Cellular Prion Protein Mediates Impairment of Synaptic Plasticity by Amyloid-β Oligomers (original) (raw)
. Author manuscript; available in PMC: 2009 Sep 22.
Published in final edited form as: Nature. 2009 Feb 26;457(7233):1128–1132. doi: 10.1038/nature07761
Abstract
A pathological hallmark of Alzheimer’s disease (AD) is an accumulation of insoluble plaque containing the amyloid-β peptide (Aβ) of 40–42 aa residues1. Prefibrillar, soluble oligomers of Aβ have been recognized to be early and key intermediates in AD-related synaptic dysfunction2–9. At nanomolar concentrations, soluble Aβ-oligomers block hippocampal long-term potentiation7, cause dendritic spine retraction from pyramidal cells5,8 and impair rodent spatial memory2. Soluble Aβ-oligomers have been prepared from chemical syntheses, from transfected cell culture supernatants, from transgenic mouse brain and from human AD brain2,4,7,9. Together, these data imply a high affinity cell surface receptor for soluble Aβ-oligomers on neurons, one that is central to the pathophysiological process in AD. Here, we identify the cellular Prion Protein (PrPC) as an Aβ-oligomer receptor by expression cloning. Aβ-oligomers bind with nanomolar affinity to PrPC, but the interaction does not require the infectious PrPSc conformation. Synaptic responsiveness in hippocampal slices from young adult PrP null mice is normal, but the Aβ-oligomer blockade of long-term potentiation is absent. Anti-PrP antibodies prevent Aβ-oligomer binding to PrPC and rescue synaptic plasticity in hippocampal slices from oligomeric β. Thus, PrPC is a mediator of Aβoligomer induced synaptic dysfunction, and PrPC-specific pharmaceuticals may have therapeutic potential for Alzheimer’s disease.
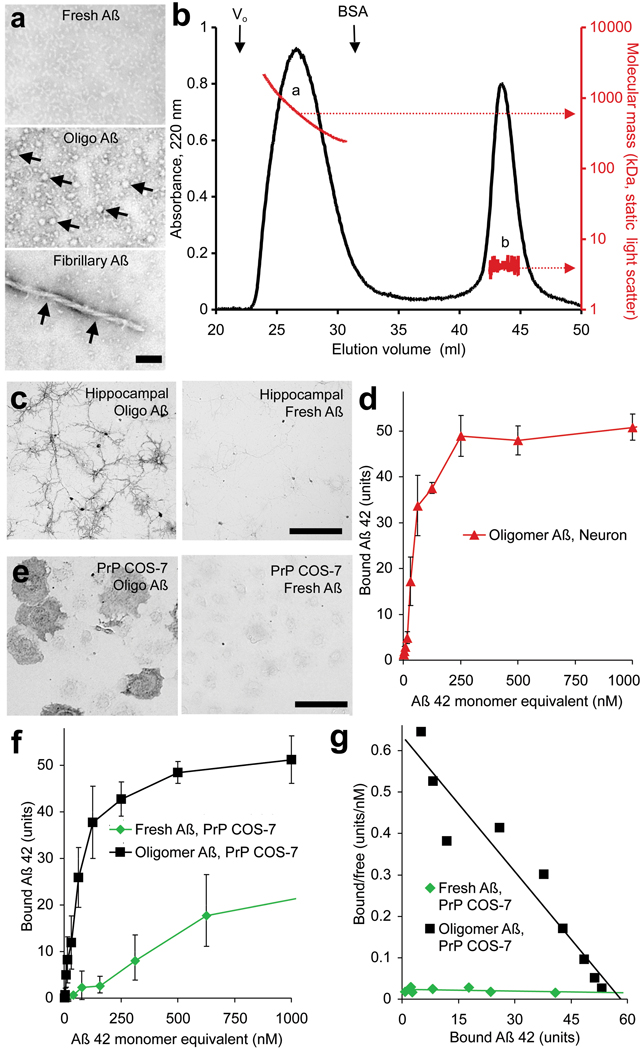
To characterize Aβ-oligomer binding sites, we synthesized biotin-Aβ42 peptide, denatured the peptide and allowed oligomers to form as described for ADDLs4. Consistent with findings for untagged Aβ42-oligomers5, biotin-Aβ42-oligomer preparations contain spherical particles of 5–6 nm diameter visible by negative staining in transmission electron microscopy, with rare protofibrils and no larger fibrils (Fig. 1a). Approximately 50% of peptide migrates by size exclusion chromatography (SEC) as a distinct assembly with a size of approximately 500 kDa corresponding to 50–100 Aβ monomers (Fig. 1b). Low molecular weight forms of Aβ42 in either oligomeric and fresh preparations migrate by SEC as monomers (Fig. 1b), demonstrating that the trimers or tetramers observed by SDS-PAGE (Suppl. Fig. 1) are not present under native conditions (and ref.10). Aβ42-oligomer binds to hippocampal neurons, whereas freshly prepared biotin-Aβ42 does not (Fig. 1c; Suppl. Fig. 2). Biotin-Aβ42-oligomer binding is enriched in MAP2-positive dendrites, with lower levels in βIII-tubulin positive axons, and very low levels in astroglial cells (Suppl. Fig. 3a, c, not shown and ref.6). The Aβ42-oligomer binding is most concentrated at post-synaptic densities marked by immunoreactive PSD-95 (Suppl. Fig. 3b). Binding to neurons is saturable, with an apparent KD of 50–100 nM monomer equivalent (Fig. 1d). The KD of the relevant Aβ42 assembly must be much less than 100 nM because minimal binding is detected with freshly prepared Aβ42. If the Aβ42 species responsible for binding contains 100 monomers and represents 50% of all biotin-Aβ42 in the preparation, the corrected affinity would be ∼0.4 nM. While this formulation of Aβ42-oligomer is not chromatographically identical to Aβ42-oligomer from brain2,3,9, it affords detection of high affinity binding sites likely to share pathological actions with sites for other Aβ42-oligomer preparations5,6,11.
Figure 1. Oligomeric Aβ42 binds to neurons and to cells expressing PrPC.
a, Freshly prepared, oligomeric, or fibrillary preparations of Aβ42 were examined by transmission electron microscopy with negative staining. The arrows indicate globular oligomers in the middle segment and a fibril in the lower segment. Scale bar, 25 nm. b, Oligomeric Aβ42 peptide was analyzed by size exclusion chromatography, monitoring absorbance at 220 nm (black) and light scattering (red). The void volume (Vo) and elution of bovine serum albumin (BSA) from a separate run are shown. c, Oligomeric Aβ42 peptide (200 nM total peptide) binds to 21 DIV hippocampal neurons, whereas fresh Aβ42 (200 nM) does not. Bound biotin-Aβ42 was visualized by alkaline phosphatase conjugated streptavidin. d, Dose dependence of oligomeric Aβ42 binding to hippocampal neurons.e, The binding of 40 nM oligomeric or freshly prepared Aβ42 to COS-7 expressing PrPC. f, g, Fresh or oligomeric Aβ42 binding to PrPC-expressing COS-7 cells as a function of Aβ42 total concentration (monomer equivalent for oligomer preparations). Data are mean ± sem, and the Scatchard analysis is presented in g. Scale bars, 100 µm for c and e.
A key requirement for expression cloning of Aβ42-oligomer binding sites is the existence of a cell line with low background binding. COS-7 cells exhibit <5% of the biotin-Aβ42-oligomer binding level in hippocampal neurons. We expressed cDNAs from an adult mouse brain library in COS-7 cells and screened for biotin-Aβ42-oligomer binding. From 225,000 clones, two independent positive clones were isolated and both were found to encode full-length mouse PrP (Fig. 1e). Aβ42-oligomers bind to cells expressing the PrPC conformation; interaction is not dependent on the PrPSc conformation required for infectious prion disease12. PrPC is known to interact with copper ion but this does not alter Aβ42-oligomer binding (Suppl. Fig. 4). Like hippocampal neurons, PrPC-expressing COS-7 cells have much lower affinity for freshly prepared low molecular weight biotin-Aβ42 (Fig. 1e, Fig. 2a). The apparent dissociation constant for biotin-Aβ42-oligomer binding to PrPC-expressing COS-7 cells is indistinguishable from that for biotin-Aβ42-oligomer binding to hippocampal neurons (Fig. 1f, g, Fig. 2a). The selectivity of PrP for binding Aβ42-oligomer versus fresh Aβ42 is reflected in the ratio of KDs and must be greater than 20 (>2000 nM / 92 nM) based on the total peptide monomer concentration in the Aβ42-oligomer preparation (Fig. 2a), or as great as 5000 (>2000 nM / 0.4 nM) based on the molar concentration of Aβ42-oligomer estimated by SEC/LS (Fig. 1b).
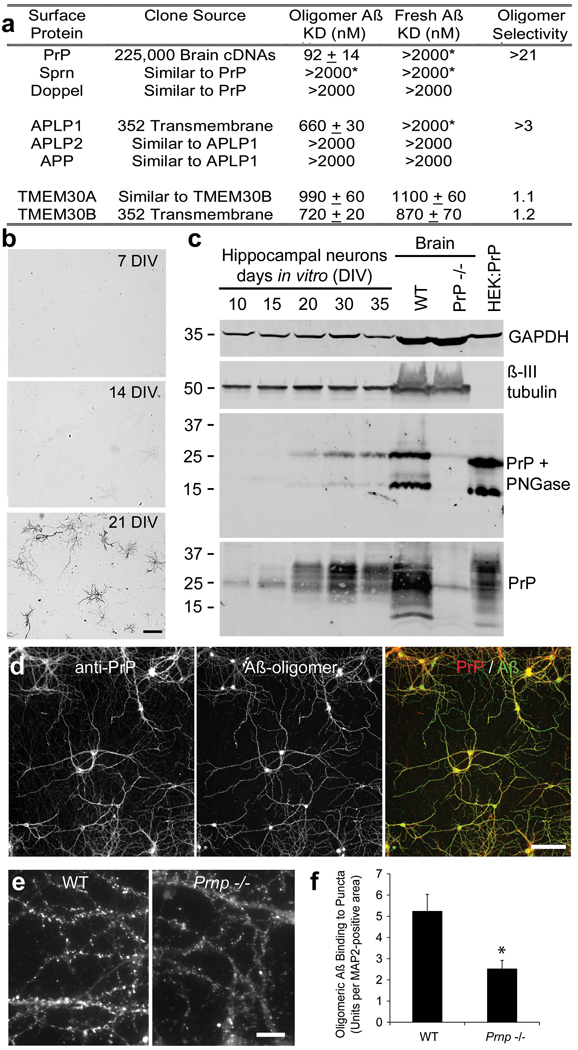
Figure 2. Characterization of Aβ42 oligomer binding sites.
a, The table summarizes the Aβ42 binding to COS-7 cells expressing the indicated proteins. KD values are mean ± sem. APLP1 and TMEM30B were identified by examination of individual clones from a 352-member pre-existing collection of expression vectors for transmembrane proteins (Origene). *, For KD values indicated with an asterisk, binding of Aβ42 was detected at 2 µM ligand, but binding was not saturated. Oligomer specificity is the ratio of monomer KD to oligomer KD. b, Oligomeric Aβ42 (30 nM) binding to hippocampal neurons after culture for the indicated days. Neuronal cell density is similar in the three panels. Scale bar, 100 µm. c, Total protein (20 µg) from hippocampal cultures or from whole brain of the indicated genotype or from HEK293T cells transfected with a PrP expression vector was analyzed by immunoblot with anti-PrP antibody (8H4), with anti-βIII tubulin antibody or with anti-GAPDH antibody. Samples for the middle panel were pretreated with endoglycosidase (PNGase F) before gel electrophoresis through a 4–20% polyacrylamide gel in tris-glycine-SDS. Mol wt standards at left. d, Hippocampal neurons from E18 mice after 21 DIV were incubated with biotinylated Aβ42-oligomer (130 nM monomer equivalent) for 1 hour at 37C and then fixed. Bound Aβ was detected with fluorescent avidin (green), and PrPC with anti-PrP immunocytochemistry (red). Scale bar, 100 µm. e, Cultures were prepared from wild type or Prnp −/− mice and then binding of Aβ42-oligomer (130 nM monomer equivalent) was detected as in d. Scale bar, 10µm. f, Binding of 100 nM Aβ42-oligomers to puncta in hippocampal cultures as shown in e. Data from n = 6 pairs of cultures from wild type and _Prnp_−/− embryos. Mean ± sem; *, P < 0.05, two-tailed t test.
To explore any contribution of the biotin tag to PrP affinity, we prepared untagged Aβ42-oligomer and examined binding to PrPC-expressing cells with an anti-Aβ antibody (Suppl. Fig. 5). Untagged Aβ42 binding is localized to PrPC-expressing cells. Thus, binding is mediated by the Aβ aa residues. The simplest model for PrPC expression inducing Aβ42-oligomer binding is a direct interaction between the two polypeptides. To verify this, we examined the interaction of purified PrP-Fc with Aβ42 (Suppl. Fig. 6). A control Fc protein, immobilized on a resin, retained neither freshly prepared nor oligomeric preparations of Aβ42. In contrast, PrP-Fc protein retained Aβ42 peptide through a direct physical interaction. The pre-incubated oligomeric form of Aβ42 was retained to a 2.5-fold greater degree than the freshly prepared peptide. The preference of PrP for Aβ-oligomer versus fresh Aβ42 is less complete here than in the cellular assays, perhaps due to the use of concentrated solid-phase purified reagents and higher concentrations.
Although the PrP cDNA is the only clone to support oligomeric Aβ42 binding isolated from the brain cDNA library, we considered whether other Aβ binding sites might exist. First, we examined two clones sharing sequence similarity with PrP, Doppel and Sprn, but neither exhibited affinity for oligomeric Aβ42 (Fig. 2a, Suppl. Fig. 7). Second, we screened a pre-existing collection of 352 cDNAs encoding transmembrane proteins one-by-one. In this format, weaker affinity interactions are detectable than in the initial pooled brain library screen. Amyloid precursor-like protein 1 (APLP1) and Transmembrane protein 30B (TMEM30B) were isolated through this focused screen and demonstrate KDs for oligomeric Aβ42 of 660 and 720 nM, respectively (Fig. 2a, Suppl. Fig. 7). These lower affinity binding proteins exhibit limited specificity for oligomeric Aβ42, as compared to fresh Aβ42. APLP1 shares similarity with APP and with APLP2, but neither of these proteins binds Aβ42 (Fig. 2a). TMEM30B is similar to TMEM30A, which is expressed at high levels in the brain. TMEM30A supports Aβ42 binding with an affinity similar to TMEM30B and shows no preference for oligomeric species (Fig. 2a). The receptor for advanced glycation endproducts (RAGE) and the α7 nicotinic acetylcholine receptor (nAChRα7) have been reported to bind Aβ13,14. In this heterologous COS-7 cell binding assay, expression of RAGE yielded less Aβ42-oligomer binding signal than did PrP, APLP1 or TMEM30B, and we failed to detect binding to nAChRα7 (Suppl. Fig. 7). Thus, while several proteins exhibit Aβ42 binding, only PrP has high affinity and high selectivity for the oligomeric peptide.
Aβ42-oligomer binding to neurons depends on developmental stage, with minimal binding to neurons immediately after dissociation from E18 hippocampus. Not until 15–20 days have elapsed in vitro does Aβ42-oligomer binding to neurons become robust (Fig. 2b). The immunoblot level of PrPC expression closely matches this developmental pattern (Fig. 2c). Immunocytochemically, PrPC expression is largely restricted to MAP2+ dendrites of differentiated neurons (Suppl. Fig. 3d, Fig. 2d). Furthermore, localization of PrP immunoreactivity and Aβ42-oligomer binding overlap extensively (Fig. 2d). If PrPC were the only cellular binding site for Aβ42-oligomers, then no binding would be detected in cultures from Prnp −/− mice at 20 DIV. Because we observe 50% reduction of punctate Aβ42-oligomer binding in such cultures (Fig. 2e, f), PrPC cannot be the only cell surface molecule binding Aβ42-oligomers. Multiple alternative sites, including APLP1, TMEM30A, TMEM30B, RAGE and unidentified proteins, may explain Aβ42 binding to Prnp −/− neurons.
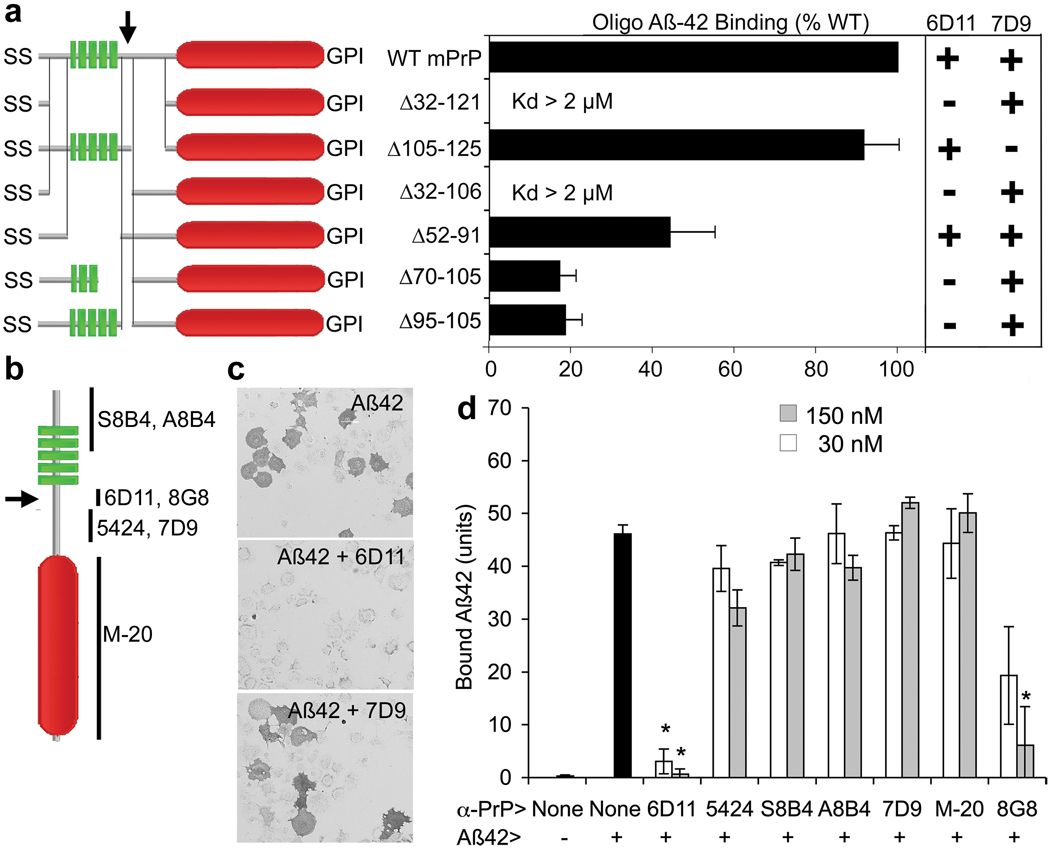
Different domains of PrPC have been associated with various activities. The N-terminal octapeptide repeat domain (aa 60–95) contributes to extracellular copper ion binding15,16. The unstructured central domain (aa 95–134) includes a charge cluster (aa 95–110) and a segment with hydrophobic character (aa 112–134). This central domain has been implicated in masking a neurodegenerative activity of PrPC17,18. The C-terminal domain is globular (aa 134–231)19 and the protein is GPI-anchored to the plasma membrane. We mapped Aβ42-oligomer binding using PrP deletion mutants (Fig. 3). Each mutant protein was expressed at the COS-7 surface by live anti-PrP immunostaining (Fig. 3a, Suppl. Fig. 8). Deletion of the octapeptide repeat domain and the central domain (Δ32–121) abrogates binding, indicating that the globular domain alone cannot mediate binding. The hydrophobic 105–125 region is not a major determinant, since Δ105–125 protein binds Aβ42-oligomers indistinguishably from full length PrPC, and since the Δ32–106 variant behaves like the Δ32–121 variant, having no Aβ42-oligomer affinity. To distinguish whether the 95–110 charge cluster or the octapeptide repeat domain is crucial for Aβ42 binding, a mutant lacking the 52–91 segment was expressed. The Δ52–91 mutant exhibits significant Aβ42 binding, implicating the 95–110 region as a principal site for Aβ42-oligomer binding. Consistent with this hypothesis, deletion of 11 aa in the Δ95–105 variant reduces binding by 80%, and there was no further reduction in the Δ70–105 variant.
Figure 3. Aβ42 oligomers bind to residues 95–110 of PrPC.
a, COS-7 cells were transfected with expression plasmids directing the expression of each of the indicated PrP deletion mutants (green, octapeptide repeats; red, globular domain). Transfected cells were assessed for binding of oligomeric Aβ42, or by live cell immunocytochemistry with 6D11 and 7D9 anti-PrP antibodies. Mean ± sem from 4 experiments. b, Schematic of antibody epitopes. c, d, PrPC-expressing COS-7 cells were analyzed for oligomeric Aβ42 binding after exposure to the various anti-PrP antibodies for one hour. 6D11 and 8G8 but not other antibodies block Aβ42-oligomer binding. Data are mean ± sem from 4 experiments. Inhibitalicion of binding by 6D11 or 8G8 is significant (*, P < 0.02, ANOVA).
As an alternative method to localize Aβ42 binding within PrPC, we employed anti-PrP antibodies (Fig 3b–d). Of six antibodies initially tested, only one (6D11) blocked the binding of Aβ42 assemblies to PrPC with an IC50 of 1 nM (Fig. 3b–d, Suppl. Fig. 8–10). The 6D11 blockade is epitope-specific since the 7D9 antibody binds avidly to a different epitope but fails to block Aβ42 binding (Fig. 3a–d, Suppl. Fig. 8, 9). The epitope for 6D11 corresponds to aa 93–109 of mouse PrPC, matching the conclusion that the 95–105 region is a primary determinant for binding. To confirm this hypothesis, we examined the effect of an additional antibody (8G8) with an overlapping epitope, aa 95–110. The 8G8 antibody blocked Aβ42-PrPC interaction, though with a lesser potency than 6D11. The effect of 6D11 was not caused by internalization of PrPC, since similar cell surface levels of PrPC were detectable after 6D11 pre-incubation (Suppl. Fig. 11). The 6D11 antibody is highly specific for PrPC, as no immunoreactivity was observed in Prnp null brain sections nor was there any reactivity to Aβ42 (Suppl. Fig. 12 and not shown). We conclude that the 95–105 segment of PrPC contributes to Aβ42-oligomer binding in a 6D11-sensitive manner.
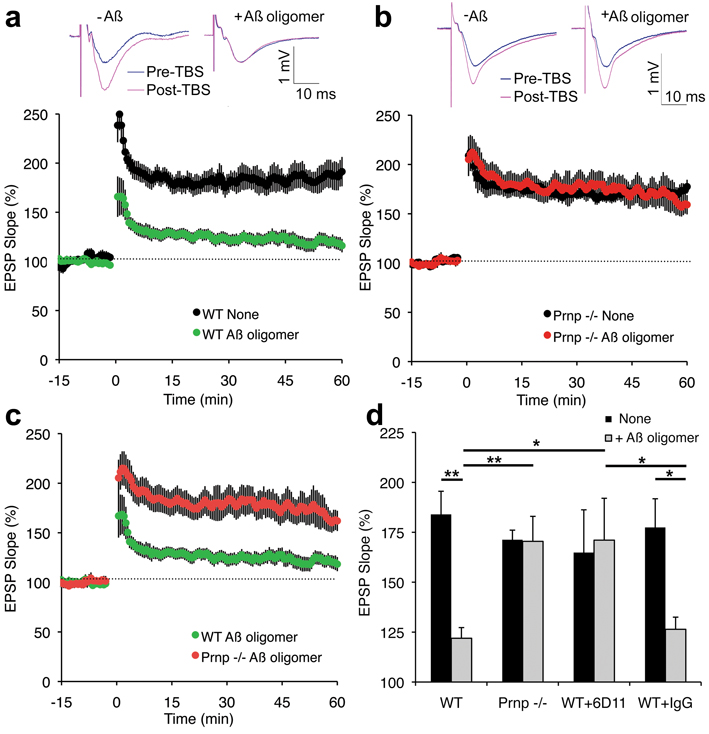
While these data demonstrate that PrPC is a high affinity binding site for Aβ42-oligomers, they do not assess its role in the pathological actions of Aβ42. It has been noted that soluble Aβ42-oligomers suppress long-term potentiation (LTP) of the Schaffer collateral pathway between hippocampal CA3 and CA1 pyramidal cells 7,11. Therefore, we compared the effects of soluble Aβ42-oligomers on LTP from slices of wild-type versus Prnp −/− mice 20,21. As reported previously, soluble Aβ42-oligomers (500 nM total peptide, estimated 2 nM Aβ42-oligomer) reduce LTP in hippocampal slices from wild-type mice (Fig. 4a, d). The slope of the excitatory postsynaptic potential (EPSP) after theta burst stimulation is augmented by 80% in control slices but only by 20% in slices pre-incubated with Aβ42-oligomer preparations. In slices from 2–6 month old PrP null mice without Aβ42 treatment, Schaffer collateral LTP is indistinguishable from baseline levels of wild-type mice (Fig. 4b), as described previously22,23. Strikingly, there is no inhibition of LTP by Aβ42-oligomers in the Prnp −/− slices (Fig. 4b–d).
Figure 4. PrPCis required for Aβ42 oligomer inhibition of hippocampal long-term potentiation.
a, Field potentials were recorded from the CA1 region of hippocampal slices from adult wild-type mice with or without the addition of 500 nM oligomeric Aβ42 to the perfusion 20–40 minutes prior to theta burst stimulation (TBS). The top panels show traces before and after TBS. The slope of the EPSP relative to the pre-TBS level is a plotted as a function of time in the lower panel. Data are mean ± sem from separate slices. For no peptide, n = 12 slices from 9 mice and for Aβ-oligomer, n = 31 slices from 14 mice. b, CA1 potentials were recorded from slices of mice lacking PrP expression by the same method as in a. There is no significant inhibition of LTP by oligomeric Aβ42. For no peptide, n = 10 slices from 7 mice and for Aβ-oligomer, n = 35 slices from 15 mice. c, The CA1 EPSP slope in wild-type and Prnp −/− slices was recorded in the presence of oligomeric Aβ42 by an observer blind to the genotype and is replotted from panels a and b. For the values 30–60 minutes post-TBS, the EPSPs were significantly greater in the _Prnp_−/− slices by Repeated Measures ANOVA, P = 0.005. For WT, n = 31 slices from 14 mice and for Prnp −/−, n = 35 slices from 15 mice. d, The magnitude of LTP between 30–60 minutes is plotted as a function of genotype, the addition of 6D11 antibody, control IgG and/or Aβ42 oligomer prior to the induction of LTP. Data are mean ± sem. For 6D11 without Aβ, n = 7, and for 6D11 plus Aβ, n= 6. For IgG without Aβ, n = 8, and for IgG plus Aβ, n= 6. The indicated comparisons are significant at **P < 0.01 or *P < 0.05, ANOVA. Untreated, IgG and 6D11 slices without Aβ42 did not differ significantly.
The lack of Aβ42 sensitivity for LTP in Prnp −/− slices suggests that PrPC acts as a receptor for Aβ42-oligomers mediating inhibition of LTP in wild-type slices. Alternatively, chronic loss of PrPC may lead to developmental and/or compensatory effects that account indirectly for Aβ42-oligomer ineffectiveness. To separate these possibilities, we pretreated wild-type slices with the 6D11 anti-PrP antibody (100 nM for 20 min) shown to block Aβ42 binding acutely (Fig. 3). Pretreatment with control IgG did not reduce the suppression of LTP by Aβ42-oligomer (Fig. 4d). In contrast, the 6D11-pretreated wild-type slices were protected from LTP suppression by the later addition of Aβ42-oligomer preparations (Fig. 4d). Thus, we conclude that PrPC exerts a receptor action acutely to mediate Aβ42-oligomer inhibition of synaptic plasticity in the hippocampal slice.
The major finding of this study is that PrPC functions as a receptor to mediate deleterious effects of the Aβ42-oligomer. This hypothesis is supported by our isolation of PrPC as an Aβ42-oligomer binding site in an unbiased genome-wide screen, by the match between PrPC expression and the properties of Aβ42-oligomer binding sites and by the localization of Aβ binding to a neurodegeneration-associated domain of PrPC. Although PrPC is not the sole binding site for Aβ-oligomers on hippocampal neurons, it is essential for Aβ42-oligomer inhibition of hippocampal LTP. Several publications indirectly support coupling of Aβ42-oligomers and PrPC. For example, a polymorphism in Prnp gene is associated with Alzheimer’s disease in certain populations24 and with long-term memory formation in the general population25. Amongst several proteins found in PrPC immunoprecipitates are APP and the related proteins, APLP1/226,27.
Glutamate receptors are central to LTP and their modulation has been implicated in deleterious synaptic Aβ action5,8,28,29. Very recently, PrPC has been shown to interact with NMDA receptor subunit 2D (NR2D), and to modulate its function30. We assessed whether Aβ42 interaction might regulate glutamate receptors directly through PrPc. When expressed in a heterologous X. laevis oocyte system, GluR1–4 receptors and NR-2B and -2D containing receptors are insensitive to Aβ42-oligomers, with or without PrPC co-expression (Suppl. Fig. 14, Suppl. Fig. 15). This is consistent with previous observations that Aβ drives glutamate receptor redistribution in neurons together with morphologic changes in dendrites5,8,9. Thus, Aβ42-oligomer interaction with PrPC is likely to initiate a signaling cascade that is not operative in oocytes, but one that is capable of modifying synaptic morphology and function in brain. The mechanism by which Aβ42-oligomer binding to PrPC participates in AD appears unrelated to the infectious PrPSc conformation of PrP. In this regard, the neurodegeneration reported in transgenic mice expressing truncated forms of PrPC may be more relevant17,18. A putative PrPC-associated transmembrane co-receptor is likely to play a central role in AD-mediated neurodegeneration. PrPC-specific reagents will provide molecular tools to dissect the cellular basis for Aβ42-oligomer induced changes in synaptic function. The interaction between Aβ and PrPC provides a novel site for the development of therapeutics designed to relieve AD symptoms.
METHODS SUMMARY
Mouse strains
Prnp −/− mice (Edinburgh strain)21 on an inbred C57Bl6 background were obtained from Dr. Chesebro of the Rocky Mountain Laboratories and Prnp −/− mice (Zurich I)20 on a mixed strain background from the European Mutant Mouse Archive.
Aβ42 preparation and cellular binding
Aβ42 oligomer preparations were generated from synthetic peptide4. For binding assays, COS-7 cells were transiently transfected with cDNA expression plasmids or isolated hippocampal neurons were cultured from E18 embryos. Bound biotin-Aβ42 was detected using avidin conjugates.
Electrophysiology
Hippocampal slices (400 µm) from C57Bl6J or _Prnp_−/− mice were bathed in oxygenated artificial cerebrospinal fluid. The Schaffer collateral pathway was stimulated at 0.033 Hz at levels that evoked less than 50% of maximal field EPSPs. Evoked CA1 field potentials were recorded and the slope of the EPSP determined (Clampfit, Molecular Devices). Aβ42 or antibodies were bath applied for 20–40 minutes before inducing LTP with ten 100 Hz trains at five pulses delivered at 5 Hz.
Details are provided in the On-line Supplement.
Supplementary Material
1
2
3
4
5
01
Acknowledgements
We thank Dr. Susumu Tomita for cRNAs encoding GluR1–4 and stargazin, Dr. Chesebro for providing us the Prnp null mice, Dr. Melitta Schachner for providing the PrP-Fc expression vector, Dr. Eckhard Flechsig, Dr. Charles Weismann and Dr. David Harris for providing the PrPc deletion expression plasmids, Dr. David Westaway for Sprn expression plasmid and Dr. Peter Seeburg for NMDA receptor subunit cDNAs. We thank Stefano Sodi for assistance with mouse husbandry. We thank Dr. Ewa Folta-Stogniew for size exclusion chromatography, Dr. Christoph Rahner and Ms. Morven Graham for electron microscopy. J.L. is a Brown-Coxe Postdoctoral Fellow, J.W.G. is supported by NIH Medical Scientist training Program grant 5T32GN07205, and S.M.S. is a member of the Kavli Institute for Neuroscience at Yale University. This work was supported by research grants from the Falk Medical Research Trust, an anonymous donor and the NIH to S.M.S. The size exclusion chromatography was supported by a NIDA-funded Neuroproteomic Center.
Footnotes
Author Information. The authors declare competing financial interests.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Lesne S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 3.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 4.Chromy BA, et al. Self-assembly of Abeta(1–42) into globular neurotoxins. Biochemistry. 2003;42(44):12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- 5.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacor PN, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24(45):10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 8.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hepler RW, et al. Solution State Characterization of Amyloid β-Derived Diffusible Ligands. Biochemistry. 2006;45(51):15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- 11.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95(23):13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan SD, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 14.Wang HY, et al. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 15.Viles JH, et al. Copper binding to the prion protein: structural implications of four identical cooperative binding sites. Proc Natl Acad Sci U S A. 1999;96(5):2042–2047. doi: 10.1073/pnas.96.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson GS, et al. Location and properties of metal-binding sites on the human prion protein. Proceedings of the National Academy of Sciences of the United states of America. 2001;98(15):8531–8535. doi: 10.1073/pnas.151038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann F, et al. Lethal recessive myelin toxicity of prion protein lacking its central domain. Embo J. 2007;26(2):538–547. doi: 10.1038/sj.emboj.7601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li A, et al. Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. Embo J. 2007;26(2):548–558. doi: 10.1038/sj.emboj.7601507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riek R, et al. NMR structure of the mouse prion protein domain PrP(121–321) Nature. 1996;382(6587):180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 20.Bueler H, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 21.Manson JC, et al. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Molecular neurobiology. 1994;8((2–3)):121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 22.Lledo PM, Tremblay P, DeArmond SJ, Prusiner SB, Nicoll RA. Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc Natl Acad Sci U S A. 1996;93(6):2403–2407. doi: 10.1073/pnas.93.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis J, Errington M, Bliss T, Voss K, MacLeod N. Age-dependent loss of PTP and LTP in the hippocampus of PrP-null mice. Neurobiology of disease. 2003;13(1):55–62. doi: 10.1016/s0969-9961(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 24.Riemenschneider M, et al. Prion protein codon 129 polymorphism and risk of Alzheimer disease. Neurology. 2004;63(2):364–366. doi: 10.1212/01.wnl.0000130198.72589.69. [DOI] [PubMed] [Google Scholar]
- 25.Papassotiropoulos A, et al. The prion gene is associated with human long-term memory. Human molecular genetics. 2005;14(15):2241–2246. doi: 10.1093/hmg/ddi228. [DOI] [PubMed] [Google Scholar]
- 26.Yehiely F, et al. Identification of candidate proteins binding to prion protein. Neurobiology of disease. 1997;3(4):339–355. doi: 10.1006/nbdi.1997.0130. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt-Ulms G, et al. Time-controlled transcardiac perfusion cross-linking for the study of protein interactions in complex tissues. Nature biotechnology. 2004;22(6):724–731. doi: 10.1038/nbt969. [DOI] [PubMed] [Google Scholar]
- 28.Venkitaramani DV, et al. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27(44):11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosravani H, et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. The Journal of cell biology. 2008;181(3):551–565. doi: 10.1083/jcb.200711002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
01