (Mulloy et al., 2007; Schiffer et al., 2007; Vikis et al., 2007; Yuza et al., 2007), indicating a need for increased therapeutic efficacy of the next generation of EGFR inhibitors.
Additional mechanisms of resistance to gefitinib and erlotinib have been reported, including primary resistance due to small in-frame insertions in exon 20 of the kinase domains of EGFR or HER2 (Greulich et al., 2005; Shimamura et al., 2006; Wang et al., 2006). A parallel situation exists in glioblastoma, in which extracellular domain point mutations and variant III deletions of EGFR occur that are relatively insensitive to first-generation EGFR inhibitors (Lee et al., 2006). These mechanisms of acquired and primary resistance indicate the need for a small molecule tyrosine kinase inhibitor that is more broadly active against ERBB receptor tyrosine kinases, yet retains the exquisite overall selectivity of first-generation EGFR TKIs within the human kinome that has afforded an acceptable drug safety and tolerability profile.
Irreversible inhibitors that covalently modify EGFR and/or HER2 exhibit increased efficacy against mutants resistant to gefitinib and erlotinib in cell-based assays (Greulich et al., 2005; Kwak et al., 2005; Kobayashi et al., 2005a, b; Shimamura et al., 2006; Yuza et al., 2007). One such inhibitor, BIBW2992, is a new irreversible dual specificity EGFR/HER2 inhibitor derived from the anilino-quinazoline chemical series that was designed to covalently bind to Cys 773 of EGFR and Cys 805 of HER2. We have investigated the efficacy of BIBW2992 in a battery of preclinical assays and demonstrate improvements over both first-generation EGFR inhibitors and other irreversible inhibitors that have yielded mixed results in transgenic mouse models of lung adenocarcinoma (Li et al., 2007b).
Results
BIBW2992 inhibits EGFR and HER2 kinase activity in vitro
BIBW2992, an anilino-quinazoline designed as an irreversible, dual EGFR/HER2 inhibitor, possesses a functional Michael acceptor group similar to the one found in the quinoline-derived irreversible EGFR inhibitors EKB-569 and HKI-272, allowing covalent modification of the ATP-binding site of the kinase domains of EGFR (Cys 773) and HER2 (Cys 805) (Rabindran et al., 2004; Eskens et al., 2008). In cell-free in vitro kinase assays, BIBW2992 shows potent activity against wild-type and mutant forms of EGFR and HER2, similar to gefitinib in potency for L858R EGFR, but about 100-fold more active against the gefitinib-resistant L858R-T790M EGFR double mutant, with an IC50 of 10 nM (Table 1). BIBW2992 is furthermore comparable to lapatinib and canertinib for in vitro potency against HER2, with an IC50 of 14 nM. Importantly, the overall kinase selectivity of BIBW2992 is in line with first-generation EGFR inhibitors (Table 1). An extended evaluation of BIBW2992 on a broad panel of additional tyrosine and serine/threonine kinases (_N_=52) confirms selectivity of BIBW2992 (Supplementary Table 1A and 1B). The most sensitive kinase in this evaluation was lyn with an IC50 of 736 nM.
Table 1.
In vitro inhibitory activity of BIBW2992
| BIBW2992 | Lapatinib | Canertinib | Gefitinib | |
|---|---|---|---|---|
| EGFRwt | 0.5 | 3 | 0.3 | 3 |
| EGFRL858R | 0.4 | 8 | 0.4 | 0.8 |
| EGFRL858R/T790M | 10 | >4000 | 26 | 1013 |
| HER2 | 14 | 15 | 30 | 1830 |
| β-InsRK | >100 000 | >100 000 | >100 000 | >100 000 |
| HGFR | 13 000 | >20 000 | >20 000 | >20 000 |
| c-SRC | >4000 | >20 000 | 1480 | >10 000 |
| VEGFR-2 | >100 000 | >100 000 | 24 900 | >100 000 |
Effects of BIBW2992 on cellular receptor phosphorylation
To determine the potency of BIBW2992 against EGFR and HER2 autophosphorylation in intact cells, we performed ELISA tests with EGFR- and HER2-specific antibodies and measured levels of receptor phosphor-ylation with increasing drug concentrations. Cell lines assayed include the human epidermoid carcinoma cell line A431 expressing wt EGFR, murine NIH-3T3 cells transfected with wt HER2, as well as breast cancer cell line BT-474 and gastric cancer cell line NCI-N87, which express endogenous HER2. BIBW2992 displays potent cellular effects on both EGFR and HER2 phosphorylation in line with the in vitro kinase results, comparing favorably to reference compounds in all cell types tested (Supplementary Table 2).
Because HER3 has recently been identified as the mediator of activation of the PI3-K- AKT survival pathway in NSCLC cell lines sensitive to gefitinib (Engelman et al., 2005), the activity of BIBW2992 against HER3 present in the human breast cancer cell line T47D was assessed. HER3 is devoid of intrinsic kinase activity and relies on trans-phosphorylation by heterodimerization partners, including EGFR, HER2 and MET (Hynes and Lane, 2005; Engelman et al., 2007), suggesting that HER3 phosphorylation is an appropriate biomarker for inhibition of EGFR and HER2. Anti-phospho-HER3 immunoblotting showed that treatment with 100 nM BIBW2992 was sufficient to prevent heregulin-stimulated HER3 phosphorylation (Supplementary Figure 1).
Inhibition of EGFR-dependent phenotypes in isogenic cell-based assays
The activity of BIBW2992 against oncogenic EGFR mutants found in human cancers, including lung adenocarcinomas and glioblastomas, was assessed in two mechanism-based models strictly responsive to these mutations; namely, anchorage-independent proliferation of NIH-3T3 cells expressing mutant EGFR (Greulich et al., 2005; Lee et al., 2006), and IL-3 independent proliferation of Ba/F3 cells (Jiang et al., 2005; Yuza et al., 2007).
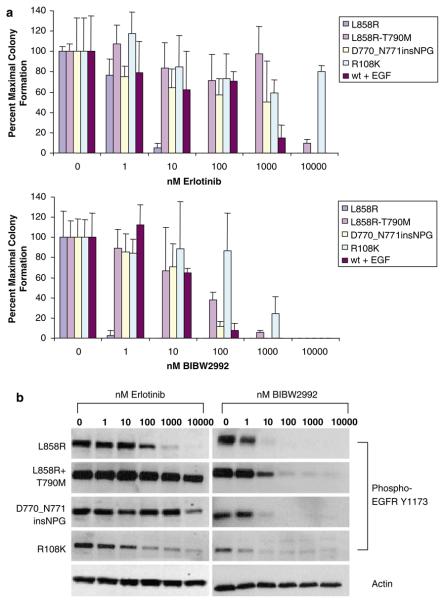
In addition to the erlotinib-sensitive L858R kinase domain mutation found in lung adenocarcinoma, NIH-3T3 cell lines ectopically expressing four EGFR iso-forms that are partially or completely resistant to erlotinib were assayed for proliferation in soft agar, including the double mutation L858R/T790M, which is associated with acquired resistance in NSCLC patients (Kobayashi et al., 2005a; Pao et al., 2005a); a primary resistance exon 20 insertion mutant, D770-771insNPG (Greulich et al., 2005); the R108K extracellular domain point mutation found in glioblastoma (Lee et al., 2006); and EGF-stimulated wild-type EGFR (Greulich et al., 2005). In each setting, BIBW2992 efficiently inhibited colony formation in soft agar (Figure 1a). The effective concentrations of BIBW2992 were found to be one to two orders of magnitude below those needed for inhibition of colony formation by erlotinib in the soft agar assays. These cellular effects were corroborated by testing the dose–responses for BIBW2992 and erlotinib on EGFR autophosphorylation, a proxy for enzymatic activity (Figure 1b).
Figure 1.
BIBW2992 effectively inhibits anchorage-independent proliferation of NIH-3T3 cells ectopically expressing EGFR mutants. (a) NIH-3T3 cells expressing wild-type or mutant EGFR constructs were treated with the indicated concentrations of BIBW2992 or erlotinib and suspended in soft agar. Values shown are averages of triplicate samples normalized to absence of drug treatment; error bars represent s.d. Expression of mutant EGFR was confirmed by immunoblotting (data not shown). wt + EGF, wild-type _EGFR_-expressing NIH-3T3 cells supplemented with 100 ng/ml EGF. (b) Anti-phospho-EGFR Y1173 immunoblots of NIH-3T3 cell lysates treated with the indicated concentrations of BIBW2992 or erlotinib for 2 h. A representative anti-actin immunoblot is shown as a loading control.
To further evaluate the potency of BIBW2992 against clinically relevant EGFR mutations, we performed cytotoxicity assays on Ba/F3 cells, which depend on IL-3 signaling for survival and can be rendered growth factor independent by ectopically expressing oncogenic EGFR mutants (Jiang et al., 2005; Yuza et al., 2007). In addition to the L858R and L858R/T790M EGFR, the variant III deletion was included in the Ba/F3 experiments (Ji et al., 2006b; Vikis et al., 2007), as well as an extensive panel of exon 19 deletion mutants alone or in combination with the T790M resistance mutation. BIBW2992 was at least two orders of magnitude more effective than erlotinib against the erlotinib-resistant EGFR mutants in this Ba/F3 assay (Supplementary Table 3), as well as extracellular domain point mutations A289V and R108K, T790M alone and EGF-supported wild-type EGFR (data not shown).
BIBW2992 inhibits survival of human NSCLC cell lines
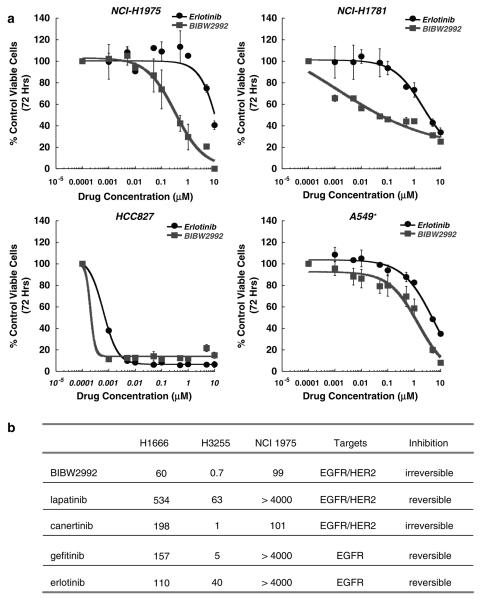
We investigated the effects of BIBW2992 on the more clinically-relevant model of human NSCLC cell lines expressing wild-type or mutant EGFR in the physiological context of additional genomic aberrations. Similar to the results from the isogenic transformation models, we found that BIBW2992 was more effective than erlotinib, gefitinib, or lapatinib in inhibiting survival of lung cancer cell lines harboring wild-type (H1666) or L858R/T790M (NCI-H1975) EGFR, with IC50s below 100 nM for these isoforms resistant to first-generation inhibitors and a subnanomolar IC50 for the gefitinib-sensitive L858R expressed by H3255 (Figure 2b). BIBW2992 was similarly effective against NSCLC lines expressing HER2 776insV (NCI-H1781) or EGFR E746_A750del (HCC827), but showed no activity toward A549 cells, which express wild-type EGFR and HER2, but simultaneously harbor an oncogenic Kras G12S point mutation (Figure 2a).
Figure 2.
Growth inhibition of lung cancer cell lines by BIBW2992 and other EGFR and/or HER2 inhibitors (a) Sensitivity of NSCLC cell lines that express EGFR WT, Exon 19 Del, L858R/T790M and HER2 INS776V to BIBW2992 and erlotinib. Dose-dependent growth inhibition was determined by MTS assay. Only HCC827 cells are sensitive to erlotinib whereas NCI-H1781 and NCI-H1975 cells are also sensitive to BIBW2992. Points, average of two independent experiments, each done in triplicate; bars, s.d. *A549 cells harbor a Kras G12S mutation. See also in Supplementary Table 4. (b) Inhibition of anchorage independent cell proliferation of various lung cancer cell lines treated with BIBW2992, lapatinib, canertinib, gefitinib or erlotinib. The IC50 values were determined as described in ‘Materials and methods’, confirmed in independent experiments and reported as nM values. H1666 express wild-type EGFR, H3255 express the EGFR L858R mutant and NCI 1975 express the EGFR L858R/T790M double mutant.
In vivo anti-tumor activity of BIBW2992
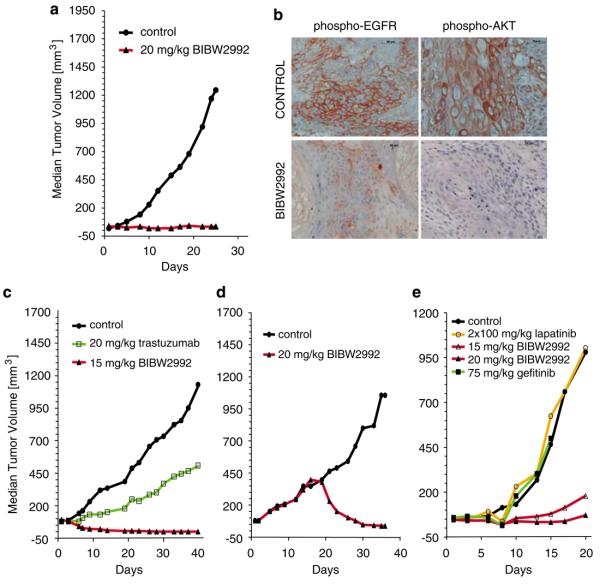
The in vivo activity of BIBW2992 was first assessed in a standard xenograft model of the epidermoid carcinoma cell line A431, expressing high levels of wild-type EGFR but also detectable levels of HER2 and previously validated with EGFR-targeted antibody therapy (Fan et al., 1993). Daily oral treatment with BIBW2992 at 20 mg/kg for 25 days resulted in dramatic tumor regression with a cumulative treated/control tumor volume ratio (T/C ratio) of 2% (Figure 3a), and downregulation of EGFR and AKT phosphorylation, as detected by immunohistochemical staining of tissue sections (Figure 3b). With this dose schedule, the animals showed no clinical signs of intolerability and gained weight similar to untreated littermates, with systemic exposure (_C_max, AUC0–24h in Supplementary Table 5) comparable to the pharmacokinetic data seen in Phase I studies with effective doses of BIBW2992 (Eskens et al., 2008). When drugs were given at respective maximum tolerated doses in the A431 model, BIBW2992 was more potent than either gefitinib (T/C=46% at 75 mg/kg/day) or lapatinib (T/C=32% at 2×100 mg/kg/day) (data not shown).
Figure 3.
In vivo activity of BIBW 2992 in xenograft models. (a) Animals carrying tumors established from A431 cells were treated daily with BIBW2992 by oral gavage at indicated doses. Statistical analysis of the tumor volumes in each group was performed using one-way analysis of variance (Dunnett's multiple test) with _P_-values consistently below 0.01. (b) Immunohistochemical analysis of A431 tumor sections obtained 6 h after the last dose confirms in vivo modulation of phosphorylated EGFR and AKT by BIBW2992. (c) Animals carrying tumors established from NCI-N87 cells were treated daily with BIBW2992 or weekly with an intravenous bolus of 20 mg/kg trastuzumab and analysed as in a. (d) 14 days after NCI-N87 xenograft formation, animals carrying tumors were treated daily with BIBW2992 and analysed as in Figure 3a. (e) Animals carrying tumors established from H1975 cells were treated daily with BIBW2992, gefitinib, or lapatinib and analysed as in a.
Efficacy of BIBW2992 was additionally demonstrated in xenograft models resistant to first-generation EGFR inhibitors. Growth of the NCI-N87 gastric cancer cell line, which overexpresses HER2 and responds to anti-HER2 antibody therapy (Hurwitz et al., 2000), was completely inhibited by BIBW2992 (Figure 3c, Supplementary Table 5). BIBW2992 even induced regression of large tumors in this HER2-driven model (Figure 3d). Similarly, xenograft tumor formation by the NCIH1975 cell line, expressing EGFR L858R/T790M, was effectively controlled by BIBW2992, with a T/C value of 12% for doses of 20 mg/kg (Figure 3e).
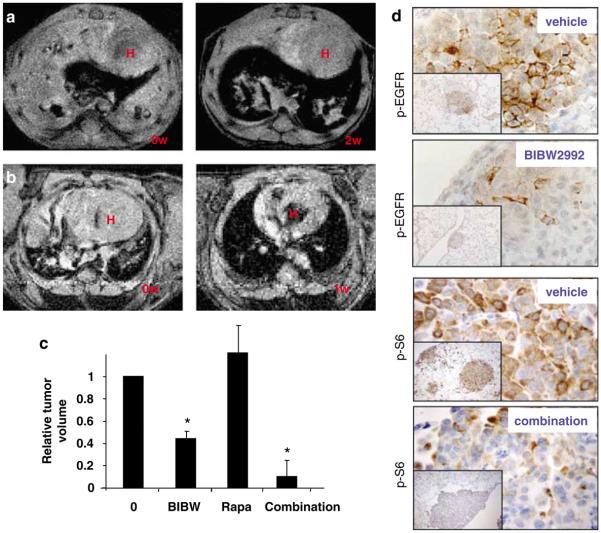
In our previous analysis of EGFR TKIs, we have described a model of de novo EGFR L858R/T790M driven lung cancer (Li et al., 2007b). This system represents a more disease-relevant and challenging model as compared to standard subcutaneous xenografts. Although the de novo murine lung tumors proved to be resistant to erlotinib (Li et al., 2007b), we found that a well-tolerated daily dose of 20 mg/kg BIBW2992 induced more than 50% percent tumor reduction after a 4-week treatment period (Figures 4a and c), as evaluated by magnetic resonance imaging (MRI). Histological analysis of lungs from treated mice revealed significant therapeutic effects, with decreased numbers of tumor foci and increased tissue spaces and fibrosis inside tumor nodules and an increase of pigment-laden macrophages indicative of tissue damage and repair (Supplementary Figure 2 and data not shown).
Figure 4.
BIBW2992 induces dramatic tumor regression in the _L858R/T790M EGFR_—driven lung cancer model. (a) Radiographic response induced by BIBW2992 in L858R/T790M EGFR mice. After approximately 6 weeks of doxycycline administration, tumor-bearing mice were identified by MRI and treatment with 20 mg/kg/day BIBW2992 was initiated with continuous doxycycline diet. MRI scanning was repeated 2 and 4 weeks later to monitor tumor development, and mice were sacrificed at 2 or 4 weeks after re-imaging. Representative MRI photos from one of four mice before treatment and after 2 weeks of BIBW2992 treatment are shown. H: heart area. (b) Radiographic response in L858R/T790M EGFR mice after 1 week of BIBW2992 (20 mg/kg, gavage) and rapamycin (2 mg/kg, intraperitoneally) combination treatment. Mice were on continuous doxycycline diet during treatment. Representative MRI photos from one of six treated mice are shown. H: heart area. (c) Histogram expressed as mean±s.d. illustrating the tumor regression in all treated mice in (a) and (b) at time point indicated below as evaluated by MRI. Statistical analyses were performed using Student's exact _t_-test. BIBW: BIBW2992 at 20 mg/kg/day for 2 weeks; Rapa: rapamycin at 2 mg/kg/day for 2 weeks; combination: BIBW2992 and rapamycin combination at above dose for 1 week; *P<0.05 for tumor regression rates of BIBW2992 alone and BIBW2992 combined with rapamycin group. (d) Immunohistochemistry staining showed inhibited EGFR and also downstream signaling after BIBW2992 alone treatment and combination therapy of BIBW2992 and rapamycin. L858R/T790M EGFR mice were treated with BIBW2992 (20 mg/kg, gavage) and rapamycin (2 mg/kg, intraperitoneally) for 48 h and lung sections were then stained with antibodies recognizing phospho-EGFR, and phospho-S6. Pictures shown are representative fields from two mice. Photographs were taken at low (×100, insets) and high magnification (×800), respectively.
Because the regression of the EGFR L858R/T790M tumors by BIBW2992 alone was not complete, we tested BIBW2992 in combination with rapamycin, which inhibits the EGFR-PI3K-mTOR axis. Although treatment with rapamycin alone is not effective in this particular animal model (Li et al., 2007b), 20 mg/kg BIBW2992 together with 2 mg/kg of rapamycin result in almost complete tumor regression in six EGFR L858R/T790M mice within 1 week of treatment (Figures 4b and c). Histology of lung from treated mice reveals grossly normal lung structure, albeit with foci of localized inflammatory cells in the alveolar space (Supplementary Figure 2). BIBW2992 alone was sufficient for downregulation of EGFR, HER2 and HER3 phosphorylation, and the combination of BIBW2992 with rapamycin resulted in dramatic downregulation of S6 phosphorylation, a biomarker of mTOR signaling (Figure 4d and data not shown).
Discussion
Although the discovery of oncogenic EGFR mutations that conferred sensitivity to first-generation EGFR inhibitors such as gefitinib and erlotinib generated much hope for effective therapy targeted to lung cancer patients with tumors harboring these mutations, the subsequent realization that these inhibitors provided only a marginal survival benefit due to acquired resistance indicated the need for novel inhibitors effective against a wider variety of EGFR mutants. A second generation of irreversible inhibitors that covalently modify EGFR is now emerging in the clinic. BIBW2992 is one such inhibitor active against both EGFR and HER2.
A variety of enzymological, cell-based and in vivo assays were employed to explore the efficacy of BIBW2992 against a series of EGFR mutants partially or wholly resistant to first-generation EGFR inhibitors. The resistance settings we have assessed include the prominent acquired second-site resistance mutation T790M, the primary resistance exon 20 insertion mutations, the partially resistant extracellular domain mutations found in glioblastoma, wild-type EGFR, and wild-type and mutant HER2.
We have shown that BIBW2992 are sufficient to specifically inhibit the in vitro and in vivo enzymatic activity of wild-type EGFR and HER2, as well as the erlotinib-sensitive EGFR L858R mutant and the erlotinib-insensitive L858R/T790M double mutant (Table 1, Supplementary Table 2, Figures 3b and 4d). Presumably because of this dual specificity, BIBW 2992 also inhibited heregulin-stimulated phosphorylation of HER3, a kinase-inactive heterodimerization partner of EGFR and HER2 (Supplementary Figure 1).
Three transformation assay systems were used to demonstrate the superior cell-based efficacy of BIBW2992 against the erlotinib-resistant EGFR mutants. BIBW2992 was at least 100-fold more effective than erlotinib in abolishing IL-3 independent survival of Ba/F3 cells supported by all three types of resistance mutations: T790M, extracellular domain mutations, including the variant III deletion, and overexpressed wild-type EGFR (Supplementary Table 4 and data not shown). Qualitatively similar results were observed using the NIH-3T3 anchorage-independent proliferation assay system and lung adenocarcinoma cell lines harboring EGFR and HER2 mutations (Figures 1a and 2b). Furthermore, treatment of NIH-3T3 cells expressing the EGFR mutants with BIBW2992 inhibited receptor autophosphorylation at least 100-fold more effectively than erlotinib (Figure 1b). IC50s for inhibition in these cell-based transformation models are generally well below 300 nM, the maximum achievable drug plasma concentration (Eskens et al., 2008). One notable exception is the A549 cells, which express wild-type EGFR and HER2 and are resistant to BIBW2992. However, this cell line harbors an activating KRAS mutation and is therefore expected to be resistant in general to anti-EGFR/HER2 therapy (Pao et al., 2005b; Krypuy et al., 2006).
We have shown that BIBW2992 is also effective in both xenograft models driven by EGFR L858R/T790M or HER2 overexpression (Figure 3) and a murine lung cancer model driven by EGFR L858R/T790M (Figure 4a). Although irreversible inhibitors have not been tested head-to-head in our model systems, our data suggest that BIBW2992 is superior to another irreversible EGFR-HER2 inhibitor, HKI-272, in inducing tumor regression in the L858R/T790M murine adenocarcinoma model both alone and in combination with rapamycin (Ji et al., 2006a; Li et al., 2007b). Importantly, BIBW2992 in combination with rapamycin led to a near-complete tumor regression (Figures 4b and c), comparable to that induced by erlotinib in the erlotinib-sensitive EGFR L858R lung cancer model (Ji et al., 2006a).
To conclude, we have shown that BIBW2992 is a highly potent, irreversible dual EGFR/HER2 tyrosine kinase inhibitor potentially efficacious in the treatment of cancers dependent on EGFR/HER2 signaling. In particular, NSCLC patients with tumors that harbor either primary (exon 20 insertion) or acquired (T790M) erlotinib resistance mutations might be ideal candidates for BIBW2992 treatment. On the other hand, NSCLC patients with primary resistance to first-generation EGFR inhibitors due to the previously mentioned KRAS mutations (Pao et al., 2005b), or acquired resistance due to amplification of the MET protooncogene (Engelman et al., 2007), would not be expected to respond to treatment with BIBW2992 alone. However, because MET signaling activates the phosphatidylinositol-3-kinase pathway in a HER3-dependent manner (Engelman et al., 2007), it is possible that the combination of BIBW2992 and rapamycin would be effective as well in patients with resistance to first-generation inhibitors acquired by this mechanism. We are now in the process of generating genetically defined cells and inducible bitransgenic mouse models that harbor both EGFR kinase domain mutations and MET amplification/overexpression to precisely test the efficacy of BIBW2992 and rapamycin combination in this setting.
Lung cancer patients who initially responded to erlotinib but subsequently acquired the T790M resistance mutation and relapsed have often exhausted other conventional chemotherapeutic options and represent an urgent unmet medical need.
Phase II clinical trials of BIBW2992 are underway, and these results may ultimately attest to the predictive power of our preclinical models, and more importantly, may demonstrate clinical benefit of BIBW2992 to this subset of lung cancer patients.
Materials and methods
Synthesis of compounds
BIBW2992 was synthesized at Boehringer Ingelheim Pharma GmbH and Co. KG as described in WO 02/50043. Lapatinib was synthesized as described in Carter, Malcolm Clive; Cockerill, George Stuart; Guntrip, Stephen Barry; Lackey, Karen Elizabeth; Smith, Kathryn Jane, Bicyclic heteroaromatic compounds as protein tyrosine kinase inhibitors, WO 99/35146. Erlotinib was synthesized as described in Schnur, Rodney C; Arnold, Lee D, Quinazoline derivatives, WO 96/ 30347. Canertinib was synthesized as described in Bridges, Alexander James; Driscoll, Denise; Klohs, Wayne Daniel. _N_-{4-(3-Chloro-4-fluoro-phenylamino)-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}acrylamide, an irreversible inhibitor of tyrosine kinases, WO 00/31048. Gefitinib was synthesized as described in Gibson, Keith Hopkinson, Quinazoline derivatives, WO 96/33980.
In vitro kinase activity assays
The wild type tyrosine kinase domain of the human EGFR (Ullrich et al., 1984) as well as the EGFR L858R/T790M double mutant were fused to Glutathione-_S_-transferase (GST), and extracted as described in Supplementary methods. The L858R mutant was purchased from Upstate (Charlottesville, VA, USA). Enzyme activity was then assayed in the presence or absence of serial inhibitor dilutions performed in 50% Me2SO. A random polymer pEY (4:1) from Sigma (St Louis, MO, USA) was used as substrate. Biotinylated pEY (bio-pEY) was added as a tracer substrate. The kinase domain of HER2 (Coussens et al., 1985) was cloned using baculovirus system and extracted similarly to that of EGFR kinase domain. Detailed procedures for EGFR, HER2, SRC, BIRK and VEGFR2 kinase activity assays are included in Supplementary information.
Cell culture
Information pertaining to cell lines and culture conditions can be found in the Supplementary information. Erlotinib was obtained from WuXi Pharmatech (Shanghai, China) and diluted to the required concentrations in DMSO. Epidermal Growth Factor (EGF) was obtained from Upstate and diluted to required concentrations in PBS.
Phosphorylation of EGFR and HER2 at the cellular level
Cells (1×104) were transferred into each well of a 96-well plate and cultured over night in serum-free media for EGFR phosphorylation assay. After addition of test compounds on the next day, the plates were then incubated at 37°C for 1 hour. EGF-stimulation was done at 100 ng/ml for 10 min at room temperature. Cells were washed with ice cold PBS before extraction with 120 μl per well HEPEX buffer and shaken for 1 h at room temperature. In all 2×104 cells per well was used for HER2 phosphorylation assay.
Streptavidin precoated plates were coated with anti-EGFR-biotin (Leinco, E101) at 1:100 dilution with blocking buffer and c-erb2/HER2 oncoprotein Ab-5(Clone N24)-Biotin (Neo-marker, Fremont, CA, USA). Extracts from above steps was then transferred to the antibody-coated wells, and incubated for 1 h at room temperature. Assessment of color development is described in Supplementary information. Extinction was measured at 450 nm.
The data generated were analysed by the program PRISM (GraphPad Inc., San Diego, CA, USA). Normalized values were used to calculate the IC50 by a nonlinear regression curve fit (variable slope).
Soft-agar anchorage-independent growth assays
NIH-3T3 cells expressing wild-type or mutant EGFR were suspended in a top agar layer containing Dulbecco's modified Eagle's medium, 10% calf serum and 0.4%. Select Agar (Gibco/Invitrogen, Grand Island, NY, USA). Erlotinib, BIBW2992 and/or EGF was added to the top layer as described and plated onto a bottom agar layer containing Dulbecco's modified Eagle's medium, 10% calf serum and 0.5% Select Agar with each cell line/drug combination plated in triplicate. After a 2-week incubation period, the number of colonies was determined from 10 fields photographed at ×4 for each cell line/drug combination.
The NSCLC cells H1666, H3255 and NCI-H1975 were suspended in 0.3% sea plaque agarose (FMC Bioproducts, Philadelphia, PA, USA) made up in IMDM and 10% calf serum and layered over a 1% bottom agar prepared in the same medium. Serial dilutions of compounds prepared in duplicates were transferred onto the cell layers (controls without substance) and allowed to diffuse. The 96-well microtiter plates were incubated at 37 °C and 5% CO2 in a humidified atmosphere for 2 weeks. Alamar Bluet™ (Serotec Ltd, Düsseldorf, Germany) was added to each well and fluorescence (extinction wavelength of 544 nm and emission at 590 nm) was determined after an additional 6 h of incubation at 37 °C. Normalized data was fitted by iterative calculation using a sigmoidal curve analysis program (Graph Pad Prism version 3.0) with variable Hill slope to determine the IC50 values.
Drug treatment and immunoblotting
Cell pellets from NIH-3T3 cells and Ba/F3 cells cultured as above or drug treated were collected and protein-isolated and purified as previously described (Greulich et al., 2005; Lee et al., 2006). For drug treatment, NIH-3T3 cells and Ba/F3 cells expressing EGFR were placed overnight in media containing 0.5% serum. Erlotinib, BIBW2992 and/or EGF were then added as described for a total of 2 hours. Whole cell lysates were subjected to SDS–polyacrylamide gel electrophoresis (SDS–PAGE) on 8% polyacrylamide gels, transferred to PVDF and probed as described utilizing anti-EGFR (Cell Signaling Technologies, Beverly, MA, USA), anti-phospho-EGFR Y1173 (Upstate) and anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Mouse cohorts
Five to six-week-old athymic NMRI-nu/nu female mice (21–31 g) were purchased from Harlan (Germany). All experiments complied with the Declaration of Helsinki and European Policy Legislations (FELASA and GV-SOLAS) on the Care and Use of Laboratory Animals. After acclimatization mice were inoculated with 1–5×106 (in 100 μl) A431, FaDu, NCI-N87, SKOV-3 or H1975 cells into the right flank of the animal. The MDA-MB-453 xenografts were established by transplanting fresh fragments (2×2×2mm) into the right flank of the animal.
The generation of Tet-op-L858R/T790M EGFR/CCSP-rtTA mice was described previously (Li et al., 2007b). All mice were housed in the pathogen-free environment at Harvard School of Public Health and all mouse experiments performed were approved by the Institutional Animal Care and Use Committee (IACUC). To induce L858R/T790M EGFR expression, mice were fed with a doxycycline diet (Research Diets Inc.). Doxycycline withdrawal experiments in previous studies have shown that lung tumors in this model are critically dependent on continued presence of doxycycline.
Targeted therapies in transgenic murine lung cancer model and xenograft models
In xenograft models, mice with established tumors (40–130mm3) were randomized and treated daily p.o. with test compounds or vehicle control on the basis of individual weights. Tumors were measured 3 times a week with calipers, and tumor volumes were calculated by the formula π/6×length×(width)2. Except for trastuzumab, experimental compounds were dissolved in 1.8% HP-beta-CD (Hydroxypropyl-β-cyclodextrin, Aldrich, catalog no. 33, 259–3), 5% acetic acid (10%) and aqueous Natrosol (0.5%) and administered by intragastral gavage. The administration volume was 10 ml/kg body weight.
Four bitransgenic mice on continuous doxycycline diets for more than 6 weeks were subjected to MRI (Figure 4) to document the lung tumor burden. BIBW2992 (generated by Boehringer Ingelheim Austria GmbH) formulated in 0.5% methocellulose-0.4% polysorbate-80 (Tween 80) was administered orally by gavage at 20 mg/kg once daily dosing schedule. Rapamycin (LC laboratories, Woburn, MA, USA) was dissolved in 100% ethanol, freshly diluted in 5% PEG400 and 5% Tween 80 before treatment and administered by intraperitoneal injection at 2 mg/kg daily dosage. Mice were monitored by MRI every 1 or 2 weeks to determine reduction in tumor volume and killed for further histological and biochemical studies after drug treatment.
For immunohistochemistry staining, three tumor-bearing mice in each group were treated three times with either BIBW2992 (20 mg/kg) alone or BIBW2992 (20 mg/kg) and rapamycin 2 mg/kg at 24 h intervals and killed 1 h after the last drug delivery. All the mice were kept on the doxycycline diet throughout the experiments. Littermates were used as controls.
MRI scanning and tumor volume measurement
MRI scanning was performed as described previously (Li et al., 2007a) and in Supplementary information. On each image, the areas indicating the pulmonary tumor were manually segmented and measured to calculate tumor volumes using Image J (ver. 1.33, National Institute of Health).
Statistics
Statistical analyses were performed using unpaired 2-tailed student's _t_-test. _P_-values of less than 0.05 were considered significant.
Acknowledgements
We thank Dr Jeffrey Whitsett for providing the CCSP-rtTA transgenic mice. We also thank all present and past members of the BIBW2992 research and development team at Boehringer Ingelheim Austria GmbH and Boehringer Ingelheim Pharma GmbH & Co. KG for their respective contributions. In particular, we acknowledge Ursula Strobl, Monika Leber, Reiner Meyer, Regina Ruzicka, Franziska Popp, Christine Lam and Mei Zheng for their commitment and excellent technical assistance. TS was supported by a Career Development Award as part of the Dana-Farber/Harvard Cancer Center Specialized Program of Research Excellence (SPORE) in Lung Cancer, NIH Grant P20 CA90578. GIS was supported by NIH Grants P20 CA90578 and R01 CA90687. KKW was supported by NIH Grant K08 AG024004, R01 CA122794, R01 AG2400401, the Sidney Kimmel Foundation for Cancer Research, the Joan Scarangello Foundation to Conquer Lung Cancer, the Cecily and Robert Harris Foundation and the Flight Attendant Medical Research Institute.
Footnotes
References
- Bedano PM, Hanna NH. Salvage therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2006;1:582–587. [PubMed] [Google Scholar]
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Jänne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, et al. ErbB-3 mediates PI3K activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci USA. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer. 2008;98:80–85. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz E, Klapper LN, Wilchek M, Yarden Y, Sela M. Inhibition of tumor growth by poly(ethylene glycol) derivatives of anti-ErbB2 antibodies. Cancer Immunol Immunother. 2000;49:226–234. doi: 10.1007/s002620000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Janne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol. 2005;23:3227–3234. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006a;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Ji H, Zhao X, Yuza Y, Shimamura T, Li D, Protopopov A, et al. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci USA. 2006b;103:7817–7822. doi: 10.1073/pnas.0510284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Greulich H, Janne PA, Sellers WR, Meyerson M, Griffin JD. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65:8968–8974. doi: 10.1158/0008-5472.CAN-05-1829. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005a;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ji H, Yuza Y, Meyerson M, Wong KK, Tenen DG, et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005b;65:7096–7101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- Krypuy M, Newnham GM, Thomas DM, Conron M, Dobrovic A. High resolution melting analysis for the rapid and sensitive detection of mutations in clinical samples: KRAS codon 12 and 13 mutations in non-small cell lung cancer. BMC Cancer. 2006;6:295. doi: 10.1186/1471-2407-6-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, Debiasi RM, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Ji H, Zaghlul S, McNamara K, Liang MC, Shimamura T, et al. Therapeutic anti-EGFR antibody 806 generates responses in murine de novo EGFR mutant-dependent lung carcinomas. J Clin Invest. 2007a;117:346–352. doi: 10.1172/JCI30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007b;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Mulloy R, Ferrand A, Kim Y, Sordella R, Bell DW, Haber DA, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–2330. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib Is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005a;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005b;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Reding EC, Fuhs SR, Lu Q, Piu F, Wong S, et al. Pharmacology and signaling properties of epidermal growth factor receptor isoforms studied by bioluminescence resonance energy transfer. Mol Pharmacol. 2007;71:508–518. doi: 10.1124/mol.106.027656. [DOI] [PubMed] [Google Scholar]
- Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Ji H, Minami Y, Thomas RK, Lowell AM, Shah K, et al. Non-small-cell lung cancer and Ba/F3 transformed cells harboring the ERBB2 G776insV_G/C mutation are sensitive to the dual-specific epidermal growth factor receptor and ERBB2 inhibitor HKI-272. Cancer Res. 2006;66:6487–6491. doi: 10.1158/0008-5472.CAN-06-0971. [DOI] [PubMed] [Google Scholar]
- Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumors. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Vikis H, Sato M, James M, Wang D, Wang Y, Wang M, et al. EGFR-T790M is a rare lung cancer susceptibility allele with enhanced kinase activity. Cancer Res. 2007;67:4665–4670. doi: 10.1158/0008-5472.CAN-07-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Yuza Y, Glatt KA, Jiang J, Greulich H, Minami Y, Woo MS, et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol Ther. 2007;6:661–667. doi: 10.4161/cbt.6.5.4003. [DOI] [PubMed] [Google Scholar]