Clinical Significance of Enteric Protozoa in the Immunosuppressed Human Population (original) (raw)
Abstract
Summary: Globally, the number of immunosuppressed people increases each year, with the human immunodeficiency virus (HIV) pandemic continuing to spread unabated in many parts of the world. Immunosuppression may also occur in malnourished persons, patients undergoing chemotherapy for malignancy, and those receiving immunosuppressive therapy. Components of the immune system can be functionally or genetically abnormal as a result of acquired (e.g., caused by HIV infection, lymphoma, or high-dose steroids or other immunosuppressive medications) or congenital illnesses, with more than 120 congenital immunodeficiencies described to date that either affect humoral immunity or compromise T-cell function. All individuals affected by immunosuppression are at risk of infection by opportunistic parasites (such as the microsporidia) as well as those more commonly associated with gastrointestinal disease (such as Giardia). The outcome of infection by enteric protozoan parasites is dependent on absolute CD4+ cell counts, with lower counts being associated with more severe disease, more atypical disease, and a greater risk of disseminated disease. This review summarizes our current state of knowledge on the significance of enteric parasitic protozoa as a cause of disease in immunosuppressed persons and also provides guidance on recent advances in diagnosis and therapy for the control of these important parasites.
INTRODUCTION
Parasitic diseases continue to cause significant morbidity and mortality throughout the world irrespective of the patient's immune status. It is estimated that there are approximately 340 parasite species capable of infecting humans, with the majority of the 3 billion people currently infected residing in developing regions of the world (99). Enteric protozoan parasites remain the most commonly encountered parasitic diseases and continue to cause significant morbidity and mortality.
Risk factors for acquisition of parasitic infections are the same in both immunocompetent (IC) and immunosuppressed (IS) individuals. What, then, is the role of the immune system in parasitic infections? Through local and systemic responses, the immune system plays an integral part in modifying the establishment of infection, controlling disease once it is established, limiting the severity and dissemination of the disease, and assisting in clearance or control of the parasite. Thus, IS hosts are more likely to acquire infection after exposure, have more severe disease once the infection is established, have disseminated infection rather than localized infection, and be unable to clear parasites with chronic carriage states. These all lead to, and account for, the greater morbidity and mortality in these patients. However, the majority of IS individuals do not differ from IC hosts in their presentation, with the major determinants of clinical severity and outcome of parasitic infection in IS patients being the degree of immune deficiency. Furthermore, with immune reconstitution through effective therapy or withdrawal of immunosuppressive agents, these patients are more likely to behave like IC hosts.
The number of IS individuals worldwide continues to increase each year as the human immunodeficiency virus (HIV) pandemic continues to spread unabated in many parts of the world, with an estimated 14,000 new infections occurring daily (19). What compounds this problem is that the majority of these new infections continue to occur in regions of the world where access to active therapy is limited and thus patients progress to profound immunosuppression (i.e., AIDS). In contrast, in more developed nations the numbers of IS individuals continue to increase as a result of medical interventions with more aggressive immunosuppressive therapies for immune-mediated disorders and hematopoietic and solid organ transplants, with approximately 1 million transplants performed annually (17).
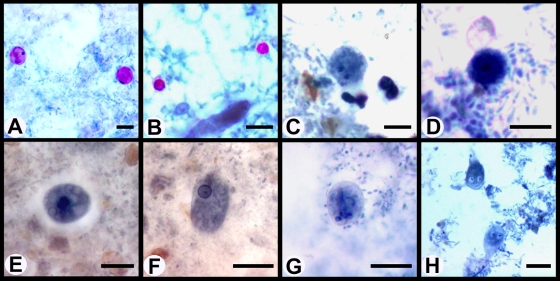
A summary of studies reporting the worldwide prevalence of pathogenic enteric protozoa in HIV-infected persons is given in Table 1, and treatment options for enteric protozoal infections are listed in Table 2. Examples of stained enteric protozoa are shown in Fig. 1.
TABLE 1.
Worldwide prevalence of pathogenic enteric protozoa in HIV-infected persons
| Reference | Location | Organism | Prevalence (%) |
|---|---|---|---|
| 31 | Apulia, southern Italy | Cryptosporidium parvum | 21.54 |
| Microsporidia | 9.23 | ||
| Giardia lamblia | 6.15 | ||
| Isospora belli | 1.54 | ||
| 60 | France | Cryptosporidium sp. | 37.3 |
| Giardia intestinalis | 5.8 | ||
| Isospora belli | 2 | ||
| Enterocytozoon bieneusi | 2 | ||
| 82 | Cuba | Cryptosporidium sp. | 11.9 |
| Giardia lamblia | 6 | ||
| E. histolytica/E. dispar | 1.5 | ||
| Isospora belli | 1.5 | ||
| Cyclospora cayetanensis | 3 | ||
| 118 | Seoul, South Korea | Cryptosporidium parvum | 10.5 |
| Giardia lamblia | 1.5 | ||
| Isospora belli | 7.5 | ||
| 122 | Ethiopia | Entamoeba histolytica | 10.3 |
| Giardia lamblia | 3.8 | ||
| 179 | San Pedro Sula, Honduras | Entamoeba histolytica | 5.8 |
| Giardia lamblia | 1.9 | ||
| Cryptosporidium parvum | 7.7 | ||
| Cyclospora cayetanensis | 1.9 | ||
| 192 | Northern India | E. histolytica/E. dispar | 1.7 |
| Giardia lamblia | 8.3 | ||
| Cryptosporidium parvum | 10.8 | ||
| Cyclospora cayetanensis | 3.3 | ||
| Enterocytozoon bieneusi | 2.5 | ||
| Isospora belli | 2.5 | ||
| 229 | Northern India | Entamoeba histolytica | 7.7 |
| Giardia lamblia | 3.8 | ||
| Cryptosporidium parvum | 11.5 | ||
| Isospora belli | 26.9 | ||
| 240 | Cameroon | Cryptosporidia | 3.8 |
| Microsporidia | 5.2 | ||
| Isospora belli | 2 | ||
| 254 | Sydney, Australia | E. histolytica/E. dispar | 3.2 |
| Giardia intestinalis | 1.5 | ||
| Cryptosporidium sp. | 2.2 | ||
| Dientamoeba fragilis | 2 | ||
| 289 | Iran | Giardia lamblia | 7.3 |
| Cryptosporidium parvum | 1.5 | ||
| 36 | Uganda | Entamoeba histolytica | 1.4 |
| Isospora belli | 0.8 | ||
| Giardia lamblia | 1.9 | ||
| Cryptosporidium parvum | 5 | ||
| 174 | Guinea-Bissau | Cryptosporidium parvum | 25 |
| Isospora belli | 11 | ||
| Microsporidia | 11 | ||
| 53 | Brazil | Giardia lamblia | 16 |
| Cryptosporidium parvum | 7 | ||
| Isospora belii | 2 | ||
| 83 | Los Angeles, CA | Giardia lamblia | 17.7 |
| Cryptosporidium parvum | 4 |
TABLE 2.
Treatment options for infections with enteric protozoa
| Intestinal parasite | Antimicrobial therapy |
|---|---|
| Cryptosporidium | Nitazoxanide (500 mg twice a day for 14 days), albendazole (400 mg twice a day for 7-14 days), or paromomycin (500 mg four times a day for 7-14 days) |
| Cyclospora cayetanensis | Co-trimoxazole (160 mg trimethoprim plus 800 mg sulfamethoxazole, twice a day for 7 days), pyrimethamine (50-75 mg daily) and leucovorin (5-10 mg daily), or ciprofloxacin (500 mg twice a day) |
| Dientamoeba fragilis | Iodoquinol (650 mg three time a day for 20 days), metronidazole (500-750 mg three time a day for 10 days), or paromomycin (25-35 mg/kg/day for 7 days) (271) |
| Microsporidia | |
| E. bieneusi | Albendazole (400 mg twice a day for 28 days) or fumagillin (20 mg three time a day for 14 days) |
| E. intestinalis | Albendazole (400 mg twice a day for 28 days) |
| Entamoeba histolytica | |
| Invasive disease | Metronidazole (750-800 mg three times a day for 6-10 days) or tinidazole (2 g once daily for 10 days) followed by paromomycin (500 mg three times a day for 7 days) |
| Intestinal disease | Paromomycin (500 mg three times a day for 7 days) |
| Giardia intestinalis | Metronidazole (2 g daily for 3 days) or tinidazole (2-g single dose) |
| Isospora belli | Co-trimoxazole (160 mg trimethoprim plus 800 mg sulfamethoxazole, four times a day for 10 days) or ciprofloxacin (500 mg twice a day for 7 days) |
FIG. 1.
Photomicrographs of enteric protozoa stained with a modified iron-hematoxylin stain (incorporating a carbol fuschin staining step). (A) Cyclospora oocysts; (B) Cryptosporidium oocysts; (C) Dientamoeba fragilis binucleated trophozoite; (D) Dientamoeba fragilis uninucleated trophozoite; (E) Entamoeba histolytica cysts; (F) Entamoeba histolytica trophozoite; (G) Giardia cysts; (H) Giardia trophozoites. Bars represent 10 μm.
IMMUNITY
The immune system can simplistically be divided into three components, namely, nonspecific immunity (e.g., skin and other mucosal barriers), the innate immune system (soluble factors and cells), and the adaptive immune system. The innate system is limited to pattern recognition immune responses based on a diverse array of sensors (e.g., Toll-like receptors or complement) which detect invading pathogens. In contrast, the adaptive immune system is able to undergo maturation. This is represented by increased affinity antibody production (immunoglobulin G [IgG] as opposed to initial IgM antibodies) and cell-mediated immunity with T-cell memory. Most pathogens have developed ways of evading the protection provided by the immune system. Thus, infection and/or persistent infection/carriage is established by the interplay between the host (immune system) and the pathogen. In the IS patient, changes often occur in favor of the pathogen. The advantage to the pathogen depends on the component of the immune system that is defective. Any component of the immune system can be functionally or genetically abnormal as a result of acquired (e.g., through HIV infection, lymphomas, or high-dose steroids or other immune-suppressive medications) or congenital illnesses, with more than 120 congenital immunodeficiencies described to date that either affect humoral immunity or compromise T-cell function (19). Immunosuppression may also occur in malnourished persons, patients undergoing chemotherapy for malignancy, and those receiving immunosuppressive therapy. However, for parasitic infections, cell-mediated (T-cell) abnormalities predominate. These patients tend to have an increased risk of acquiring common pathogens (e.g., cryptosproridia) with delayed coccidian clearance. With profound cell-mediated defects, reactivation of previously controlled pathogens (e.g., toxoplasmas) can occur. In addition, these patients are at risk of infection by “nonpathogenic” parasites (those that do not cause disease in normal hosts). With reconstitution of the cell-mediated immunity, the risk of parasitic infections reverts to that for a normal host.
HIV infection is the most common immunodeficiency state worldwide, with the hallmark of infection being depletion of CD4+ T lymphocytes, essential components of the cell-mediated immune system. After acquisition of HIV through genital secretions, the virus infects local macrophages using the CD4 receptor to mediate cell entry. These cells are transported to local lymph nodes, and viral replication commences. This is followed 1 to 3 weeks later with a seroconversion syndrome similar to a glandular fever-like illness, characterized by high viral replication and a decline in CD4+ T-cell counts. Both cellular and humoral responses ensue, which reduce the viremia, and the CD4+ T-cell count reverts to normal. This is followed by an asymptomatic phase lasting from 1 to 15 years or longer, which is characterized by viral replication and variable CD4+ cell loss of approximately 25 to 60 cells/μl per year. Once the CD4+ cell count drops below 200 cells/μl, patients are considered to have developed AIDS, with the risk of an AIDS-defining illness or opportunistic infection significantly increasing.
Developing an infection with enteric protozoan parasites is dependent on absolute CD4+ cell counts, with lower counts associated with more severe disease, more atypical disease, and a greater risk of disseminated disease (16, 36, 207, 237, 280). In addition, at counts of less than 200 (i.e., profound immunosuppression), HIV-infected patients are at risk from specific opportunistic protozoan pathogens which are usually unable to establish infection in IC hosts.
OPPORTUNISTIC INFECTIONS
Opportunistic infections are generally restricted to severely IS individuals and are considered AIDS-defining illnesses in HIV-infected patients, as they almost always occur when the CD4+ T-cell count falls below 200 cells/μl. Furthermore, with effective therapy and immune reconstitution, i.e., a rise of CD4+ T cells to above 200 cells/μl, the risk of these infections virtually disappears.
Cyclospora
Cyclospora cayetanensis is a coccidian protozoan most commonly found in developing regions of the world. However outbreaks of cyclosporiasis have been reported from North America (137, 138, 181) and Europe (76). The sources of infection in all these cases were traced to vegetables imported from areas of endemicity. Humans are the only known host for this parasite (99). Infection is via the fecal-oral route, though oocysts are not infective immediately after excretion in the feces and require sporulation in the environment to become infective.
Generally patients present with a rapid-onset, self-limiting diarrhea (178). With progressive immune suppression (CD4+ T cell counts of <200 cells/μl in HIV-infected individuals) prolonged carriage occurs, resulting in frequent severe relapses which may last from 4 to 7 weeks (11, 90, 139). These recurrences in turn result in severe malnutrition and significant morbidity and mortality in HIV-coinfected patients (201).
Episodes of diarrhea associated with C. cayetanensis infections have also been documented in other IS hosts, with cases of prolonged diarrhea in patients with compensated idiopathic hepatic cirrhosis, protein energy malnutrition, Hodgkin's lymphoma, and acute lymphoblastic leukemia being described (133, 215, 287).
Disseminated infections are uncommon, with several described cases involving the biliary tract in HIV-infected individuals (112, 291). Diagnosis was confirmed by histological confirmation of C. cayetanensis in the gallbladder epithelium following cholecystectomy for acalculous cholecystitis (291). Although C. cayetanensis oocysts have been observed in respiratory samples, it remains unclear whether these caused pulmonary disease, as no respiratory tissue samples were obtained (109).
Diagnosis of Cyclospora oocysts may be problematic, as most laboratories fail to recognize them in direct fecal smears. Special stains such as modified acid-fast auramine or modified iron-hematoxylin are usually required for definitive diagnosis. Other methods used are autofluorescence under UV epifluorescence (99) and DNA amplification by PCR (171). Histopathological examination of jejunal biopsy specimens from infected individuals showed mild to moderate acute inflammation of the lamina propria and surface epithelial disarray.
Cyclosporosis in patients can be treated effectively with a 10-day course of trimethoprim-sulfamethoxazole. Maintenance therapy with either trimethoprim-sulfamethoxazole or sulfadoxine-pyrimethamine is required to prevent recurrent disease (275). Alternatives include nitazoxanide (a new thiazolide antiparasitic agent), combination therapy with both pyrimethamine and leucovorin, or ciprofloxacin (295).
Data on the immune response to C. cayetanensis and mechanisms of immunity to this pathogen and how these relate to various immune deficiencies are lacking. However, previous exposure to Cyclospora may confer some resistance against challenge infection (98).
Cryptosporidium
Cryptosporidium species have a worldwide distribution, and the ability to infect a large range of vertebrate hosts (208, 212, 223, 236, 263). Cryptosporidium parvum and Cryptosporidium hominis are the species most commonly associated with human cryptosporidiosis (149), though infections with other species such as Cryptosporidium felis and Cryptosporidium meleagridis (121, 224) have been reported, particularly in IS patients (149, 198). Infection is via the fecal-oral route.
Studies from London and Nairobi demonstrate that cryptosporidiosis remains the most common opportunistic enteric protozoal disease encountered in IS HIV-infected patients (78, 203). This is supported by a recent prospective, comparative study comparing the prevalences of enteric protozoa among HIV-positive and -negative men in Australia (254). A total of 1,868 patients submitted stool specimens over a 36-month period for examination for the presence of enteric parasites. In this study C. parvum cases occurred exclusively in HIV-positive patients. Other IS groups at significant risk of severe cryptosporidiosis include those diagnosed with non-Hodgkin's lymphoma, leukemia, lymphoproliferative disease, or protein energy malnutrition (51, 133, 205, 215). Patients receiving immunosuppressive drugs for organ transplants or cancers are also at risk of prolonged, potentially life-threatening persistent diarrhea (67, 140, 238), as are IS patients undergoing hemodialysis (244). Cryptosporidium infection with serious clinical symptoms has also been observed in IS patients with hyper-IgM syndrome and primary CD4 lymphopenia (286). Cryptosporidium parvum infections have also been reported in transplant patients undergoing immunosuppressive therapy. One case study reports _C. parvum_-related sclerosing cholangitis in a 40-year-old renal transplant patient. After reduction of immunosuppression, C. parvum was cleared from her stool, with a marked improvement in diarrhea and general health (1).
Patients that are not IS tend to present with a self-limiting diarrhea, which may last for several weeks to months even in IC individuals. The highest burden of disease occurs in children under 5 years of age (2). Infection in the IS individual is usually associated with chronic diarrhea and wasting and can be life threatening (2, 197, 203). Disseminated infection has been described, predominantly involving the biliary tract with cases of ascending cholangitis (24, 29, 49, 288). Other manifestations include pulmonary cryptosporidiosis (163, 218). These infections may occur in the absence of gastrointestinal involvement (218).
Laboratory diagnosis of cryptosporidiosis traditionally relies on special staining techniques, such as modified acid-fast, Kinyoun's, and Giemsa stains, as oocysts are difficult to detect using basic light microscopy (99). Other alternative diagnostic techniques have also been employed. Several commercial companies have developed rapid diagnostic tests that are simple to perform and can be completed in less time than traditional methods for detecting Cryptosporidium; these rapid tests include lateral-flow immunoassays, immunochromatograhic assays, and direct fluorescent-antibody tests (151) The use of enzyme-linked immunosorbent assay (ELISA) for the detection of Cryptosporidium antigen in stools has been described (147), and PCR assays have been developed for the specific detection of Cryptosporidium species in stools. A TaqMan PCR assay targeting the 18S ribosomal DNA (rDNA) has allowed sensitive detection of Cryptosporidium species (155). Various other PCR assays for the detection of Cryptosporidium species in stool specimens have also been developed (18, 107, 136, 293). While being more expensive and time-consuming, PCR and ELISA have shown superior sensitivity for the detection of Cryptosporidium species compared to conventional staining and microscopy (158, 199).
Based upon the paucity of evidence, the effectiveness of any therapeutic agent in the treatment of cryptosporidiosis has yet to be confirmed. However, the drugs paromomycin, azithromycin (218), and nitazoxanide (249) have been used to treat cryptosporidiosis in HIV-infected patients and have been shown to reduce the parasite load. Resolution of cryptosporidiosis can be maintained with effective highly active antiretroviral therapy (HAART) (191, 218, 242, 292). There is also evidence to suggest that some antiretroviral compounds used in HAART may have a direct inhibitory effect on Cryptosporidium (197).
Both humoral and cellular immune mechanisms appear to be necessary for protection against Cryptosporidium. The exacerbation of disease by immune suppression reflects the role of the immune system in controlling replication of the organism. IS patients with cryptosporidiosis fail to develop a significant serological response to the organism (184); in contrast, seroepidemiological studies have indicated that between 30 and 80% of IC patients will elicit an antibody response (26). Reports of chronic cryptosporidiosis in children with congenital Ig deficiency but intact cellular immunity highlight the importance of antibodies in the immune response against cryptosporidium (172). Murine models have shown that gamma interferon mediates an important protective innate response against Cryptosporidium infection (188). Studies suggest that CD4+ cells in the gut epithelium also play a role in controlling infection, along with Th1 and Th2 cytokine production (187, 267).
Isospora belli
Isospora belli is a coccidian parasite that has a global distribution limited to mainly tropical regions in developing countries where it is endemic (especially Africa, the Middle East, and South America). The parasite invades the intestinal epithelium, where it completes its life cycle in the cytoplasm of the enterocyst (234). Unsporulated oocysts are excreted in feces and mature outside the host, where they develop into infective sporulated oocysts. Infection is then acquired through ingestion of these infective oocysts. Immunodeficiency was shown to increase the susceptibility to infection with I. belli (117), which accounted for up to 20% of cases of diarrhea in AIDS patients (46, 65).
The parasite may cause acute self-limiting diarrhea, fever, and abdominal pain that usually resolves spontaneously in a normal host. In severely IS patients, severe chronic diarrhea is often reported and has been associated with fulminant diarrhea leading to a wasting syndrome and sometimes death in AIDS patients (46).
Diarrhea associated with I. belli infections has also been reported in patients with other immunosuppressive diseases, such as lymphoblastic leukemia (148, 283), adult T-cell leukemia (115, 159), Hodgkin's disease (225), non-Hodgkin's lymphoma (234), lymphoproliferative disorders (159, 214), and renal transplant recipients (169), and in a liver transplant patient with chronic diarrhea (15). Patients with malabsorption syndrome, particularly malnourished children, are also at increased risk of chronic isosporiasis (21, 168).
As with other opportunistic protozoal pathogens, clinical manifestations are dependent on the balance between the immune system and the virulence of the organism, with chronic carriage states (“latency”) and clinically symptomatic reactivation during periods of severe immunodeficiency (152), as reported for a recent case of reactivation 8 years following acquisition (234).
Diagnosis is by direct visualization of the oocyst in feces; however, for some patients microscopic examination of stool samples may remain negative, even in cases of severe diarrhea (65) Therefore, a mucosal biopsy may be required for a definitive diagnosis. Detection of I. belli by PCR has been used as an additional diagnostic tool in clinical laboratories. Conventional PCR using primers based on 18S rDNA sequences shows excellent sensitivity and specificity (202). Recently, a real-time PCR targeting the internal transcribed spacer 2 region of the rRNA gene for the detection of Isospora belli DNA in fecal samples was developed. This real-time assay achieved 100% specificity and sensitivity (266).
Isospora infection responds promptly to antimicrobial therapy. Co-trimoxazole, sulfadiazine, and pyrimethamine have all been used successfully to treat isosporiasis (65, 219, 283). A number of randomized control trials conducted in areas of endemicity have shown a very high efficacy of co-trimoxazole against I. belli (275), with a 7- to 10-day course usually being efficacious in treating the infection (234). Maintenance or secondary prophylaxis should be used to prevent relapses (275).
Once again, there are limited data in the scientific literature in regard to the mechanisms of immunity to this pathogen, and it is evident that more research is needed in this area.
Microsporidia
The term microsporidia is used as general nomenclature for the obligate intracellular parasites belonging to the phylum Microsporidia. Microsporidia were originally classified as protozoa, though it has been recently suggested that they are more closely related to fungi and as such should no longer be considered protozoa (135). However, as the precise relationship between the microsporidia and fungi is yet to be defined, microsporidia are included here as protozoa.
Thirteen hundred species of microsporidia belonging to 160 genera and infecting a wide range of vertebrate and invertebrate hosts have been described. Fourteen microsporidian species have been identified as human pathogens: Anncalia algerae, Anncalia connori, Anncalia vesicularum, Encephalitozoon cuniculi, Encephalitozoon hellem, Encephalitozoon intestinalis (syn. Septata intestinalis), Enterocytozoon bieneusi, Microsporidium ceylonensis, Microsporidium africanum, Nosema ocularum, Pleistophora ronneafiei, Trachipleistophora hominis, Trachipleistophora anthropophthera, and Vittaforma corneae. Of these, E. bieneusi and E. intestinalis are the two most common causes of human enteric disease.
Microsporidia are recognized as opportunistic infectious agents worldwide in both developed and developing countries. Human microsporidiosis represents an important disease, occurring mainly but not exclusively in severely immunocompromised patients with AIDS. A recent study examined a total of 893 fecal specimens from hospitalized patients for microsporidia using a modification of the Gram-chromotrope stain technique. One hundred sixteen patients (13.0%) were positive for microsporidia, and approximately one-third of the patients were IC individuals. Microsporidiosis was commonly observed in children aged 0 to 6 years (26.4%) and adults aged ≥31 years (57.2%). Among the IS group, microsporidia were observed to be more prevalent in patients with hematological malignancy or a combination of malignancy and diabetes mellitus (210). What role microsporidia play in causing gastrointestinal symptoms in IC populations is unclear. However, another recent study found that microsporidia were more prevalent in IC individuals than in HIV-infected patients, with none of the IC group displaying gastrointestinal symptoms (209).
The mode of transmission for the various microsporidia remains obscure. It is accepted that infection occurs via the fecal-oral route for intestinal microsporidia such as E. bieneusi and E. intestinalis, as spores of these species are shed in the feces, but they can also often be shed in the urine from disseminated infections of the kidney. This is supported by the finding of E. bieneusi and E. intestinalis spores in recreational bathing water alongside oocysts of other enteric pathogens (94, 95, 114).
Enterocytozoon bieneusi is the most common microsporidian in humans (130) and the second most prevalent cause of diarrhea in IC patients, after Cryptosporidium (32). However, in countries with access to HAART, the prevalence of microsporidial infections has declined (105). In a study from Australia, van Hal et al. (273) found that the total incidence of intestinal microsporidiosis in HIV-infected patients declined from 11% in 1995 to 0% from 2004 onwards. These dramatic changes have been attributed to restoration of cell-mediated immunity and possibly secondary to the direct antiparasitic activity of some components of HAART (i.e., protease inhibitors) (228). In contrast, in developing countries with limited access to HAART, the incidence of microsporidiosis remains high (33).
The clinical manifestations of microsporidiosis are very diverse and vary according to the causal species, with diarrhea being the most common. In the case of E. bieneusi infection, the disease is usually confined to the gastrointestinal tract, though disseminated infection can occur. In HIV patients with E. bieneusi infection, chronic diarrhea associated with wasting syndromes and cachexia are common (32, 130). Dissemination to the hepatobilary system with cholangitis (29, 226), to the maxillary sinus with invasive sinusitis (131, 272), and to the respiratory system with pulmonary infections (28, 66) have all been reported for _E. bieneus_i/HIV coinfections. Intestinal disease resulting from E. bieneusi infection has also been reported in heart-lung, liver, and renal transplant recipients undergoing immunosuppressive therapy (110, 116, 190, 231, 248).
Encephalitozoon spp. are the second most common microsporidia in humans and the most common cause of disseminated microsporidiosis (130, 153, 164, 268, 278, 279). There are three species within this genus that are known to cause human disease: E. cuniculi, E. hellem, and E. intestinalis. While E. intestinalis can infect most tissues, this species is usually associated with enteric disease (14, 81).
Traditionally, light microscopy based on modified trichrome stains (e.g., Ryan's stain) has been used by most laboratories for the diagnosis of enteric microsporidiosis (70). Immunofluorescence tests using the chitin binding fluorochromes Uvitex 2B, Fungifluor, calcofluor white, and Fungiqual A are also available and offer a sensitive and rapid method for detecting microsporidial spores in stool, intestinal fluid, biopsy imprint, and tissue specimens, even from archived material (5, 58, 87, 97, 200, 294) Molecularly based PCR assays (63, 77, 96) are also available for the diagnosis of E. bieneusi and E. intestinalis infection. The advantage of molecular testing over microscopic methods is that it allows for determination of the species of microsporidia. A comparison of PCR and an immunofluorescent-antibody test (IFAT) for the detection of E. bieneusi and E. intestinalis found that the two techniques had similar sensitivities, though IFAT was cheaper and more rapid (6). However, there are currently no commercially available or FDA-approved IFAT kits. Staining of tissue sections by the Warthin-Starry staining method was assessed as an effective diagnostic tool for the microscopic detection of microsporidia and demonstrated better diagnostic capabilities than the hematoxylin and eosin stain (88, 89).
Management of microsporidial infection most often includes oral treatment with the drug albendazole (23, 52, 85, 105, 193). Albendazole has demonstrated good antimicrosporidial activity against Encephalitozoon spp., particularly E. hellem (69, 71) (in vivo and in vitro), though it is only partially active against E. bieneusi (57, 73, 85, 105, 157, 162, 193). The drug nitazoxanide has demonstrated activity against E. bieneusi, with the symptoms of one patient receiving nitazoxanide therapy resolving in the absence of antiretroviral therapy (22). The drug fumagillin has also demonstrated good anti-Enterocytozoon bieneusi activity (40, 57, 195), although various adverse side effects have been documented (56, 141, 194, 195). The drug furazolidone has also demonstrated a degree of anti-Enterocytozoon activity (74). As with most opportunistic infections, HAART plays a key role in eradicating microsporidia in HIV-infected patients (105), and effective HAART is likely to reduce the incidence of microsporidial infections in the future.
Cell-mediated immunity appears to be critical for protection against the microsporidia. An effective Th1 cytokine response is important in the immune response to infection. However, the role of humoral immune responses in human microsporidial infections is yet to be fully elucidated (72).
OTHER PATHOGENIC ENTERIC PARASITES
Infections with the following pathogenic enteric parasites generally occur in both IC and IS patients. Although greater rates of carriage of some of these pathogens (i.e., Entamoeba histolytica) are associated with HIV-infected patients, this probably reflects the higher opportunity acquisition risk secondary to various sexual practices rather than the immune deficiency per se.
Blastocystis spp.
Blastocystis spp. are enteric unicellular parasites that are the most frequently reported parasite in human fecal samples (261). Although traditionally classified as a protozoan parasite, recently, due to new molecular techniques, Blastocystis has been shown to be closely related to the stramenopiles. However, due to it being nonmotile and not possessing flagella, in contrast to other stramenopiles, it has been placed in a new class, class Blastocystea, in the subphylum Opalinata, infrakingdom Heterokonta, subkingdom Chromobiota, kingdom Chromista (45).
Blastocystis exhibits extensive genetic diversity, and this has been documented using numerous molecular techniques. Currently there are at least nine subtypes (genotypes) within Blastocystis. Recent studies have shown that no group exclusive to humans exists and that all clades have been detected in human stool (222). Consequently, human isolates of Blastocystis that were commonly referred to as Blastocystis hominis should be called Blastocystis spp. due to there not being a single subtype specific to humans.
Laboratory diagnosis of Blastocystis relies on several methods for identification, including light microscopy of fresh samples from wet preparations and concentrates, permanently stained fixed fecal smears, culture, and molecular techniques (55, 260, 261).
There is still much controversy surrounding Blastocystis and its pathogenicity in humans. There are conflicting reports in the scientific literature, where many authors view Blastocystis as a pathogen, (10, 41, 176, 177) while many other reports doubt the role of Blastocystis in human infection (175, 270). The most common symptoms associated with this parasite are diarrhea, abdominal pains, and vomiting. As no recognized animal model exists for Blastocystis, Koch's postulates are unable to be fulfilled in order to confirm or exclude the pathogenic nature of this organism. While the organism has a global distribution, the prevalence is higher in developing countries (261). Acquisition of the organism is thought to occur as a result of frequent animal-human, human-human, and human-animal transmission, with reports of carriage of Blastocystis in mammals, birds, amphibians, and even insects (264, 265).
What role Blastocystis plays in gastrointestinal disease in IS hosts is also unclear. There have been several studies of the prevalence of intestinal parasites in HIV-infected patients, with most finding higher rates of Blastocystis carriage. Several studies from Africa showed Blastocystis infection to be at a higher rate in HIV-positive patients than in negative controls. A study in Senegal found Blastocystis only in HIV-infected patients, with all but one suffering from diarrhea and with no other pathogens found in the samples. This study suggested that Blastocystis may be considered an opportunistic parasite (104). Another African study in an Ethiopian teaching hospital found there to be an incidence of Blastocystis infection of 14.1% in HIV/AIDS patients. There were no statistically significant differences in the prevalence of parasites among cases and controls except for Blastocystis, which was significantly higher in HIV/AIDS patients, which shows that it may be a possible pathogen in immunocompromised patients (122). A study in Iran showed that the occurrence of parasites in HIV-infected patients was not as high as seen in African countries, with an infection rate of only 18.4%. Of the parasites seen in that study, though, Blastocystis was the second most prevalent at 4.4%, with most of these cases being seen in patients with diarrhea (289). There are also insufficient data on the immunology of the parasite and the interactions with IS hosts.
Dientamoeba fragilis
Dientamoeba fragilis is a trichomonad parasite. Humans are probably the definitive host of this parasite even though D. fragilis trophozoites have been reported in nonhuman primates, including macaques (132, 167), baboons (204), and gorillas (255).
There is little understanding of the pathogenesis and pathology resulting from D. fragilis infection, and understanding is further hampered by the lack of a suitable animal model (75, 161, 166). However, acute and chronic diarrhea with associated abdominal pain has been documented in IC children and adults (61, 253, 285).
The impact of immune deficiency on D. fragilis infection and disease remains unclear. A study from Argentina found higher rates of D. fragilis infections in IS patients, in contrast to an Australian study, which found no difference in carriage rates in HIV-infected patients and the general population (189, 254). Clearly, further study of all aspects of this infection and in all patient groups is warranted.
Diagnosis of D. fragilis is based on prompt fixation and permanent staining, as trophozoites degenerate within hours of being passed and demonstration of the characteristic nuclear structure is achieved by use of permanently stained preparations only (284). However, as many laboratories do not routinely perform permanent staining, the incidence of D. fragilis is likely to be underestimated. Newer molecular techniques, such as conventional and real-time PCR, targeting the 18S rDNA have been developed (251-253, 257).
Multiple differing antimicrobial agents have been used for treatment of D. fragilis with varying success. These include doxycycline, iodoquinol, metronidazole, and secnidazole (25, 108, 150, 230). However, there is no general consensus on what is best practice in treating D. fragilis infections. There are also no data available on the mechanisms of immunity for this parasite.
Entamoeba histolytica
Entamoeba histolytica is a nonflagellated amoeboid protozoan parasite. The genus Entamoeba includes six species (E. histolytica, Entamoeba dispar, Entamoeba moshkovskii, Entamoeba polecki, Entamoeba coli, and Entamoeba hartmanni) that are capable of infecting the intestinal lumen of humans. All these species are considered commensal organisms and rarely (if ever) cause intestinal disease in humans, with the exception of the pathogenic E. histolytica, for which humans are the primary reservoir (258). Entamoeba histolytica is an invasive pathogen and the causative agent of amoebiasis, with approximately 50 million cases acquired annually in the developing world (274). What role E. moshkovkii plays in human infections is yet to be adequately defined, most likely in part due to diagnostic confusion with E. histolytica and E. dispar. Although previous studies have not shown any association between E. moshkovskii in clinical samples and disease (54), recent studies have reported E. moshkovskii as an enteropathogen in patients presenting with gastrointestinal symptoms (92, 93, 221). There have also been no adequate studies examining the pathogenic potential of this organism in IS groups, and it is clear that further study is needed to assess the true pathogenicity of this organism.
The factors that control the pathogenesis of E. histolytica are not completely understood. However, key features are the ability of the organism to lyse host cells and cause tissue destruction, with induced immune responses occurring in invasive disease (3). Entamoeba histolytica acquisition is via the fecal-oral route. In confined populations (e.g., men who have sex with men [MSM] visiting sex-on-premises venues), carriage rates are significantly higher than in the general population once the organism has been established secondary to various oral-anal sexual practices (173, 254, 274). As the risks for HIV acquisition and parasite acquisition in these environments overlap, apparent associations between E. histolytica and immunosuppression exist. However, in an Australian study Entamoeba infection was found to be more prevalent in HIV-negative than in HIV-positive MSM with diarrhea. In the United States E. histolytica rates in AIDS patients remain low, with amoebiasis being diagnosed more frequently in individuals exposed to HIV through male-male intercourse (182). Thus, it is probable that HIV-positive individuals are no more susceptible to gastrointestinal symptoms than HIV-negative individuals.
Following an incubation period that can vary greatly, ranging from days to years, individuals infected with E. histolytica present with symptoms of abdominal pain, tenderness, and diarrhea with >10 bowel movements/day, corresponding with colitis and ulcerative disease on histopathological examination. Bowel complications occur in 1 to 4% of patients. Invasive or extraintestinal disease is uncommon and may be present in 0.1 to 1% of symptomatic patients, with the liver being the most common site involved (>50%) (259). The lungs are the second most common site of invasive amoebiasis (245). Invasive amoebiasis involving the heart (59, 111, 245), brain (20, 143, 277), and genitourinary tract (38, 183, 217) has also been reported. Generally, when clinical symptoms develop they are limited to the gastrointestinal tract. However, the likelihood of developing invasive amoebiasis is increased in the presence of HIV infection (144-146, 245), with higher rates of amoebic colitis (144, 213) and amoebic liver abscesses (35, 142, 160) reported. Furthermore, seroprevalence studies, which are considered a sensitive marker of previous invasion, suggest that immunosuppression increases the risk of disseminated disease, with significantly higher rates of antibody positivity in HIV-positive patients (50, 269).
Entamoeba dispar, the usually free-living (occasionally pathogenic) Entamoeba moshkovskii, and E. histolytica are morphologically identical, though genetic differences have confirmed the separation of these three as independent species (62, 68, 250). Due to this conserved morphology, stained smears of stool specimens are insufficient for differentiation of these species. Staining of fixed fecal smears with iron-hematoxylin or Ziehl-Neelsen stain (47, 154) can determine the presence of the Entamoeba histolytica/ E. dispar/ E. moshkovskii complex within a stool specimen, while other techniques such as PCR or ELISA must be employed for differentiation. A number of PCR assays are available for detection and/or differentiation of Entamoeba species (62, 127, 239). A unique approach to diagnosis of E. histolytica infection is described by Britten et al. (37), which makes use of DNA probes conjugated to an antibody-detectable protein tag. Immunological assays are also useful and often employed. Commercial antigen capture and antibody-detecting ELISA kits are also available (34, 128, 129, 165), along with rapid lateral-flow cartridge tests (101). While some authors suggest that the commercial antigen capture ELISA kits are preferable to the use of PCR due to the rapidity and simplicity of these kits (84), other authors suggest that PCR is more useful than antigen capture ELISA for detection of E. histolytica in stools due to the higher sensitivities observed in PCR and the reduced chance of cross-reactivity with other Entamoeba species (256).
A number of different serological assays for the detection of E. histolytica antibodies are commercially available. Indirect hemagglutination assays, latex agglutination assays, complement fixation assays, indirect immunofluorescence assays, and ELISAs have all been developed (91). Serological testing may be helpful from a diagnostic perspective in certain situations. In patients suspected of having extraintestinal disease, serological tests are warranted; however, testing in patients with intestinal disease is normally not recommended. The serological tests are specific but have varying sensitivity depending on the presence or absence of invasive disease and the type of invasive disease. The sensitivity for serology is 100% in patients with amoebic liver abscess and 82% in those with invasive intestinal disease (91). Antibody testing to diagnose carriage or noninvasive disease is unhelpful, as the sensitivity is low at around 8% (91). Serum IgG antibodies persist for years, and antibody titers can remain high for years after successful therapy and/or eradication of the organism. This limits the usefulness of the test in areas where infection is endemic and people have been exposed to E. histolytica, due to the inability of serological tests to distinguish past from current infection (91). However, serological tests may be helpful from a diagnostic perspective in industrialized countries, where infections and exposure to E. histolytica are uncommon.
Diagnosis of invasive disease is usually based on patient symptoms (e.g., localized pain) followed by radiographic studies to identify the presence of lesions or organomegaly (245). To identify E. histolytica as the causative agent of a tissue abscess or lesion, PCR of tissue aspirates or identification of E. histolytica antigens in tissue aspirates is required (4, 220). A possible prediction of invasive disease can be made based on the level of Ig subtypes present in the circulation. One study noted that in the event of invasive disease, anti-E. histolytica IgG and IgA levels were comparatively high, while much lower anti-E. histolytica IgM and IgE antibody levels were detected (246).
Drug treatments for amoebiasis include paromomycin, diloxanide furoate, and iodoquinol. These drugs are all effective at treating luminal amoebiasis, although they are ineffective against invasive amoebiasis (85). Nitroimidazole derivatives such as metronidazole, secnidazole, tinidazole, and ornidazole are effective for treatment of invasive amoebiasis though less effective against luminal disease (85, 119). For treatment of luminal and extraintestinal amoebiasis, Farthing (85) suggests a 5-day course of metronidiazole plus a 10-day course of diloxanide furoate or a 7- to 10-day course of paromomycin for the treatment of invasive amoebiasis.
Innate immune responses are the initial mechanisms that are responsible for limiting E. histolytica infection and invasive disease. Entamoeba histolytica has been shown to evade complement-mediated lysis (232), and extracellular cysteine proteinases of E. histolytica are capable of degrading the complement anaphylatoxins C3a and C5a (233). Intestinal epithelial cells have been shown to initiate an inflammatory response to E. histolytica infection, and nuclear factor κB and proinflammatory cytokines also play a significant role in host defense (243).
Giardia intestinalis
Giardia intestinalis (synonyms, “Giardia lamblia_” and “_Giardia _duodenalis_”) is a common and ubiquitous flagellated protozoan parasite with a worldwide distribution. Giardia species are parasites of mammals and other animals, including reptiles and birds (7, 42-44, 123-126). Humans become infected by ingestion of cysts, which develop into trophozoites after excystation. Infections occur in both developed and developing regions of the world (241).
An Australian study found that HIV-infected patients were as likely to have Giardia as HIV-negative MSM (254). In this study, 3% of HIV-negative MSM were infected, compared to 4.5% of HIV-positive MSM and 1.5% of the general population. This suggests that, as with other enteric parasites, sexual practices lead to higher Giardia carriages rates (83, 196).
In developing countries the burden of disease remains high in IC individuals, especially in children less than 10 years of age, with Giardia considered normal gut flora in children in developing countries (134).
After acquisition, approximately 50% of people clear Giardia without any untoward effects, 5 to 15% of people shed cysts asymptomatically, and the remainder develop an acute and/or chronic infection (7, 241). The acute infection lasts for days to weeks and is accompanied by nausea and the sudden onset of explosive, watery, foul-smelling diarrhea. The acute phase is often followed by a subacute or chronic phase. Chronic symptoms may last for years and be continuous, intermittent, sporadic, or recurrent, with episodes of diarrhea or loose stools. Between bouts of diarrhea the patient may have normal stools. However, abdominal discomfort may be continuous and independent of the alteration in bowel habits (271).
Symptoms of giardiasis in HIV-infected individuals appear to be similar to, and no more severe than, those of giardiasis in HIV-negative individuals, with asymptomatic infection occurring commonly in the presence of HIV (39, 196). With progressive immunosuppression following reduced CD4+ counts, the risk of symptomatic Giardia infections is increased (12, 79). Despite this, giardiasis is not considered a major cause of enteritis in HIV-infected patients (12, 196).
Although there is little in the scientific literature in regard to Giardia infections in IS individuals, a number of studies have shown that Giardia is more prevalent in the stools of hypogammaglobulinemic patients than in those of IC hosts (86, 235). It has also been shown that the majority (>90%) of hypogammaglobulinemic patients passing Giardia cysts are symptomatic, with chronic diarrhea (30). Symptomatic giardiasis has been observed in X-linked infantile congenital hypogammaglobulinemia (Bruton's syndrome) and also in the common variable (late-onset) acquired hypogammaglobulinemia (30). IS patients, including nephrotic syndrome children receiving corticosteroids, protein-calorie malnutrition patients, patients with cases of marasmic kwashiorkor, and lymphoma patients, were found to be significantly more at risk for Giardia infection (211).
Enzyme immunoassays, immunochromatographic assays, and direct-fluorescence assays for detection of G. intestinalis in stool have been available in the form of commercial kits for several years (113, 216, 282). These kits are commonly used in diagnostic laboratories. Compared to microscopy, the coproantigen assays are less time-consuming and easier to perform. However, conflicting data in the exist in the scientific literature in regard to the performance of these rapid assays. Some researchers have reported excellent sensitivity and specificity (48, 100-102), while others have reported the rapid tests to be generally less sensitive than conventional microscopic methods (216, 282). A number of PCR assays are also available for the detection of Giardia in stool specimens (8, 106, 120, 276). Giardia infection can also be diagnosed microscopically by identification of cysts and trophozoites in stained or unstained fecal smears. Giardia cysts and trophozoites have a unique morphology, different from that of most other protozoa, and thus can be identified by trained lab staff in a simple wet preparation of the fecal specimen.
Giardia infection can be treated with metronidiazole, quinacrine, furazolidone, paromamycin, tinidazole, nitrazoxamide, and ornidazole (13, 85, 103, 196). Albendazole has also demonstrated good antigiardial activity and therapeutic efficacy (103). Gupta et al. (119) suggest that metronidiazole is the drug of choice for treating giardiasis and advise a dosage of 15 mg/kg/day in three divided doses for 5 days or 2 g daily for 3 days.
Antigiardial host defenses are B-cell dependent, with secretory IgA antibodies playing an important role (80). T cells also appear to be important in the intestinal elimination of the organism. However, patients with marked T-cell deficiencies do not exhibit an increased susceptibility to giardiasis (281).
CONCLUSIONS
Gastrointestinal disease, such as diarrhea, abdominal pain, and irritable bowel syndrome, are common in the world's population. For example, an estimated 1.6 to 2.5 million deaths of children under 5 years old are caused by diarrhea globally (170, 180), with approximately 73% of these deaths being in just 15 developing countries (27). In addition, diarrhea is also a major cause of morbidity and death in HIV-infected individuals (227). In coming years there is likely to be an increase in the number of HIV/AIDS deaths, with worrying projections of 6.5 million deaths in 2030 and HIV/AIDS being the main burden of disease in some developing countries by 2015 (185). In developed countries the number of IS individuals continue to increase each year with more patients undergoing hematopoietic and solid organ transplants and more aggressive treatments instigated with immunosuppressive therapies. Furthermore, although diarrheal diseases may be declining due to improvements in treatment, the total population of people continues to grow, leaving diarrheal diseases remaining as a major problem, especially in the developing world. It is thus likely that the clinical significance of enteric protozoa in the IS population will continue to grow globally.
Each year, many studies of infectious diseases and their interaction with the immune system are published. Central to most of this research is a knockout mouse, which has assumed an important role in studies investigating how the immune system functions not only in response to microbes but also in other disease conditions (186, 247). Indeed, advances in our understanding of the mechanisms by which pathogens cause diarrhea have been greatly enhanced by the availability of immune-suppressed mice generated by knockout technologies (206). Unfortunately, our knowledge of the human immune system and the way it interacts with parasites is much more limited, despite the recognition that enteric parasites are commonly associated with the onset of diarrhea in IS patients. CD4 counts play an incredibly important role in the presentation of diarrhea as well as in the control of protozoa in HIV-infected individuals. For example, chronic diarrhea is typically associated with lower CD4 counts than acute diarrhea. In addition, at counts of less than 200, HIV-infected patients are at risk from specific opportunistic protozoan pathogens which are usually unable to establish infection in IC hosts (207, 280).
Advances in the diagnosis of infectious diseases occur regularly, although the first form of diagnosis of parasite infections is still by light microscopy of stool by an experienced microscopist. Commercially available fecal immunoassays now not only are widely available for the majority of enteric protozoa but also provide a cost-effective alternative to traditional diagnosis of protozoan enteric parasites. These kits are rapid and easy to use and are far more practical for most laboratories than PCR assays. A confirmatory test such as PCR may be run subsequently. Ironically, even in today's age of modern technology being available in the developed world, the cause of gastroenteritis may still go undiagnosed in about 50% of cases (9). Even in IC individuals, the presence of parasites, including D. fragilis, is normally overlooked in cases of diarrhea, as the provision of appropriate technology in diagnostic laboratories is not yet commonplace or routine. Nine parasitic protozoan species or genera (B. hominis, Cryptosporidium spp., Cyclospora spp., D. fragilis, E. histolytica, E. bieneusi, E. intestinalis, G. intestinalis, and I. belli) are recognized as a cause of gastrointestinal disease (primarily diarrhea), with three additional species (E. dispar, E. moshkovskii, and E. polecki) warranting further investigation into their role as pathogens of the human gastrointestinal track.
Several of the parasite species discussed here are zoonotic, and animals may be the reservoir and source of the parasite species. Exposure to pets and other animals is a recognized risk factor for acquisition of enteric parasites causing diarrhea (79). Infection is likely to be acquired via ingestion (fecal-oral transmission), with contaminated food or water representing the likely vehicle of transmission (64, 156). In at-risk groups, sexual practices may also represent a contributor to the transmission of parasites (254).
Biography
 Damien Stark is a Senior Hospital Scientist in the Microbiology Department at St. Vincents Hospital, Sydney, and is also an Associate, Department of Medical and Molecular Biosciences, Institute for the Biotechnology of Infectious Disease, University of Technology, Sydney (UTS). His research interests include clinical, diagnostic, and molecular parasitology, with a special interest in all aspects of D. fragilis research.
Damien Stark is a Senior Hospital Scientist in the Microbiology Department at St. Vincents Hospital, Sydney, and is also an Associate, Department of Medical and Molecular Biosciences, Institute for the Biotechnology of Infectious Disease, University of Technology, Sydney (UTS). His research interests include clinical, diagnostic, and molecular parasitology, with a special interest in all aspects of D. fragilis research.
 Joel Barratt is a research assistant and Ph.D. student at the University of Technology, Sydney (UTS), working in collaboration with St. Vincent's hospital, under the supervision of Professor John Ellis and Dr. Damien Stark. Joel completed his honors degree at UTS under the supervision of Professor John Ellis on the protozoan parasite Neospora caninum. Joel's current research focuses on human enteric protozoa, with a particular focus on the trichomonad parasite Dientamoeba fragilis.
Joel Barratt is a research assistant and Ph.D. student at the University of Technology, Sydney (UTS), working in collaboration with St. Vincent's hospital, under the supervision of Professor John Ellis and Dr. Damien Stark. Joel completed his honors degree at UTS under the supervision of Professor John Ellis on the protozoan parasite Neospora caninum. Joel's current research focuses on human enteric protozoa, with a particular focus on the trichomonad parasite Dientamoeba fragilis.
 John L. Harkness graduated from Monash University medical school in Melbourne, Australia, in 1967. Training as a junior medical officer was at the Alfred Hospital in Melbourne, followed by training in pathology at the Alfred Hospital, Hammersmith Hospital, London, United Kingdom, and the Mayo Clinic, Rochester, NY. He was appointed Director of Microbiology at St. Vincent's Hospital, Sydney, Australia, in 1975. He is an Associate Professor in the Medical Faculty, University of NSW, and Adjunct Professor at the University of Technology in Sydney. He is actively involved in teaching medical undergraduates and postgraduates. He is interested in infections in the immunocompromised host, antimicrobial therapy, and parasitology. Research interests in the laboratory in particular are in parasitology and mycology.
John L. Harkness graduated from Monash University medical school in Melbourne, Australia, in 1967. Training as a junior medical officer was at the Alfred Hospital in Melbourne, followed by training in pathology at the Alfred Hospital, Hammersmith Hospital, London, United Kingdom, and the Mayo Clinic, Rochester, NY. He was appointed Director of Microbiology at St. Vincent's Hospital, Sydney, Australia, in 1975. He is an Associate Professor in the Medical Faculty, University of NSW, and Adjunct Professor at the University of Technology in Sydney. He is actively involved in teaching medical undergraduates and postgraduates. He is interested in infections in the immunocompromised host, antimicrobial therapy, and parasitology. Research interests in the laboratory in particular are in parasitology and mycology.
 Deborah J. E. Marriott graduated from the University of New South Wales Medical School in 1978 and was awarded FRACP in 1985 and FRCPA in 1986. She is an Associate Professor, School of Medicine, UNSW, and Adjunct Professor at the University of Technology, Sydney. Deborah has a long-standing interest in infections in immunocompromised patients and was a founding member and past president of the Australasian Society for HIV Medicine. She is active in clinical research, particularly mycology and parasitology, and is an enthusiastic teacher and clinician.
Deborah J. E. Marriott graduated from the University of New South Wales Medical School in 1978 and was awarded FRACP in 1985 and FRCPA in 1986. She is an Associate Professor, School of Medicine, UNSW, and Adjunct Professor at the University of Technology, Sydney. Deborah has a long-standing interest in infections in immunocompromised patients and was a founding member and past president of the Australasian Society for HIV Medicine. She is active in clinical research, particularly mycology and parasitology, and is an enthusiastic teacher and clinician.
 John Ellis completed a Ph.D. on leishmaniasis with Professor J. M. Crampton at the Liverpool School of Tropical Medicine in 1986, and subsequently did postdoctoral research on Eimeria vaccines at Houghton Poultry Research Station (with Dr. M. Elaine Rose and colleagues) and parasite phylogeny (with Professor A. M. Johnson at the Flinders University of South Australia). John was appointed to the academic staff of the University Technology Sydney in 1992 as a Lecturer in Microbiology. Over the last 19 years he has continued to study parasitic protozoa of both veterinary and medical importance. John Ellis is currently Professor of Molecular Biology, and his present research interests include development of vaccines and diagnostics for protozoal diseases of economic importance. He was awarded the degree of D.Sc. by Liverpool University in 2006 for his pioneering research on the biology of cyst-forming coccidia. He is a Fellow of the Royal Society for Tropical Medicine and Hygiene and a member of the Australian, British, and American Societies for Parasitology.
John Ellis completed a Ph.D. on leishmaniasis with Professor J. M. Crampton at the Liverpool School of Tropical Medicine in 1986, and subsequently did postdoctoral research on Eimeria vaccines at Houghton Poultry Research Station (with Dr. M. Elaine Rose and colleagues) and parasite phylogeny (with Professor A. M. Johnson at the Flinders University of South Australia). John was appointed to the academic staff of the University Technology Sydney in 1992 as a Lecturer in Microbiology. Over the last 19 years he has continued to study parasitic protozoa of both veterinary and medical importance. John Ellis is currently Professor of Molecular Biology, and his present research interests include development of vaccines and diagnostics for protozoal diseases of economic importance. He was awarded the degree of D.Sc. by Liverpool University in 2006 for his pioneering research on the biology of cyst-forming coccidia. He is a Fellow of the Royal Society for Tropical Medicine and Hygiene and a member of the Australian, British, and American Societies for Parasitology.
 Sebastiaan J. van Hal graduated from University of Cape Town in South Africa with honors in 1996. He worked extensively in Africa and England before moving to Australia in 1999. While completing his training for the Fellowship of the Royal Australasian College of Physicians (ID) and for the Royal College of Pathologists of Australasia (Microbiology), he has focused on developing his interests in infections in the immunocompromised host. His laboratory research interests include exploring how a patient's immune status influences not only the clinical presentation but also the utility and development of laboratory tests. He currently is working as a full-time staff specialist in infectious diseases and microbiology at Liverpool Hospital, Sydney.
Sebastiaan J. van Hal graduated from University of Cape Town in South Africa with honors in 1996. He worked extensively in Africa and England before moving to Australia in 1999. While completing his training for the Fellowship of the Royal Australasian College of Physicians (ID) and for the Royal College of Pathologists of Australasia (Microbiology), he has focused on developing his interests in infections in the immunocompromised host. His laboratory research interests include exploring how a patient's immune status influences not only the clinical presentation but also the utility and development of laboratory tests. He currently is working as a full-time staff specialist in infectious diseases and microbiology at Liverpool Hospital, Sydney.
REFERENCES
- 1.Abdo, A., J. Klassen, S. Urbanski, E. Raber, and M. G. Swain. 2003. Reversible sclerosing cholangitis secondary to cryptosporidiosis in a renal transplant patient. J. Hepatol. 38**:**688-691. [DOI] [PubMed] [Google Scholar]
- 2.Abubakar, I., S. H. Aliyu, C. Arumugam, P. R. Hunter, and N. K. Usman. 2008. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. doi: 10.1002/14651858.CD004932.pub2. [DOI] [PubMed]
- 3.Ackers, J. P., and D. Mirelman. 2006. Progress in research on Entamoeba histolytica pathogenesis. Curr. Opin. Microbiol. 9**:**367-373. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad, N., M. Khan, M. I. Hoque, R. Haque, and D. Mondol. 2007. Detection of Entamoeba histolytica DNA from liver abscess aspirate using polymerase chain reaction (PCR): a diagnostic tool for amoebic liver abscess. Bangladesh Med. Res. Counc. Bull. 33**:**13-20. [PubMed] [Google Scholar]
- 5.Aldras, A. M., J. M. Orenstein, D. P. Kotler, J. A. Shadduck, and E. S. Didier. 1994. Detection of microsporidia by indirect immunofluorescence antibody test using polyclonal and monoclonal antibodies. J. Clin. Microbiol. 32**:**608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfa Cisse, O., A. Ouattara, M. Thellier, I. Accoceberry, S. Biligui, D. Minta, O. Doumbo, I. Desportes-Livage, M. A. Thera, M. Danis, and A. Datry. 2002. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali). J. Clin. Microbiol. 40**:**1715-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali, S. A., and D. R. Hill. 2003. Giardia intestinalis. Curr. Opin. Infect. Dis. 16**:**453-460. [DOI] [PubMed] [Google Scholar]
- 8.Amar, C. F., P. H. Dear, and J. McLauchlin. 2003. Detection and genotyping by real-time PCR/RFLP analyses of Giardia duodenalis from human faeces. J. Med. Microbiol. 52**:**681-683. [DOI] [PubMed] [Google Scholar]
- 9.Amar, C. F. L., C. L. East, J. Gray, M. Iturriza-Gomara, E. A. Maclure, and J. McLauchlin. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993-1996). Eur. J. Clin. Microbiol. Infect. Dis. 26**:**311-323. [DOI] [PubMed] [Google Scholar]
- 10.Andiran, N., Z. C. Acikgoz, S. Turkay, and F. Andiran. 2006. Blastocystis hominis—an emerging and imitating cause of acute abdomen in children. J. Pediatr. Surg. 41**:**1489-1491. [DOI] [PubMed] [Google Scholar]
- 11.Añé, M. S., F. A. N. Fernández, J. P. Avila, M. B. Bringuez, and B. V. Viamontes. 2000. Emergence of a new pathogen: Cyclospora cayetanensis in patients infected with human immunodeficiency virus. Rev. Cubana Med. Trop. 52**:**66-69. [PubMed] [Google Scholar]
- 12.Angarano, G., P. Maggi, M. A. Di Bari, A. M. V. Larocca, P. Congedo, C. De Bari, O. Brandonisio, and F. Chiodo. 1997. Giardiasis in HIV: a possible role in patients with severe immune deficiency. Eur. J. Epidemiol. 13**:**485-487. [DOI] [PubMed] [Google Scholar]
- 13.Aslam, S., and D. M. Musher. 2007. Nitazoxanide: clinical studies of a broad-spectrum anti-infective agent. Future Microbiol. 2**:**583-590. [DOI] [PubMed] [Google Scholar]
- 14.Asmuth, D. M. 1994. Microsporidia and diarrhea in AIDS patients. Clin. Microbiol. Newsl. 16**:**179-186. [Google Scholar]
- 15.Atambay, M., M. R. Bayraktar, U. Kayabas, S. Yilmaz, and Y. Bayindir. 2007. A rare diarrheic parasite in a liver transplant patient: Isospora belli. Transplant. Proc. 39**:**1693-1695. [DOI] [PubMed] [Google Scholar]
- 16.Attili, S. V., A. K. Gulati, V. P. Singh, D. V. Varma, M. Rai, and S. Sundar. 2006. Diarrhea, CD4 counts and enteric infections in a hospital-based cohort of HIV-infected patients around Varanasi, India. BMC Infect. Dis. 6**:**39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachur, T. P., J. M. Vale, I. C. Coêlho, T. R. Queiroz, and C. D. S. Chaves. 2008. Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Braz. J. Infect. Dis. 12**:**115-122. [DOI] [PubMed] [Google Scholar]
- 18.Balatbat, A. B., G. W. Jordan, Y. J. Tang, and J. Silva, Jr. 1996. Detection of Cryptosporidium parvum DNA in human feces by nested PCR. J. Clin. Microbiol. 34**:**1769-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barsoum, R. S. 2006. Parasitic infections in transplant recipients. Nat. Clin. Pract. Nephrol. 2**:**490-503. [DOI] [PubMed] [Google Scholar]
- 20.Becker, G. L., Jr., S. Knep, K. P. Lance, and L. Kaufman. 1980. Amebic abscess of the brain. Neurosurgery 6**:**192-194. [PubMed] [Google Scholar]
- 21.Behera, B., B. R. Mirdha, G. K. Makharia, S. Bhatnagar, S. Dattagupta, and J. C. Samantaray. 2008. Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig. Dis. Sci. 53**:**672-679. [DOI] [PubMed] [Google Scholar]
- 22.Bicart-See, A., P. Massip, M. D. Linas, and A. Datry. 2000. Successful treatment with nitazoxanide of Enterocytozoon bieneusi microsporidiosis in a patient with AIDS. Antimicrob. Agents Chemother. 44**:**167-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanshard, C., D. S. Ellis, D. G. Tovey, S. Dowell, and B. G. Gazzard. 1992. Treatment of intestinal microsporidiosis with albendazole in patients with AIDS. AIDS 6**:**311-313. [DOI] [PubMed] [Google Scholar]
- 24.Bonacini, M. 1992. Hepatobiliary complications in patients with human immunodeficiency virus infection. Am. J. Med. 92**:**404-411. [DOI] [PubMed] [Google Scholar]
- 25.Borody, T. J., E. F. Warren, A. Wettstein, G. Robertson, P. Recabarren, A. Fontella, K. Herdnman, and R. Surace. 2002. Eradication of Dientamoeba fragilis can resolve IBS-like symptoms. J. Gastroenterol. Hepatol. 17(Suppl.)**:**A103. [Google Scholar]
- 26.Borowitz, S. M., and F. T. Saulsbury. 1991. Treatment of chronic cryptosporidial infection with orally administered human serum immune globulin. J. Pediatr. 119**:**593-595. [DOI] [PubMed] [Google Scholar]
- 27.Boschi-Pinto, C., L. Velebit, and K. Shibuya. 2008. Estimating child mortality due to diarrhoea in developing countries. Bull. W. H. O. 86**:**710-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botterel, F., C. Minozzi, D. Vittecoq, and P. Bouree. 2002. Pulmonary localization of Enterocytozoon bieneusi in an AIDS patient: case report and review. J. Clin. Microbiol. 40**:**4800-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouche, H., C. Housset, J. L. Dumont, F. Carnot, Y. Menu, B. Aveline, J. Belghiti, B. Boboc, S. Erlinger, P. Berthelot, and S. Pol. 1993. AIDS-related cholangitis: diagnostic features and course in 15 patients. J. Hepatol. 17**:**34-39. [DOI] [PubMed] [Google Scholar]
- 30.Boyd, W. P., Jr., and B. A. Bachman. 1982. Gastrointestinal infections in the compromised host. Med. Clin. N. Am. 66**:**743-753. [DOI] [PubMed] [Google Scholar]
- 31.Brandonisio, O., P. Maggi, M. A. Panaro, S. Lisi, A. Andriola, A. Acquafredda, and G. Angarano. 1999. Intestinal protozoa in HIV-infected patients in Apulia, South Italy. Epidemiol. Infect. 123**:**457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brasil, P., D. B. de Lima, D. D. de Paiva, M. S. Lobo, F. C. Sodre, S. P. Silva, E. V. Villela, E. J. Silva, J. M. Peralta, M. Morgado, and H. Moura. 2000. Clinical and diagnostic aspects of intestinal microsporidiosis in HIV-infected patients with chronic diarrhea in Rio de Janeiro, Brazil. Rev. Inst. Med. Trop. Sao Paulo 42**:**299-304. [DOI] [PubMed] [Google Scholar]
- 33.Breton, J., E. Bart-Delabesse, S. Biligui, A. Carbone, X. Seiller, M. Okome-Nkoumou, C. Nzamba, M. Kombila, I. Accoceberry, and M. Thellier. 2007. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. J. Clin. Microbiol. 45**:**2580-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer, L. A., M. C. Denver, M. Whitney, and D. J. Eichinger. 2008. Analysis of commercial Entamoeba histolytica ELISA kits for the detection of Entamoeba invadens in reptiles. J. Zoo Wildl. Med. 39**:**493-495. [DOI] [PubMed] [Google Scholar]
- 35.Brindicci, G., C. Picciarelli, L. Fumarola, S. Carbonara, F. Stano, E. Ciraci, M. Gramiccia, A. R. Sannella, M. Milella, D. De Vito, R. Monno, and L. Monno. 2006. Amoebic hepatic abscesses in an HIV-positive patient. AIDS Patient Care STDS 20**:**606-611. [DOI] [PubMed] [Google Scholar]
- 36.Brink, A. K., C. Mahe, C. Watera, E. Lugada, C. Gilks, J. Whitworth, and N. French. 2002. Diarrhoea, CD4 counts and enteric infections in a community-based cohort of HIV-infected adults in Uganda. J. Infect. 45**:**99-106. [DOI] [PubMed] [Google Scholar]
- 37.Britten, D., S. M. Wilson, R. McNerney, A. H. Moody, P. L. Chiodini, and J. P. Ackers. 1997. An improved colorimetric PCR-based method for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in feces. J. Clin. Microbiol. 35**:**1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calore, E. E., N. M. Calore, and M. J. Cavaliere. 2002. Salpingitis due to Entamoeba histolytica. Braz. J. Infect. Dis. 6**:**97-99. [DOI] [PubMed] [Google Scholar]
- 39.Carcamo, C., T. Hooton, M. H. Wener, N. S. Weiss, R. Gilman, J. Arevalo, J. Carrasco, C. Seas, M. Caballero, and K. K. Holmes. 2005. Etiologies and manifestations of persistent diarrhea in adults with HIV-1 infection: a case-control study in Lima, Peru. J. Infect. Dis. 191**:**11-19. [DOI] [PubMed] [Google Scholar]
- 40.Carr, A., and D. A. Cooper. 2002. Fumagillin for intestinal microsporidiosis. N. Engl. J. Med. 347**:**1381. (Author reply, **347:**1381.) [DOI] [PubMed] [Google Scholar]
- 41.Carrascosa, M., J. Martinez, and J. L. Perez-Castrillon. 1996. Hemorrhagic proctosigmoiditis and Blastocystis hominis infection. Ann. Intern. Med. 124**:**278-279. [DOI] [PubMed] [Google Scholar]
- 42.Castro-Hermida, J. A., A. Almeid, M. Gonzalez-Warleta, J. M. Da Costa, and M. Mezo. 2006. Prevalence and preliminary genetic analysis of Giardia isolated from adult sheep in Galicia (northwest Spain). J. Eukaryot. Microbiol. 53(Suppl. 1)**:**S172-S173. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Hermida, J. A., A. Almeida, M. Gonzalez-Warleta, J. M. Da Costa, and M. Mezo. 2006. Prevalence and preliminary genetic characterization of Cryptosporidium spp. isolated from asymptomatic heifers in Galicia (NW, Spain). J. Eukaryot. Microbiol. 53(Suppl. 1)**:**S22-S23. [DOI] [PubMed] [Google Scholar]
- 44.Castro-Hermida, J. A., A. Delafosse, I. Pors, E. Ares-Mazas, and C. Chartier. 2005. Giardia duodenalis and Cryptosporidium parvum infections in adult goats and their implications for neonatal kids. Vet. Rec. 157**:**623-627. [DOI] [PubMed] [Google Scholar]
- 45.Cavalier-Smith, T. 1998. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc. 73**:**203-266. [DOI] [PubMed] [Google Scholar]
- 46.Certad, G., A. Arenas-Pinto, L. Pocaterra, G. Ferrara, J. Castro, A. Bello, and L. Nunez. 2003. Isosporiasis in Venezuelan adults infected with human immunodeficiency virus: clinical characterization. Am. J. Trop. Med. Hyg. 69**:**217-222. [PubMed] [Google Scholar]
- 47.Chacin-Bonilla, L., N. Guanipa, G. Cano, X. Raleigh, and L. Quijada. 1992. Cryptosporidiosis among patients with acquired immunodeficiency syndrome in Zulia State, Venezuela. Am. J. Trop. Med. Hyg. 47**:**582-586. [DOI] [PubMed] [Google Scholar]
- 48.Chan, R., J. Chen, M. K. York, N. Setijono, R. L. Kaplan, F. Graham, and H. B. Tanowitz. 2000. Evaluation of a combination rapid immunoassay for detection of Giardia and Cryptosporidium antigens. J. Clin. Microbiol. 38**:**393-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen, X. M., and N. F. LaRusso. 2002. Cryptosporidiosis and the pathogenesis of AIDS-cholangiopathy. Semin. Liver Dis. 22**:**277-289. [DOI] [PubMed] [Google Scholar]
- 50.Chen, Y., Y. Zhang, B. Yang, T. Qi, H. Lu, X. Cheng, and H. Tachibana. 2007. Seroprevalence of Entamoeba histolytica infection in HIV-infected patients in China. Am. J. Trop. Med. Hyg. 77**:**825-828. [PubMed] [Google Scholar]
- 51.Chieffi, P. P., M. A. Paschoalotti, C. S. Vergueiro, and C. S. Chiattone. 2005. Infection by Cryptosporidium sp. in immunocompromised haematological patients. Rev. Inst. Med. Trop. Sao Paulo 47**:**301-302. [DOI] [PubMed] [Google Scholar]
- 52.Chui, D. W., and R. L. Owen. 1994. AIDS and the gut. J. Gastroenterol. Hepatol. 9**:**291-303. [DOI] [PubMed] [Google Scholar]
- 53.Cimerman, S., B. Cimerman, and D. Salomao Lewi. 1999. Prevalence of intestinal parasitic infections in patients with acquired immunodeficiency syndrome in Brazil. Int. J. Infect. Dis. 3**:**203-206. [DOI] [PubMed] [Google Scholar]
- 54.Clark, C. G., and L. S. Diamond. 1997. Intraspecific variation and phylogenetic relationships in the genus Entamoeba as revealed by riboprinting. J. Eukaryot. Microbiol. 44**:**142-154. [DOI] [PubMed] [Google Scholar]
- 55.Clark, C. G., and L. S. Diamond. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 15**:**329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Comer, E. O. 1956. Leukopenia following fumagillin therapy for amebiasis. Gastroenterology 30**:**294-296. [PubMed] [Google Scholar]
- 57.Conteas, C. N., O. G. Berlin, L. R. Ash, and J. S. Pruthi. 2000. Therapy for human gastrointestinal microsporidiosis. Am. J. Trop. Med. Hyg. 63**:**121-127. [DOI] [PubMed] [Google Scholar]
- 58.Conteas, C. N., T. Sowerby, G. W. Berlin, F. Dahlan, A. Nguyen, R. Porschen, J. Donovan, M. LaRiviere, and J. M. Orenstein. 1996. Fluorescence techniques for diagnosing intestinal microsporidiosis in stool, enteric fluid, and biopsy specimens from acquired immunodeficiency syndrome patients with chronic diarrhea. Arch. Pathol. Lab. Med. 120**:**847-853. [PubMed] [Google Scholar]
- 59.Costa Guimaraes, A., L. Azevedo Vinhaes, A. Santos Filho, J. Pericles Esteves, and W. Neves Abreu. 1974. Acute suppurative amebic pericarditis. Am. J. Cardiol. 34**:**103-106. [DOI] [PubMed] [Google Scholar]
- 60.Cotte, L., M. Rabodonirina, M. A. Piens, M. Perreard, M. Mojon, and C. Trepo. 1993. Prevalence of intestinal protozoans in French patients infected with HIV. J. Acquir. Immune Defic. Syndr. 6**:**1024-1029. [PubMed] [Google Scholar]
- 61.Crotti, D., M. L. D'Annibale, G. Fonzo, M. Lalle, S. M. Caccio, and E. Pozio. 2005. Dientamoeba fragilis is more prevalent than Giardia duodenalis in children and adults attending a day care centre in Central Italy. Parasite 12**:**165-170. [DOI] [PubMed] [Google Scholar]
- 62.Cruz-Reyes, J. A., W. M. Spice, T. Rehman, E. Gisborne, and J. P. Ackers. 1992. Ribosomal DNA sequences in the differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Parasitology 104**:**239-246. [DOI] [PubMed] [Google Scholar]
- 63.Da Silva, A. J., S. B. Slemenda, G. S. Visvesvara, D. A. Schwartz, C. M. Wilcox, S. Wallace, and N. J. Pieniazek. 1997. Detection of Septata intestinalis (Microsporidia) Cali et al. 1993 Using polymerase chain reaction primers targeting the small submit subunit ribosomal RNA coding region. Mol. Diagn. 2**:**47-52. [DOI] [PubMed] [Google Scholar]
- 64.Dawson, D. 2005. Foodborne protozoan parasites. Int. J. Food Microbiol. 103**:**207-227. [DOI] [PubMed] [Google Scholar]
- 65.DeHovitz, J. A., J. W. Pape, M. Boncy, and W. D. Johnson, Jr. 1986. Clinical manifestations and therapy of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 315**:**87-90. [DOI] [PubMed] [Google Scholar]
- 66.del Aguila, C., R. Lopez-Velez, S. Fenoy, C. Turrientes, J. Cobo, R. Navajas, G. S. Visvesvara, G. P. Croppo, A. J. Da Silva, and N. J. Pieniazek. 1997. Identification of Enterocytozoon bieneusi spores in respiratory samples from an AIDS patient with a 2-year history of intestinal microsporidiosis. J. Clin. Microbiol. 35**:**1862-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denkinger, C. M., P. Harigopal, P. Ruiz, and L. M. Dowdy. 2008. Cryptosporidium parvum-associated sclerosing cholangitis in a liver transplant patient. Transpl. Infect. Dis. 10**:**133-136. [DOI] [PubMed] [Google Scholar]
- 68.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40**:**340-344. [DOI] [PubMed] [Google Scholar]
- 69.Didier, E. S. 1997. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob. Agents Chemother. 41**:**1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Didier, E. S., J. M. Orenstein, A. Aldras, D. Bertucci, L. B. Rogers, and F. A. Janney. 1995. Comparison of three staining methods for detecting microsporidia in fluids. J. Clin. Microbiol. 33**:**3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Didier, E. S., L. B. Rogers, A. D. Brush, S. Wong, V. Traina-Dorge, and D. Bertucci. 1996. Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR-Southern analysis and successful treatment with albendazole and fumagillin. J. Clin. Microbiol. 34**:**947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Didier, E. S., P. W. Varner, P. J. Didier, A. M. Aldras, N. J. Millichamp, M. Murphey-Corb, R. Bohm, and J. A. Shadduck. 1994. Experimental microsporidiosis in immunocompetent and immunodeficient mice and monkeys. Folia Parasitol. (Prague) 41**:**1-11. [PubMed] [Google Scholar]
- 73.Didier, P. J., J. N. Phillips, D. J. Kuebler, M. Nasr, P. J. Brindley, M. E. Stovall, L. C. Bowers, and E. S. Didier. 2006. Antimicrosporidial activities of fumagillin, TNP-470, ovalicin, and ovalicin derivatives in vitro and in vivo. Antimicrob. Agents Chemother. 50**:**2146-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dionisio, D., L. I. Manneschi, S. Di Lollo, A. Orsi, G. Sterrantino, M. Meli, M. Gabbrielli, A. Tani, A. Papucci, and F. Leoncini. 1997. Enterocytozoon bieneusi in AIDS: symptomatic relief and parasite changes after furazolidone. J. Clin. Pathol. 50**:**472-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dobell, C. 1940. Researches on the intestinal protozoa of monkeys and man. X. The life history of Dientamoeba fragilis: observations, experiments and speculations. Parasitology 32**:**417-461. [Google Scholar]
- 76.Doller, P. C., K. Dietrich, N. Filipp, S. Brockmann, C. Dreweck, R. Vonthein, C. Wagner-Wiening, and A. Wiedenmann. 2002. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerg. Infect. Dis. 8**:**992-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dowd, S. E., C. P. Gerba, F. J. Enriquez, and I. L. Pepper. 1998. PCR amplification and species determination of microsporidia in formalin-fixed feces after immunomagnetic separation. Appl. Environ. Microbiol. 64**:**333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dryden, M. S., and D. C. Shanson. 1988. The microbial causes of diarrhoea in patients infected with the human immunodeficiency virus. J. Infect. 17**:**107-114. [DOI] [PubMed] [Google Scholar]
- 79.Dwivedi, K. K., G. Prasad, S. Saini, S. Mahajan, S. Lal, and U. K. Baveja. 2007. Enteric opportunistic parasites among HIV infected individuals: associated risk factors and immune status. Jpn. J. Infect. Dis. 60**:**76-81. [PubMed] [Google Scholar]
- 80.Eckmann, L. 2003. Mucosal defences against Giardia. Parasite Immunol. 25**:**259-270. [DOI] [PubMed] [Google Scholar]
- 81.Endeshaw, T., A. Kebede, J. J. Verweij, A. Zewide, K. Tsige, Y. Abraham, D. Wolday, T. Woldemichael, T. Messele, A. M. Polderman, and B. Petros. 2006. Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn. J. Infect. Dis. 59**:**306-310. [PubMed] [Google Scholar]
- 82.Escobedo, A. A., and F. A. Nunez. 1999. Prevalence of intestinal parasites in Cuban acquired immunodeficiency syndrome (AIDS) patients. Acta Trop. 72**:**125-130. [DOI] [PubMed] [Google Scholar]
- 83.Esfandiari, A., J. Swartz, and S. Teklehaimanot. 1997. Clustering of giardiosis among AIDS patients in Los Angeles County. Cell Mol. Biol. 43**:**1077-1083. [PubMed] [Google Scholar]
- 84.Evangelopoulos, A., N. Legakis, and N. Vakalis. 2001. Microscopy, PCR and ELISA applied to the epidemiology of amoebiasis in Greece. Parasitol. Int. 50**:**185-189. [DOI] [PubMed] [Google Scholar]
- 85.Farthing, M. J. G. 2006. Treatment options for the eradication of intestinal protozoa. Nat. Clin. Practice Gastroenterol. Hepatol. 3**:**436-445. [DOI] [PubMed] [Google Scholar]
- 86.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13**:**35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferreira, F. M., L. Bezerra, M. B. Santos, R. M. Bernardes, I. Avelino, and M. L. Silva. 2001. Intestinal microsporidiosis: a current infection in HIV-seropositive patients in Portugal. Microbes Infect. 3**:**1015-1019. [DOI] [PubMed] [Google Scholar]
- 88.Field, A. S., M. C. Hing, S. T. Milliken, and D. J. Marriott. 1993. Microsporidia in the small intestine of HIV-infected patients. A new diagnostic technique and a new species. Med. J. Aust. 158**:**390-394. [PubMed] [Google Scholar]
- 89.Field, A. S., D. J. Marriott, and M. C. Hing. 1993. The Warthin-Starry stain in the diagnosis of small intestinal microsporidiosis in HIV-infected patients. Folia Parasitol. (Prague) 40**:**261-266. [PubMed] [Google Scholar]
- 90.Fleming, C. A., D. Caron, J. E. Gunn, and M. A. Barry. 1998. A foodborne outbreak of Cyclospora cayetanesis at a wedding: clinical features and risk factors for illness. Arch. Intern. Med. 158**:**1121-1125. [DOI] [PubMed] [Google Scholar]
- 91.Fotedar, R., D. Stark, N. Beebe, D. Marriott, J. Ellis, and J. Harkness. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin. Microbiol. Rev. 20**:**511-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fotedar, R., D. Stark, N. Beebe, D. Marriott, J. Ellis, and J. Harkness. 2007. PCR detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii in stool samples from Sydney, Australia. J. Clin. Microbiol. 45**:**1035-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fotedar, R., D. Stark, D. Marriott, J. Ellis, and J. Harkness. 2008. Entamoeba moshkovskii infections in Sydney, Australia. Eur. J. Clin. Microbiol. Infect. Dis. 27**:**133-137. [DOI] [PubMed] [Google Scholar]
- 94.Fournier, S., S. Dubrou, O. Liguory, F. Gaussin, M. Santillana-Hayat, C. Sarfati, J. M. Molina, and F. Derouin. 2002. Detection of microsporidia, cryptosporidia and giardia in swimming pools: a one-year prospective study. FEMS Immunol. Med. Microbiol. 33**:**209-213. [DOI] [PubMed] [Google Scholar]
- 95.Fournier, S., O. Liguory, M. Santillana-Hayat, E. Guillot, C. Sarfati, N. Dumoutier, J. Molina, and F. Derouin. 2000. Detection of microsporidia in surface water: a one-year follow-up study. FEMS Immunol. Med. Microbiol. 29**:**95-100. [DOI] [PubMed] [Google Scholar]
- 96.Franzen, C., A. Muller, P. Hartmann, P. Hegener, M. Schrappe, V. Diehl, G. Fatkenheuer, and B. Salzberger. 1998. Polymerase chain reaction for diagnosis and species differentiation of microsporidia. Folia Parasitol. (Prague) 45**:**140-148. [PubMed] [Google Scholar]
- 97.Franzen, C., A. Muller, B. Salzberger, G. Fatkenheuer, S. Eidt, G. Mahrle, V. Diehl, and M. Schrappe. 1995. Tissue diagnosis of intestinal microsporidiosis using a fluorescent stain with Uvitex 2B. J. Clin. Pathol. 48**:**1009-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fryauff, D. J., R. Krippner, P. Prodjodipuro, C. Ewald, S. Kawengian, K. Pegelow, T. Yun, C. von Heydwolff-Wehnert, B. Oyofo, and R. Gross. 1999. Cyclospora cayetanensis among expatriate and indigenous populations of West Java, Indonesia. Emerg. Infect. Dis. 5**:**585-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia, L. S. 2001. Diagnostic medical parasitology. ASM Press,Washington, DC.
- 100.Garcia, L. S., and R. Y. Shimizu. 2000. Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay. J. Clin. Microbiol. 38**:**1267-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garcia, L. S., R. Y. Shimizu, and C. N. Bernard. 2000. Detection of Giardia lamblia, Entamoeba histolytica/Entamoeba dispar, and Cryptosporidium parvum antigens in human fecal specimens using the triage parasite panel enzyme immunoassay. J. Clin. Microbiol. 38**:**3337-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia, L. S., R. Y. Shimizu, S. Novak, M. Carroll, and F. Chan. 2003. Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J. Clin. Microbiol. 41**:**209-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gardner, T. B., and D. R. Hill. 2001. Treatment of giardiasis. Clin. Microbiol. Rev. 14**:**114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gassama, A., P. S. Sow, F. Fall, P. Camara, A. Gueye-N′diaye, R. Seng, B. Samb, S. M'Boup, and A. Aidara-Kane. 2001. Ordinary and opportunistic enteropathogens associated with diarrhea in Senegalese adults in relation to human immunodeficiency virus serostatus. Int. J. Infect. Dis. 5**:**192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gazzard, B. 2005. AIDS and the gastrointestinal tract. Medicine 33**:**24-26. [Google Scholar]
- 106.Ghosh, S., A. Debnath, A. Sil, S. De, D. J. Chattopadhyay, and P. Das. 2000. PCR detection of Giardia lamblia in stool: targeting intergenic spacer region of multicopy rRNA gene. Mol. Cell Probes 14**:**181-189. [DOI] [PubMed] [Google Scholar]
- 107.Gile, M., D. C. Warhurst, K. A. Webster, D. M. West, and J. A. Marshall. 2002. A multiplex allele specific polymerase chain reaction (MAS-PCR) on the dihydrofolate reductase gene for the detection of Cryptosporidium parvum genotypes 1 and 2. Parasitology 125**:**35-44. [DOI] [PubMed] [Google Scholar]
- 108.Girginkardesler, N., S. Coskun, I. Cuneyt Balcioglu, P. Ertan, and U. Z. Ok. 2003. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin. Microbiol. Infect. 9**:**110-113. [DOI] [PubMed] [Google Scholar]
- 109.Gliullo, A. B. D., M. S. Cribari, A. J. Bava, J. S. Cicconetti, and R. Collazos. 2000. Cyclospora cayetanensis in sputum and stool samples. Rev. Inst. Med. Trop. Sao Paulo 42**:**115-117. [DOI] [PubMed] [Google Scholar]
- 110.Goetz, M., S. Eichenlaub, G. R. Pape, and R. M. Hoffmann. 2001. Chronic diarrhea as a result of intestinal microsposidiosis in a liver transplant recipient. Transplantation 71**:**334-337. [DOI] [PubMed] [Google Scholar]
- 111.Gomersall, L. N., J. Currie, and R. Jeffrey. 1994. Amoebiasis: a rare cause of cardiac tamponade. Br. Heart J. 71**:**368-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gorgolas, M. D., J. Fortes, and M. L. F. Guerrero. 2001. Cyclospora cayetanensis cholecystitis in a patient with AIDS Ann. Intern. Med. 134**:**166. [DOI] [PubMed] [Google Scholar]
- 113.Graczyk, T. K., M. R. Cranfield, and R. Fayer. 1996. Evaluation of commercial enzyme immunoassay (EIA) and immunofluorescent antibody (FA) test kits for detection of Cryptosporidium oocysts of species other than Cryptosporidium parvum. Am. J. Trop. Med. Hyg. 54**:**274-279. [DOI] [PubMed] [Google Scholar]
- 114.Graczyk, T. K., D. Sunderland, L. Tamang, F. E. Lucy, and P. N. Breysse. 2007. Bather density and levels of Cryptosporidium, Giardia, and pathogenic microsporidian spores in recreational bathing water. Parasitol. Res. 101**:**1729-1731. [DOI] [PubMed] [Google Scholar]
- 115.Greenberg, S. J., M. P. Davey, W. S. Zierdt, and T. A. Waldmann. 1988. Isospora belli enteric infection in patients with human T-cell leukemia virus type I-associated adult T-cell leukemia. Am. J. Med. 85**:**435-438. [DOI] [PubMed] [Google Scholar]
- 116.Guerard, A., M. Rabodonirina, L. Cotte, O. Liguory, M. A. Piens, S. Daoud, S. Picot, and J. L. Touraine. 1999. Intestinal microsporidiosis occurring in two renal transplant recipients treated with mycophenolate mofetil. Transplantation 68**:**699-707. [DOI] [PubMed] [Google Scholar]
- 117.Guiguet, M., A. Furco, P. Tattevin, D. Costagliola, and J. M. Molina. 2007. HIV-associated Isospora belli infection: incidence and risk factors in the French Hospital Database on HIV. HIV Med. 8**:**124-130. [DOI] [PubMed] [Google Scholar]
- 118.Guk, S.-M., M. Seo, Y.-K. Park, M.-D. Oh, K.-W. Choe, J.-L. Kim, M.-H. Choi, S.-T. Hong, and J.-Y. Chai. 2005. Parasitic infections in HIV-infected patients who visited Seoul National University Hospital during the period 1995-2003. Korean J. Parasitol. 43**:**1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gupta, Y. K., M. Gupta, S. Aneja, and K. Kohli. 2004. Current drug therapy of protozoal diarrhoea. Indian J. Pediatr. 71**:**55-58. [DOI] [PubMed] [Google Scholar]
- 120.Guy, R. A., C. Xiao, and P. A. Horgen. 2004. Real-time PCR assay for detection and genotype differentiation of Giardia lamblia in stool specimens. J. Clin. Microbiol. 42**:**3317-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guyot, K., A. Follet-Dumoulin, E. Lelievre, C. Sarfati, M. Rabodonirina, G. Nevez, J. C. Cailliez, D. Camus, and E. Dei-Cas. 2001. Molecular characterization of Cryptosporidium isolates obtained from humans in France. J. Clin. Microbiol. 39**:**3472-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hailemariam, G., A. Kassu, G. Abebe, E. Abate, D. Damte, E. Mekonnen, and F. Ota. 2004. Intestinal parasitic infections in HIV/AIDS and HIV seronegative individuals in a teaching hospital, Ethiopia. Jpn. J. Infect. Dis. 57**:**41-43. [PubMed] [Google Scholar]
- 123.Hamnes, I. S., B. Gjerde, and L. Robertson. 2006. Prevalence of Giardia and Cryptosporidium in dairy calves in three areas of Norway. Vet. Parasitol. 140**:**204-216. [DOI] [PubMed] [Google Scholar]
- 124.Hamnes, I. S., B. Gjerde, L. Robertson, T. Vikoren, and K. Handeland. 2006. Prevalence of Cryptosporidium and Giardia in free-ranging wild cervids in Norway. Vet. Parasitol. 141**:**30-41. [DOI] [PubMed] [Google Scholar]
- 125.Hamnes, I. S., B. K. Gjerde, T. Forberg, and L. J. Robertson. 2007. Occurrence of Cryptosporidium and Giardia in suckling piglets in Norway. Vet. Parasitol. 144**:**222-233. [DOI] [PubMed] [Google Scholar]
- 126.Hamnes, I. S., B. K. Gjerde, T. Forberg, and L. J. Robertson. 2007. Occurrence of Giardia and Cryptosporidium in Norwegian red foxes (Vulpes vulpes). Vet. Parasitol. 143**:**347-353. [DOI] [PubMed] [Google Scholar]
- 127.Hamzah, Z., S. Petmitr, M. Mungthin, S. Leelayoova, and P. Chavalitshewinkoon-Petmitr. 2006. Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single-round PCR assay. J. Clin. Microbiol. 44**:**3196-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haque, R., N. U. Mollah, I. K. Ali, K. Alam, A. Eubanks, D. Lyerly, and W. A. Petri, Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38**:**3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haque, R., L. M. Neville, P. Hahn, and W. A. Petri, Jr. 1995. Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J. Clin. Microbiol. 33**:**2558-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hart, C. A., N. J. Beeching, B. I. Duerden, A. Curry, N. French, S. Kariuki, S. M. Graham, M. A. Gordon, P. G. Hoggard, S. Kewn, and D. J. Back. 2000. Infections in AIDS. J. Med. Microbiol. 49**:**947-967. [DOI] [PubMed] [Google Scholar]
- 131.Hartskeerl, R. A., A. R. Schuitema, T. van Gool, and W. J. Terpstra. 1993. Genetic evidence for the occurrence of extra-intestinal Enterocytozoon bieneusi infections. Nucleic Acids Res. 21**:**4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hegner, R., and H. J. Chu. 1930. A comparative study of the intestinal protozoa of wild monkeys and man. Am. J. Hyg. 12**:**62-108. [Google Scholar]
- 133.Helmy, M. M., L. A. Rashed, and H. S. Abdel-Fattah. 2006. Co-infection with Cryptosporidium parvum and Cyclospora cayetanensis in immunocompromised patients. J. Egypt. Soc. Parasitol. 36**:**613-627. [PubMed] [Google Scholar]
- 134.Heresi, G. P., J. R. Murphy, and T. G. Cleary. 2000. Giardiasis. Semin. Pediatr. Infect. Dis. 11**:**189-195. [Google Scholar]
- 135.Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lucking, H. Thorsten Lumbsch, F. Lutzoni, P. B. Matheny, D. J. McLaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y. C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. D. Hyde, J. E. Ironside, U. Koljalg, C. P. Kurtzman, K. H. Larsson, R. Lichtwardt, J. Longcore, J. Miadlikowska, A. Miller, J. M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schussler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiss, M. M. White, K. Winka, Y. J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the fungi. Mycol. Res. 111**:**509-547. [DOI] [PubMed] [Google Scholar]
- 136.Higgins, J. A., R. Fayer, J. M. Trout, L. Xiao, A. A. Lal, S. Kerby, and M. C. Jenkins. 2001. Real-time PCR for the detection of Cryptosporidium parvum. J. Microbiol. Methods 47**:**323-337. [DOI] [PubMed] [Google Scholar]
- 137.Ho, A. Y., A. S. Lopez, M. G. Eberhart, R. Levenson, B. S. Finkel, A. J. da Silva, J. M. Roberts, P. A. Orlandi, C. C. Johnson, and B. L. Herwaldt. 2002. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg. Infect. Dis. 8**:**783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoang, L. M., M. Fyfe, C. Ong, J. Harb, S. Champagne, B. Dixon, and J. Isaac-Renton. 2005. Outbreak of cyclosporiasis in British Columbia associated with imported Thai basil. Epidemiol. Infect. 133**:**23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hodge, C. W., D. R. Shilm, M. Ghimire, J. G. Rabold, P. Pandey, A. Walch, R. Rajah, P. Gaudio, and P. Echeverria. 1995. Placebo-controlled trial of cotrimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet 345**:**691-693. [DOI] [PubMed] [Google Scholar]
- 140.Hong, D. K., C. J. Wong, and K. Gutierrez. 2007. Severe cryptosporidiosis in a seven-year-old renal transplant recipient: case report and review of the literature. Pediatr. Transplant. 11**:**94-100. [DOI] [PubMed] [Google Scholar]
- 141.Hoza, J. 1966. Secondary reactions of some new antibiotics. Fumagillin. Lek Veda Zahr 8**:**156-157. [PubMed] [Google Scholar]
- 142.Hsieh, S. M., M. Y. Chen, S. C. Pan, C. C. Hung, and S. C. Chang. 2007. Aberrant induction of regulatory activity of CD4+CD25+ T cells by dendritic cells in HIV-infected persons with amebic liver abscess. J. Acquir. Immune Defic. Syndr. 44**:**6-13. [DOI] [PubMed] [Google Scholar]
- 143.Hughes, F. B., S. T. Faehnle, and J. L. Simon. 1975. Multiple cerebral abscesses complicating hepatopulmonary amebiasis. J. Pediatr. 86**:**95-96. [DOI] [PubMed] [Google Scholar]
- 144.Hung, C. C., P. J. Chen, S. M. Hsieh, J. M. Wong, C. T. Fang, S. C. Chang, and M. Y. Chen. 1999. Invasive amoebiasis: an emerging parasitic disease in patients infected with HIV in an area endemic for amoebic infection. AIDS 13**:**2421-2428. [DOI] [PubMed] [Google Scholar]
- 145.Hung, C. C., H. Y. Deng, W. H. Hsiao, S. M. Hsieh, C. F. Hsiao, M. Y. Chen, S. C. Chang, and K. E. Su. 2005. Invasive amebiasis as an emerging parasitic disease in patients with human immunodeficiency virus type 1 infection in Taiwan. Arch. Intern. Med. 165**:**409-415. [DOI] [PubMed] [Google Scholar]
- 146.Hung, C. C., D. D. Ji, H. Y. Sun, Y. T. Lee, S. Y. Hsu, S. Y. Chang, C. H. Wu, Y. H. Chan, C. F. Hsiao, W. C. Liu, and R. Colebunders. 2008. Increased risk for Entamoeba histolytica infection and invasive amebiasis in HIV seropositive men who have sex with men in Taiwan. PLoS Negl. Trop. Dis. 2**:**e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jayalakshmi, J., B. Appalaraju, and K. Mahadevan. 2008. Evaluation of an enzyme-linked immunoassay for the detection of Cryptosporidium antigen in fecal specimens of HIV/AIDS patients. Indian J. Pathol. Microbiol. 51**:**137-138. [DOI] [PubMed] [Google Scholar]
- 148.Jayshree, R. S., R. S. Acharya, and H. Sridhar. 1996. Isospora belli infection in a patient with acute lymphoblastic leukaemia in India. J. Diarrhoeal Dis. Res. 14**:**44-45. [PubMed] [Google Scholar]
- 149.Joachim, A. 2004. Human cryptosporidiosis: an update with special emphasis on the situation in Europe. J. Vet. Med. B 51**:**251-259. [DOI] [PubMed] [Google Scholar]
- 150.Johnson, E. H., J. J. Windsor, and C. G. Clark. 2004. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clin. Microbiol. Rev. 17**:**553-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnston, S. P., M. M. Ballard, M. J. Beach, L. Causer, and P. P. Wilkins. 2003. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J. Clin. Microbiol. 41**:**623-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jongwutiwes, S., P. Sampatanukul, and C. Putaporntip. 2002. Recurrent isosporiasis over a decade in an immunocompetent host successfully treated with pyrimethamine. Scand. J. Infect. Dis. 34**:**859-862. [DOI] [PubMed] [Google Scholar]
- 153.Joseph, J., G. K. Vemuganti, and S. Sharma. 2005. Microsporidia: emerging ocular pathogens. Indian J. Med. Microbiol. 23**:**80-91. [DOI] [PubMed] [Google Scholar]
- 154.Joshi, M., A. S. Chowdhary, P. J. Dalal, and J. K. Maniar. 2002. Parasitic diarrhoea in patients with AIDS. Natl. Med. J. India 15**:**72-74. [PubMed] [Google Scholar]
- 155.Jothikumar, N., A. J. da Silva, I. Moura, Y. Qvarnstrom, and V. R. Hill. 2008. Detection and differentiation of Cryptosporidium hominis and Cryptosporidium parvum by dual TaqMan assays. J. Med. Microbiol. 57**:**1099-1105. [DOI] [PubMed] [Google Scholar]
- 156.Karanis, P., C. Kourenti, and H. Smith. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5**:**1-38. [DOI] [PubMed] [Google Scholar]
- 157.Katiyar, S. K., V. R. Gordon, G. L. McLaughlin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob. Agents Chemother. 38**:**2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaushik, K., S. Khurana, A. Wanchu, and N. Malla. 2008. Evaluation of staining techniques, antigen detection and nested PCR for the diagnosis of cryptosporidiosis in HIV seropositive and seronegative patients. Acta Trop. 107**:**1-7. [DOI] [PubMed] [Google Scholar]
- 159.Kawano, F., K. Nishida, H. Kurisaki, A. Tsukamoto, M. Satoh, I. Sanada, T. Shido, S. Obata, K. Kimura, Y. Sasaki, et al. 1992. Isospora belli infection in a patient with adult T-cell leukemia. Rinsho Ketsueki 33**:**683-687. [PubMed] [Google Scholar]
- 160.Kawashima, I., H. Fusegawa, M. Obana, and Y. Matsuoka. 2000. A case of AIDS complicated with liver tuberculosis. Kansenshogaku Zasshi 74**:**984-988. [DOI] [PubMed] [Google Scholar]
- 161.Kean, B. H., and C. L. Malloch. 1966. The neglected ameba: Dientamoeba fragilis. A report of 100 “pure” infections. Am. J. Dig. Dis. 11**:**735-746. [DOI] [PubMed] [Google Scholar]
- 162.Kelly, P., F. Lungu, E. Keane, R. Baggaley, F. Kazembe, J. Pobee, and M. Farthing. 1996. Albendazole chemotherapy for treatment of diarrhoea in patients with AIDS in Zambia: a randomised double blind controlled trial. BMJ 312**:**1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kemper, C. A. 1997. Pulmonary disease in selected protozoal infections. Semin. Respir. Infect. 12**:**113-121. [PubMed] [Google Scholar]
- 164.Kester, K. E., G. W. Turiansky, and P. L. McEvoy. 1998. Nodular cutaneous microsporidiosis in a patient with AIDS and successful treatment with long-term oral clindamycin therapy. Ann. Intern. Med. 128**:**911-914. [DOI] [PubMed] [Google Scholar]
- 165.Knappik, M., U. Borner, and T. Jelinek. 2005. Sensitivity and specificity of a new commercial enzyme-linked immunoassay kit for detecting Entamoeba histolytica IgG antibodies in serum samples. Eur. J. Clin. Microbiol. Infect. Dis. 24**:**701-703. [DOI] [PubMed] [Google Scholar]
- 166.Knoll, E. H., and K. M. Howell. 1945. Studies on Dientamoeba fragilis: its incidence and possible pathogenicity. Am. J. Clin. Pathol. 15**:**178-183. [PubMed] [Google Scholar]
- 167.Knowles, R., and B. M. D. Gupta. 1936. Some observations on the intestinal protozoa of Macaques. Indian J. Med. Res. 24**:**547-556. [Google Scholar]
- 168.Kochhar, A., S. Saxena, V. L. Malhotra, and M. Deb. 2007. Isospora belli infection in a malnourished child. J. Commun. Dis. 39**:**141-143. [PubMed] [Google Scholar]
- 169.Koru, O., R. E. Araz, Y. A. Yilmaz, S. Erguven, M. Yenicesu, B. Pektas, and M. Tanyuksel. 2007. Isospora belli infection in a renal transplant recipent. Turkiye Parazitol. Derg. 31**:**98-100. [PubMed] [Google Scholar]
- 170.Kosek, M., C. Bern, and R. L. Guerrant. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. W. H. O. 81**:**197-204. [PMC free article] [PubMed] [Google Scholar]
- 171.Lalonde, L. F., and A. A. Gajadhar. 2008. Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayetanensis oocysts. Appl. Environ. Microbiol. 74**:**4354-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lasser, K. H., K. J. Lewin, and F. W. Ryning. 1979. Cryptosporidial enteritis in a patient with congenital hypogammaglobulinemia. Hum. Pathol. 10**:**234-240. [DOI] [PubMed] [Google Scholar]
- 173.Law, C. L., J. Walker, and M. H. Qassim. 1991. Factors associated with the detection of Entamoeba histolytica in homosexual men. Int. J. STD AIDS 2**:**346-350. [DOI] [PubMed] [Google Scholar]
- 174.Lebbad, M., H. Norrgren, A. Naucler, F. Dias, S. Andersson, and E. Linder. 2001. Intestinal parasites in HIV-2 associated AIDS cases with chronic diarrhoea in Guinea-Bissau. Acta Trop. 80**:**45-49. [DOI] [PubMed] [Google Scholar]
- 175.Leder, K., M. E. Hellard, M. I. Sinclair, C. K. Fairley, and R. Wolfe. 2005. No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals. J. Gastroenterol. Hepatol. 20**:**1390-1394. [DOI] [PubMed] [Google Scholar]
- 176.Leelayoova, S., R. Rangsin, P. Taamasri, T. Naaglor, U. Thathaisong, and M. Mungthin. 2004. Evidence of waterborne transmission of Blastocystis hominis. Am. J. Trop. Med. Hyg. 70**:**658-662. [PubMed] [Google Scholar]
- 177.Levy, Y., J. George, and Y. Shoenfeld. 1996. Severe Blastocystis hominis in an elderly man. J. Infect. 33**:**57-59. [DOI] [PubMed] [Google Scholar]
- 178.Lewthwaite, P., G. V. Gill, C. A. Hart, and N. J. Beeching. 2005. Gastrointestinal parasites in the immunocompromised. Curr. Opin. Infect. Dis. 18**:**427-435. [DOI] [PubMed] [Google Scholar]
- 179.Lindo, J. F., J. M. Dubon, A. L. Ager, E. M. De Gourville, H. Solo-Gabriele, W. I. Klaskala, M. K. Baum, and C. J. Palmer. 1998. Intestinal parasitic infections in human immunodeficiency virus (HIV)-positive and HIV-negative individuals in San Pedro Sula, Honduras. Am. J. Trop. Med. Hyg. 58**:**431-435. [DOI] [PubMed] [Google Scholar]
- 180.Lopez, A. D., C. D. Mathers, M. Ezzati, D. T. Jamison, and C. J. Murray. 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367**:**1747-1757. [DOI] [PubMed] [Google Scholar]
- 181.Lopez, A. S., D. R. Dodson, M. J. Arrowood, P. A. Orlandi Jr., A. J. da Silva, J. W. Bier, S. D. Hanauer, R. L. Kuster, S. Oltman, M. S. Baldwin, K. Y. Won, E. M. Nace, M. L. Eberhard, and B. L. Herwaldt. 2001. Outbreak of cyclosporiasis associated with basil in Missouri in 1999. Clin. Infect. Dis. 32**:**1010-1017. [DOI] [PubMed] [Google Scholar]
- 182.Lowther, S. A., M. S. Dworkin, and D. L. Hanson. 2000. Entamoeba histolytica/Entamoeba dispar infections in human immunodeficiency virus-infected patients in the United States. Clin. Infect. Dis. 30**:**955-959. [DOI] [PubMed] [Google Scholar]
- 183.Majmudar, B., M. L. Chaiken, and K. U. Lee. 1976. Amebiasis of clitoris mimicking carcinoma. JAMA 236**:**1145-1146. [PubMed] [Google Scholar]
- 184.Martino, P., G. Gentile, A. Caprioli, L. Baldassarri, G. Donelli, W. Arcese, S. Fenu, A. Micozzi, M. Venditti, and F. Mandelli. 1988. Hospital-acquired cryptosporidiosis in a bone marrow transplantation unit. J. Infect. Dis. 158**:**647-648. [DOI] [PubMed] [Google Scholar]
- 185.Mathers, C. D., and D. Loncar. 2006. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3**:**e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Matsukawa, A. 2007. STAT proteins in innate immunity during sepsis: lessons from gene knockout mice. Acta Med. Okayama 61**:**239-245. [DOI] [PubMed] [Google Scholar]
- 187.McDonald, V. 2000. Host cell-mediated responses to infection with Cryptosporidium. Parasite Immunol. 22**:**597-604. [DOI] [PubMed] [Google Scholar]
- 188.McDonald, V., R. Smith, H. Robinson, and G. Bancroft. 2000. Host immune responses against Cryptosporidium. Contrib. Microbiol. 6**:**75-91. [DOI] [PubMed] [Google Scholar]
- 189.Mendez, O. C., G. Szmulewicz, C. Menghi, S. Torres, G. Gonzalez, and C. Gatta. 1994. Comparison of intestinal parasite infestation indexes among HIV positive and negative populations. Medicina (Buenos Aires) 54**:**307-310. [PubMed] [Google Scholar]
- 190.Metge, S., J. T. Van Nhieu, D. Dahmane, P. Grimbert, F. Foulet, C. Sarfati, and S. Bretagne. 2000. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur. J. Clin. Microbiol. Infect. Dis. 19**:**221-223. [DOI] [PubMed] [Google Scholar]
- 191.Miao, Y. M., F. M. Awad-El-Kariem, C. Franzen, D. S. Ellis, A. Muller, H. M. Counihan, P. J. Hayes, and B. G. Gazzard. 2000. Eradication of cryptosporidia and microsporidia following successful antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 25**:**124-129. [DOI] [PubMed] [Google Scholar]
- 192.Mohandas, K., R. Sehgal, A. Sud, and N. Malla. 2002. Prevalence of intestinal parasitic pathogens in HIV-seropositive individuals in Northern India. Jpn. J. Infect. Dis. 55**:**83-84. [PubMed] [Google Scholar]
- 193.Molina, J. M., C. Chastang, J. Goguel, J. F. Michiels, C. Sarfati, I. Desportes-Livage, J. Horton, F. Derouin, and J. Modai. 1998. Albendazole for treatment and prophylaxis of microsporidiosis due to Encephalitozoon intestinalis in patients with AIDS: a randomized double-blind controlled trial. J. Infect. Dis. 177**:**1373-1377. [DOI] [PubMed] [Google Scholar]
- 194.Molina, J. M., J. Goguel, C. Sarfati, C. Chastang, I. Desportes-Livage, J. F. Michiels, C. Maslo, C. Katlama, L. Cotte, C. Leport, F. Raffi, F. Derouin, J. Modai, et al. 1997. Potential efficacy of fumagillin in intestinal microsporidiosis due to Enterocytozoon bieneusi in patients with HIV infection: results of a drug screening study. AIDS 11**:**1603-1610. [DOI] [PubMed] [Google Scholar]
- 195.Molina, J. M., M. Tourneur, C. Sarfati, S. Chevret, A. de Gouvello, J. G. Gobert, S. Balkan, and F. Derouin. 2002. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 346**:**1963-1969. [DOI] [PubMed] [Google Scholar]
- 196.Moolasart, P. 1999. Giardia lamblia in AIDS patients with diarrhea. J. Med. Assoc. Thailand 82**:**654-659. [PubMed] [Google Scholar]
- 197.Morales Gomez, M. A. 2004. Highly active antiretroviral therapy and cryptosporidiosis. Parassitologia 46**:**95-99. [PubMed] [Google Scholar]
- 198.Morgan, U., R. Weber, L. Xiao, I. Sulaiman, R. C. Thompson, W. Ndiritu, A. Lal, A. Moore, and P. Deplazes. 2000. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J. Clin. Microbiol. 38**:**1180-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morgan, U. M., L. Pallant, B. W. Dwyer, D. A. Forbes, G. Rich, and R. C. Thompson. 1998. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J. Clin. Microbiol. 36**:**995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Moura, H., F. C. Sodre, F. J. Bornay-Llinares, G. J. Leitch, T. Navin, S. Wahlquist, R. Bryan, I. Meseguer, and G. S. Visvesvara. 1999. Detection by an immunofluorescence test of Encephalitozoon intestinalis spores in routinely formalin-fixed stool samples stored at room temperature. J. Clin. Microbiol. 37**:**2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mukhopadhyay, C., G. Wilson, D. Pradhan, and P. G. Shivananda. 2007. Intestinal protozoal infestation profile in persistent diarrhea in children below age 5 years in western Nepal. Southeast Asian J. Trop. Med. Public Health 38**:**13-19. [PubMed] [Google Scholar]
- 202.Muller, A., R. Bialek, G. Fatkenheuer, B. Salzberger, V. Diehl, and C. Franzen. 2000. Detection of Isospora belli by polymerase chain reaction using primers based on small-subunit ribosomal RNA sequences. Eur. J. Clin. Microbiol. Infect. Dis. 19**:**631-634. [DOI] [PubMed] [Google Scholar]
- 203.Mwachari, C., B. I. F. Batchelor, J. Paul, P. G. Waiyaki, and C. F. Gilks. 1998. Chronic diarrhoea among HIV-infected adult patients in Nairobi, Kenya. J. Infect. 37**:**48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Myers, B. J., and R. E. Kuntz. 1968. Intestinal protozoa of the baboon Papio doguera Pucheran, 1856. J. Protozool. 15**:**363-365. [DOI] [PubMed] [Google Scholar]
- 205.Nahrevanian, H., and M. Assmar. 2008. Cryptosporidiosis in immunocompromised patients in the Islamic Republic of Iran. J. Microbiol. Immunol. Infect. 41**:**74-77. [PubMed] [Google Scholar]
- 206.Navaneethan, U., and R. A. Giannella. 2008. Mechanisms of infectious diarrhea. Nat. Clin. Pract. Gastroenterol. Hepatol. 5**:**637-647. [DOI] [PubMed] [Google Scholar]
- 207.Navin, T. R., R. Weber, D. J. Vugia, D. Rimland, J. M. Roberts, D. G. Addiss, G. S. Visvesvara, S. P. Wahlquist, S. E. Hogan, L. E. Gallagher, D. D. Juranek, D. A. Schwartz, C. M. Wilcox, J. M. Stewart, S. E. Thompson III, and R. T. Bryan. 1999. Declining CD4+ T-lymphocyte counts are associated with increased risk of enteric parasitosis and chronic diarrhea: results of a 3-year longitudinal study. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20**:**154-159. [DOI] [PubMed] [Google Scholar]
- 208.Ng, J., I. Pavlasek, and U. Ryan. 2006. Identification of novel Cryptosporidium genotypes from avian hosts. Appl. Environ. Microbiol. 72**:**7548-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nkinin, S. W., T. Asonganyi, E. S. Didier, and E. S. Kaneshiro. 2007. Microsporidian infection is prevalent in healthy people in Cameroon. J. Clin. Microbiol. 45**:**2841-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Norhayati, M., M. Azlin, M. H. Al-Mekhlafi, N. Anisah, U. Nor Aini, M. S. Fatmah, and A. R. Rozlida. 2008. A preliminary study on the prevalence of intestinal microsporidiosis in patients with and without gastrointestinal symptoms in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 102**:**1274-1278. [DOI] [PubMed] [Google Scholar]
- 211.Noureldin, M. S., A. A. Shaltout, E. M. El Hamshary, and M. E. Ali. 1999. Opportunistic intestinal protozoal infections in immunocompromised children. J. Egypt. Soc. Parasitol. 29**:**951-961. [PubMed] [Google Scholar]
- 212.O'Donoghue, P. J. 1985. Cryptosporidium infections in man, animals, birds and fish. Aust. Vet. J. 62**:**253-258. [DOI] [PubMed] [Google Scholar]
- 213.Ohnishi, K., M. Murata, and E. Okuzawa. 1994. Symptomatic amebic colitis in a Japanese homosexual AIDS patient. Intern. Med. 33**:**120-122. [DOI] [PubMed] [Google Scholar]
- 214.Ohtaki, M., Y. Michimata, T. Suzuki, K. Oikawa, and M. Mikami. 1976. Malignant lymphoma initiated with malabsorption syndrome due to Isospora belli infection and lymphocytosis. Tohoku J. Exp. Med. 120**:**43-51. [DOI] [PubMed] [Google Scholar]
- 215.Osman, G. A., K. M. Makled, H. M. El-Shakankiry, D. M. Metwali, S. S. Abdel-Aziz, and H. H. Saafan. 1999. Coccidian parasites as a cause of watery diarrhoea among protein energy malnourished and other immunocompromised Egyptian children. J. Egypt. Soc. Parasitol. 29**:**653-668. [PubMed] [Google Scholar]
- 216.Oster, N., H. Gehrig-Feistel, H. Jung, J. Kammer, J. E. McLean, and M. Lanzer. 2006. Evaluation of the immunochromatographic CORIS Giardia-Strip test for rapid diagnosis of Giardia lamblia. Eur. J. Clin. Microbiol. Infect. Dis. 25**:**112-115. [DOI] [PubMed] [Google Scholar]
- 217.Othman, N. H., and A. N. Ismail. 1993. Endometrial amoebiasis. Eur. J. Obstet. Gynecol. Reprod. Biol. 52**:**135-137. [DOI] [PubMed] [Google Scholar]
- 218.Palmieri, F., S. Cicalini, N. Froio, E. B. Rizzi, D. Goletti, A. Festa, G. Macri, and N. Petrosillo. 2005. Pulmonary cryptosporidiosis in an AIDS patient: successful treatment with paromomycin plus azithromycin. Int. J. STD AIDS 16**:**515-517. [DOI] [PubMed] [Google Scholar]
- 219.Pape, J. W., R. I. Verdier, and W. D. Johnson, Jr. 1989. Treatment and prophylaxis of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 320**:**1044-1047. [DOI] [PubMed] [Google Scholar]
- 220.Parija, S. C., and K. Khairnar. 2007. Detection of excretory Entamoeba histolytica DNA in the urine, and detection of E. histolytica DNA and lectin antigen in the liver abscess pus for the diagnosis of amoebic liver abscess. BMC Microbiol. 7**:**41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Parija, S. C., and K. Khairnar. 2005. Entamoeba moshkovskii and Entamoeba dispar-associated infections in pondicherry, India. J. Health Popul. Nutr. 23**:**292-295. [PubMed] [Google Scholar]
- 222.Parkar, U., R. J. Traub, S. Kumar, M. Mungthin, S. Vitali, S. Leelayoova, K. Morris, and R. C. Thompson. 2007. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134**:**359-367. [DOI] [PubMed] [Google Scholar]
- 223.Paziewska, A., M. Bednarska, H. Nieweglowski, G. Karbowiak, and A. Bajer. 2007. Distribution of Cryptosporidium and Giardia spp. in selected species of protected and game mammals from north-eastern Poland. Ann. Agric. Environ. Med. 14**:**265-270. [PubMed] [Google Scholar]
- 224.Pedraza-Diaz, S., C. F. L. Amar, J. McLauchlin, G. L. Nichols, K. M. Cotton, P. Godwin, A. M. Iversen, L. Milne, J. R. Mulla, K. Nye, H. Panigrahl, S. R. Venn, R. Wiggins, M. Williams, and E. R. Youngs. 2001. Cryptosporidium meleagridis from humans: molecular analysis and description of affected patients. J. Infect. 42**:**243-250. [DOI] [PubMed] [Google Scholar]
- 225.Peng, C. Y., and W. Tsai. 1991. Isospora belli infection in a patient with Hodgkin's disease: report of a case. J. Formos. Med. Assoc. 90**:**260-263. [PubMed] [Google Scholar]
- 226.Pol, S., C. A. Romana, S. Richard, P. Amouyal, I. Desportes-Livage, F. Carnot, J. F. Pays, and P. Berthelot. 1993. Microsporidia infection in patients with the human immunodeficiency virus and unexplained cholangitis. N. Engl. J. Med. 328**:**95-99. [DOI] [PubMed] [Google Scholar]
- 227.Pollok, R. C., and M. J. Farthing. 2000. Enteric viruses in HIV-related diarrhoea. Mol. Med. Today 6**:**483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pozio, E. 2004. Highly active antiretroviral therapy and opportunistic protozoan infections. Parassitologia 46**:**89-93. [PubMed] [Google Scholar]
- 229.Prasad, K. N., V. L. Nag, T. N. Dhole, and A. Ayyagari. 2000. Identification of enteric pathogens in HIV-positive patients with diarrhoea in northern India. J. Health Popul. Nutr. 18**:**23-26. [PubMed] [Google Scholar]
- 230.Preiss, U., G. Ockert, S. Broemme, and A. Otto. 1991. On the clinical importance of Dientamoeba fragilis infections in childhood. J. Hyg. Epidemiol. Microbiol. Immunol. 35**:**27-34. [PubMed] [Google Scholar]
- 231.Rabodonirina, M., M. Bertocchi, I. Desportes-Livage, L. Cotte, H. Levrey, M. A. Piens, G. Monneret, M. Celard, J. F. Mornex, and M. Mojon. 1996. Enterocytozoon bieneusi as a cause of chronic diarrhea in a heart-lung transplant recipient who was seronegative for human immunodeficiency virus. Clin. Infect. Dis. 23**:**114-117. [DOI] [PubMed] [Google Scholar]
- 232.Reed, S. L., J. G. Curd, I. Gigli, F. D. Gillin, and A. I. Braude. 1986. Activation of complement by pathogenic and nonpathogenic Entamoeba histolytica. J. Immunol. 136**:**2265-2270. [PubMed] [Google Scholar]
- 233.Reed, S. L., J. A. Ember, D. S. Herdman, R. G. DiScipio, T. E. Hugli, and I. Gigli. 1995. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J. Immunol. 155**:**266-274. [PubMed] [Google Scholar]
- 234.Resiere, D., J. M. Vantelon, P. Bouree, E. Chachaty, G. Nitenberg, and F. Blot. 2003. Isospora belli infection in a patient with non-Hodgkin's lymphoma. Clin. Microbiol. Infect. 9**:**1065-1067. [DOI] [PubMed] [Google Scholar]
- 235.Ruttenberg, D., S. R. Ress, S. K. Price, A. H. Girdwood, and I. N. Marks. 1990. Common variable hypogammaglobulinemia. A case report. J. Clin. Gastroenterol. 12**:**336-340. [DOI] [PubMed] [Google Scholar]
- 236.Ryan, U., L. Xiao, C. Read, L. Zhou, A. A. Lal, and I. Pavlasek. 2003. Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl. Environ. Microbiol. 69**:**4302-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sadraei, J., M. A. Rizvi, and U. K. Baveja. 2005. Diarrhea, CD4+ cell counts and opportunistic protozoa in Indian HIV-infected patients. Parasitol. Res. 97**:**270-273. [DOI] [PubMed] [Google Scholar]
- 238.Sanad, M. M., and J. S. Al-Malki. 2007. Cryptosporidiosis among immunocompromised patients in Saudi Arabia. J. Egypt. Soc. Parasitol. 37**:**765-774. [PubMed] [Google Scholar]
- 239.Sanchez-Guillen Mdel, C., R. Perez-Fuentes, H. Salgado-Rosas, A. Ruiz-Arguelles, J. Ackers, A. Shire, and P. Talamas-Rohana. 2002. Differentiation of entamoeba histolytica/entamoeba dispar by PCR and their correlation with humoral and cellular immunity in individuals with clinical variants of amoebiasis. Am. J. Trop. Med. Hyg. 66**:**731-737. [DOI] [PubMed] [Google Scholar]
- 240.Sarfati, C., A. Bourgeois, J. Menotti, F. Liegeois, R. Moyou-Somo, E. Delaporte, F. Derouin, E. M. Ngole, and J. M. Molina. 2006. Prevalence of intestinal parasites including microsporidia in human immunodeficiency virus-infected adults in Cameroon: a cross-sectional study. Am. J. Trop. Med. Hyg. 74**:**162-164. [PubMed] [Google Scholar]
- 241.Savioli, L., H. Smith, and A. Thompson. 2006. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative.’ Trends Parasitol. 22**:**203-208. [DOI] [PubMed] [Google Scholar]
- 242.Schmidt, W., U. Wahnschaffe, M. Schafer, T. Zippel, M. Arvand, A. Meyerhans, E.-O. Riecken, and R. Ullrich. 2001. Rapid increase of mucosal CD4 T cells followed by clearance of intestinal cryptosporidiosis in an AIDS patient receiving highly active antiretroviral therapy. Gastroenterology 120**:**984-987. [DOI] [PubMed] [Google Scholar]
- 243.Seydel, K. B., E. Li, Z. Zhang, and S. L. Stanley, Jr. 1998. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 115**:**1446-1453. [DOI] [PubMed] [Google Scholar]
- 244.Seyrafian, S., N. Pestehchian, M. Kerdegari, H. A. Yousefi, and B. Bastani. 2006. Prevalence rate of Cryptosporidium infection in hemodialysis patients in Iran. Hemodial Int. 10**:**375-379. [DOI] [PubMed] [Google Scholar]
- 245.Shamsuzzaman, S. M., and Y. Hashiguchi. 2002. Thoracic amebiasis. Clin. Chest Med. 23**:**479-492. [DOI] [PubMed] [Google Scholar]
- 246.Shetty, N., S. Nagpal, P. V. Rao, and H. Schroder. 1990. Detection of IgG, IgA, IgM and IgE antibodies in invasive amoebiasis in endemic areas. Scand. J. Infect. Dis. 22**:**485-491. [DOI] [PubMed] [Google Scholar]
- 247.Shum, B. O., M. S. Rolph, and W. A. Sewell. 2008. Mechanisms in allergic airway inflammation-lessons from studies in the mouse. Expert Rev. Mol. Med. 10**:**e15. [DOI] [PubMed] [Google Scholar]
- 248.Sing, A., K. Tybus, J. Heesemann, and A. Mathis. 2001. Molecular diagnosis of an Enterocytozoon bieneusi human genotype C infection in a moderately immunosuppressed human immunodeficiency virus seronegative liver-transplant recipient with severe chronic diarrhea. J. Clin. Microbiol. 39**:**2371-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Smith, H. V., and G. D. Corcoran. 2004. New drugs and treatment for cryptosporidiosis. Curr. Opin. Infect. Dis. 17**:**557-564. [DOI] [PubMed] [Google Scholar]
- 250.Som, I., A. Azam, A. Bhattacharya, and S. Bhattacharya. 2000. Inter- and intra-strain variation in the 5.8S ribosomal RNA and internal transcribed spacer sequences of Entamoeba histolytica and comparison with Entamoeba dispar, Entamoeba moshkovskii and Entamoeba invadens. Int. J. Parasitol. 30**:**723-728. [DOI] [PubMed] [Google Scholar]
- 251.Stark, D., N. Beebe, D. Marriott, J. Ellis, and J. Harkness. 2005. Detection of Dientamoeba fragilis in fresh stool specimens using PCR. Int. J. Parasitol. 35**:**57-62. [DOI] [PubMed] [Google Scholar]
- 252.Stark, D., N. Beebe, D. Marriott, J. Ellis, and J. Harkness. 2006. Evaluation of three diagnostic methods, including real-time PCR, for detection of Dientamoeba fragilis in stool specimens. J. Clin. Microbiol. 44**:**232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stark, D., N. Beebe, D. Marriott, J. Ellis, and J. Harkness. 2005. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. J. Clin. Microbiol. 43**:**2718-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Stark, D., R. Fotedar, S. van Hal, N. Beebe, D. Marriott, J. T. Ellis, and J. Harkness. 2007. Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV-negative men who have sex with men from Sydney, Australia. Am. J. Trop. Med. Hyg. 76**:**549-552. [PubMed] [Google Scholar]
- 255.Stark, D., O. Phillips, D. Peckett, U. Munro, D. Marriott, J. Harkness, and J. Ellis. 2008. Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Vet. Parasitol. 151**:**21-26. [DOI] [PubMed] [Google Scholar]
- 256.Stark, D., S. van Hal, R. Fotedar, A. Butcher, D. Marriott, J. Ellis, and J. Harkness. 2008. Comparison of stool antigen detection kits to PCR for diagnosis of amebiasis. J. Clin. Microbiol. 46**:**1678-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stark, D. J., N. Beebe, D. Marriott, J. T. Ellis, and J. Harkness. 2006. Dientamoebiasis: clinical importance and recent advances. Trends Parasitol. 22**:**92-96. [DOI] [PubMed] [Google Scholar]
- 258.Stauffer, W., M. Abd-Alla, and J. I. Ravdin. 2006. Prevalence and Incidence of Entamoeba histolytica Infection in South Africa and Egypt. Arch. Med. Res. 37**:**265-268. [DOI] [PubMed] [Google Scholar]
- 259.Stauffer, W., and J. I. Ravdin. 2003. Entamoeba histolytica: an update. Curr. Opin. Infect. Dis. 16**:**479-485. [DOI] [PubMed] [Google Scholar]
- 260.Stensvold, R., A. Brillowska-Dabrowska, H. V. Nielsen, and M. C. Arendrup. 2006. Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J. Parasitol. 92**:**1081-1087. [DOI] [PubMed] [Google Scholar]
- 261.Stenzel, D. J., and P. F. Boreham. 1996. Blastocystis hominis revisited. Clin. Microbiol. Rev. 9**:**563-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Reference deleted.
- 263.Sturdee, A. P., R. M. Chalmers, and S. A. Bull. 1999. Detection of Cryptosporidium oocysts in wild mammals of mainland Britain. Vet. Parasitol. 80**:**273-280. [DOI] [PubMed] [Google Scholar]
- 264.Tan, K. S. 2004. Blastocystis in humans and animals: new insights using modern methodologies. Vet. Parasitol. 126**:**121-144. [DOI] [PubMed] [Google Scholar]
- 265.Tanizaki, A., H. Yoshikawa, S. Iwatani, and I. Kimata. 2005. Infectivity of Blastocystis isolates from chickens, quails and geese in chickens. Parasitol. Res. 96**:**57-61. [DOI] [PubMed] [Google Scholar]
- 266.ten Hove, R. J., L. van Lieshout, E. A. Brienen, M. A. Perez, and J. J. Verweij. 2008. Real-time polymerase chain reaction for detection of Isospora belli in stool samples. Diagn. Microbiol. Infect. Dis. 61**:**280-283. [DOI] [PubMed] [Google Scholar]
- 267.Tilley, M., V. McDonald, and G. J. Bancroft. 1995. Resolution of cryptosporidial infection in mice correlates with parasite-specific lymphocyte proliferation associated with both Th1 and Th2 cytokine secretion. Parasite Immunol. 17**:**459-464. [DOI] [PubMed] [Google Scholar]
- 268.Tosoni, A., M. Nebuloni, A. Ferri, S. Bonetto, S. Antinori, M. Scaglia, L. Xiao, H. Moura, G. S. Visvesvara, L. Vago, and G. Costanzi. 2002. Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (dog type) in an Italian AIDS patient: a retrospective study. Mod. Pathol. 15**:**577-583. [DOI] [PubMed] [Google Scholar]
- 269.Tsai, J. J., H. Y. Sun, L. Y. Ke, K. S. Tsai, S. Y. Chang, S. M. Hsieh, C. F. Hsiao, J. H. Yen, C. C. Hung, and S. C. Chang. 2006. Higher seroprevalence of Entamoeba histolytica infection is associated with human immunodeficiency virus type 1 infection in Taiwan. Am. J. Trop. Med. Hyg. 74**:**1016-1019. [PubMed] [Google Scholar]
- 270.Tungtrongchitr, A., S. Manatsathit, C. Kositchaiwat, J. Ongrotchanakun, N. Munkong, P. Chinabutr, S. Leelakusolvong, and W. Chaicumpa. 2004. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J. Trop. Med. Public Health 35**:**705-710. [PubMed] [Google Scholar]
- 271.Vandenberg, O., R. Peek, H. Souayah, A. Dediste, M. Buset, R. Scheen, P. Retore, G. Zissis, and T. van Gool. 2006. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int. J. Infect. Dis. 10**:**255-261. [DOI] [PubMed] [Google Scholar]
- 272.van Gool, T., and J. Dankert. 1995. Human microsporidiosis: clinical, diagnostic and therapeutic aspects of an increasing infection. Clin. Microbiol. Infect. 1**:**75-85. [DOI] [PubMed] [Google Scholar]
- 273.van Hal, S. J., K. Muthiah, G. Matthews, J. Harkness, D. Stark, D. Cooper, and D. Marriott. 2007. Declining incidence of intestinal microsporidiosis and reduction in AIDS-related mortality following introduction of HAART in Sydney, Australia. Trans. R. Soc. Trop. Med. Hyg. 101**:**1096-1100. [DOI] [PubMed] [Google Scholar]
- 274.van Hal, S. J., D. J. Stark, R. Fotedar, D. Marriott, J. T. Ellis, and J. L. Harkness. 2007. Amoebiasis: current status in Australia. Med. J. Aust. 186**:**412-416. [DOI] [PubMed] [Google Scholar]
- 275.Verdier, R. I., D. W. Fitzgerald, W. D. J. Jr., and J. W. Pape. 2000. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann. Intern. Med. 132**:**885-888. [DOI] [PubMed] [Google Scholar]
- 276.Verweij, J. J., J. Schinkel, D. Laeijendecker, M. A. van Rooyen, L. van Lieshout, and A. M. Polderman. 2003. Real-time PCR for the detection of Giardia lamblia. Mol. Cell Probes 17**:**223-225. [DOI] [PubMed] [Google Scholar]
- 277.Villegas Gonzalez, J., A. Mercado Cruz, and L. Jasso Gutierrez. 1970. Cerebral amebiasis. Pathogenic hypothesis. Report of 4 new cases. Rev. Investig. Salud Publica 30**:**165-184. [PubMed] [Google Scholar]
- 278.Visvesvara, G. S., A. J. da Silva, G. P. Croppo, N. J. Pieniazek, G. J. Leitch, D. Ferguson, H. de Moura, S. Wallace, S. B. Slemenda, I. Tyrrell, and et al. 1995. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J. Clin. Microbiol. 33**:**930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7**:**426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Weber, R., B. Ledergerber, R. Zbinden, M. Altwegg, G. E. Pfyffer, M. A. Spycher, J. Briner, L. Kaiser, M. Opravil, C. Meyenberger, M. Flepp, et al. 1999. Enteric infections and diarrhea in human immunodeficiency virus-infected persons: prospective community-based cohort study. Arch. Intern. Med. 159**:**1473-1480. [DOI] [PubMed] [Google Scholar]
- 281.Webster, A. D. 1980. Giardiasis and immunodeficiency diseases. Trans. R. Soc. Trop. Med. Hyg. 74**:**440-443. [DOI] [PubMed] [Google Scholar]
- 282.Weitzel, T., S. Dittrich, I. Mohl, E. Adusu, and T. Jelinek. 2006. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin. Microbiol. Infect. 12**:**656-659. [DOI] [PubMed] [Google Scholar]
- 283.Westerman, E. L., and R. P. Christensen. 1979. Chronic Isospora belli infection treated with co-trimoxazole. Ann. Intern. Med. 91**:**413-414. [DOI] [PubMed] [Google Scholar]
- 284.Windsor, J. J., and E. H. Johnson. 1999. More laboratories should test for Dientamoeba fragilis infection. BMJ 318**:**735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Windsor, J. J., A. M. Rafay, A. K. Shenoy, and E. H. Johnson. 1998. Incidence of Dientamoeba fragilis in faecal samples submitted for routine microbiological analysis. Br J. Biomed. Sci. 55**:**172-175. [PubMed] [Google Scholar]
- 286.Wolska-Kusnierz, B., A. Bajer, S. Caccio, E. Heropolitanska-Pliszka, E. Bernatowska, P. Socha, J. van Dongen, M. Bednarska, A. Paziewska, and E. Sinski. 2007. Cryptosporidium infection in patients with primary immunodeficiencies. J. Pediatr. Gastroenterol. Nutr. 45**:**458-464. [DOI] [PubMed] [Google Scholar]
- 287.Yazar, S., O. Yaman, F. Demirtaçs, S. Yalçin, M. Yücesoy, and I. Sahin. 2002. Cyclospora cayetanensis associated with diarrhea in a patient with idiopathic compensated hepatic cirrhosis. Acta Gastroenterol. Belg. 65**:**241-244. [PubMed] [Google Scholar]
- 288.Yusuf, T. E., and T. H. Baron. 2004. AIDS cholangiopathy. Curr. Treat. Options Gastroenterol. 7**:**111-117. [DOI] [PubMed] [Google Scholar]
- 289.Zali, M. R., A. J. Mehr, M. Rezaian, A. R. Meamar, S. Vaziri, and M. Mohraz. 2004. Prevalence of intestinal parasitic pathogens among HIV-positive individuals in Iran. Jpn. J. Infect. Dis. 57**:**268-270. [PubMed] [Google Scholar]
- 290.Reference deleted.
- 291.Zar, F. A., E. El-Bayoumi, and M. M. Yungbluth. 2001. Histologic proof of acalculous cholecystitis due to Cyclospora cayetanensis. Clin. Infect. Dis. 33**:**140-141. [DOI] [PubMed] [Google Scholar]
- 292.Zardi, E. M., A. Picardi, and A. Afeltra. 2005. Treatment of cryptosporidiosis in immunocompromised hosts. Chemotherapy 51**:**193-196. [DOI] [PubMed] [Google Scholar]
- 293.Zhu, G., M. J. Marchewka, J. G. Ennis, and J. S. Keithly. 1998. Direct isolation of DNA from patient stools for polymerase chain reaction detection of Cryptosporidium parvum. J. Infect. Dis. 177**:**1443-1446. [DOI] [PubMed] [Google Scholar]
- 294.Zierdt, C. H., V. J. Gill, and W. S. Zierdt. 1993. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J. Clin. Microbiol. 31**:**3071-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zimmer, S. M., A. N. Schuetz, and C. Franco-Paredes. 2007. Efficacy of nitazoxanide for cyclosporiasis in patients with sulfa allergy. Clin. Infect. Dis. 44**:**466-467. [DOI] [PubMed] [Google Scholar]