Phase I Study of the Safety, Tolerability, and Pharmacokinetics of Oral CP-868,596, a Highly Specific Platelet-Derived Growth Factor Receptor Tyrosine Kinase Inhibitor in Patients With Advanced Cancers (original) (raw)
Abstract
Purpose
This phase I, first-in-human study evaluated the safety, tolerability, pharmacokinetics, and maximum-tolerated dose (MTD) of an oral platelet-derived growth factor receptor inhibitor, CP-868,596.
Patients and Methods
Patients with advanced solid tumors were eligible. Dose escalations were performed in three groups with two formulations: uncoated on an empty stomach (UES), uncoated with food (UFED), and film-coated (FC) without food. Initial dose escalation in the UES group was followed by parallel escalations in the UFED and FC groups.
Results
Fifty-nine patients enrolled. CP-868,596 was escalated from 100 mg to 340 mg daily in the UES group, from 60 mg to 100 mg twice daily in the UFED group, and from 100 mg once daily to 140 mg twice daily in the FC group. MTDs were 200 mg daily in the UES group and 100 mg twice daily in the FC group; MTD was not reached at 100 mg twice daily in the UFED group. Dose-limiting toxicities included hematuria, increased γ-glutamyltransferase or ALT, insomnia, and nausea/vomiting. Most treatment-related AEs were of grades 1 to 2 severity; nausea, vomiting, and diarrhea were reported most frequently. Administration with food generally improved tolerability. CP-868,596 was absorbed slowly; systemic exposure parameters appeared to increase greater than proportionally with dose. Mean serum concentrations exceeded the preclinically predicted minimal efficacious concentration (ie, 16 ng/mL) at all dosages. Food and film coating apparently increased interpatient variability of the maximum observed plasma concentration and the area under the concentration-time curve. No objective responses were reported, and eight patients achieved stable disease (mean duration, 5.7 months).
Conclusion
CP-868,596 potentially demonstrated greater than dose-proportional pharmacokinetics. The recommended dosage of 100 mg twice daily with food was well tolerated. Additional development as a single agent in selected populations or in combination with chemotherapy in broader populations is warranted.
INTRODUCTION
The platelet-derived growth factor (PDGF) receptors (PDGFRs) and their ligands play key roles in several cellular processes, including angiogenesis.1 In angiogenesis, recruitment of pericytes, the supportive and stabilizing cells of the vasculature, is driven by PDGF acting via the PDGFR β.1 Angiogenesis is an established target pathway in cancer therapeutics. Additionally, tumor dissemination may occur via the lymphatic system.2 Tumors produce a variety of factors that stimulate lymphangiogenesis. PDGFs are thought to function as lymphangiogenic factors; both PDGFR α and PDGFR β are believed to be involved in lymphatic vessel growth.2 Certain tumors are associated with genetic alterations that result in constitutive activation of PDGFR (Data Supplement, online only). Expression of PDGFRs is common in other solid tumors and in tumor-associated pericytes.1,3,4 Therefore, PDGFR inhibition may decrease tumor growth by directly targeting tumor cells as well as tumor vasculature. In preclinical models, PDGFR inhibition augmented chemotherapy delivery by its demonstrated effect of reducing interstitial fluid pressure within tumors.5–7 In animal tumor models, efficacy of chemotherapeutic agents was significantly enhanced when the agents were combined with PDGFR inhibitors, and up to three-fold increases in tumor concentration of cytotoxic agents were demonstrated.6,7 As such, PDGFR represents an attractive target for anticancer drugs.4
CP-868,596 is a potent, selective inhibitor of PDGFR β tyrosine kinase, and it has 50% inhibitory concentrations of about 1 ng/mL and 0.4 ng/mL for α and β receptor types, respectively.8 CP-868,596 is greater than 100-fold more selective for PDGFR than for other kinases, such as c-KIT, VEGFR-2, TIE-2, FGFR-2, EGFR, erbB2, and src.8 Preclinical studies have demonstrated the antiangiogenic properties of CP-868,596. (data on file, Pfizer, New London, CT). As solid tumors are dependent on angiogenesis, CP-868,596, alone or in combination with cytotoxic agents, may be effective in tumor growth inhibition and prevention of metastases for a broad range of solid tumors.
We report the first clinical evaluation of CP-868,596 in humans. This was a phase I, open-label, dose-escalation study designed to evaluate the safety, tolerability, and pharmacokinetics of oral CP-868,596 given daily to patients with advanced solid tumors. The maximum-tolerated doses (MTDs) on an empty stomach, with food, and with a film-coated formulation were determined. In addition, preliminary antitumor activity was observed.
PATIENTS AND METHODS
Patient Selection
Adults age 18 years or older with histologically or cytologically confirmed advanced solid tumors unresponsive to currently available therapies, or for which there was no standard therapy, were candidates for this study. Patients had adequate bone marrow, hepatic, and renal function. Details and additional inclusion/exclusion criteria are available (Data Supplement, online only).
Study Design
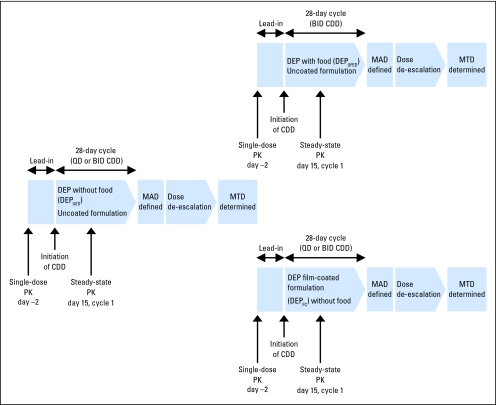
The trial design and assessments are presented in Figure 1. Each patient was treated either once daily or twice daily during a 28-day cycle, and treatment was continued in the absence of disease progression or unacceptable toxicity. Clinic visits were conducted at screening (within 2 weeks before lead-in dose), at enrollment, at lead-in dose, on day 1 of cycle 1, weekly in cycles 1 and 2, biweekly in subsequent cycles, at the end of study, and at follow-up.
Fig 1.
Study design. BID, twice daily; CDD, continuous daily dosing; DEP, dose-escalation process; UFED, uncoated formulation and fed; MAD, maximum-administered dose; MTD, maximum-tolerated dose; QD, once daily; PK, pharmacokinetics; UES, uncoated formulation and empty stomach.
Treatment
The dose-escalation process (DEP) was conducted initially by using an uncoated formulation administered on an empty stomach (DEPUES). The starting dosage of 100 mg daily for the DEPUES was based on one tenth of the severely toxic dose in rats and the predicted half-life of CP-868,596 in humans (approximately 16 hours). Nausea and vomiting, manageable with prophylactic antiemetics, were common adverse events. As these events were possibly related to the maximum observed serum concentration (Cmax), parallel escalations utilizing a pH-sensitive, film-coated formulation (DEPFC) and exploring the effect of dosing with food (DEPUFED) were conducted subsequently (Fig 1) in an effort to control nausea and vomiting and to allow administration of CP-868,596 without the need for a concomitant antiemetic. Once-daily dosing was initiated in the DEPFC cohort at a dose that was half of the MTD in the DEPUES cohort (ie, 100 mg). Twice-daily dosing was investigated on the basis of the hypothesis that twice-daily dosing would maintain overall drug exposure (ie, area under the serum concentration-time curve [AUC]) while reducing the Cmax. Twice-daily administration was initiated simultaneously in both the DEPUES (without antiemetics) and DEPUFED cohorts at 60 mg twice daily, which is a dosage that was approximately half the single-dose MTD in the DEPUES cohort. Given the favorable adverse effect profile observed in the 60-mg twice-daily DEPUFED cohort, dose escalation on the twice-daily schedule was not pursued in the DEPUES cohort.
Dose-escalation cohorts enrolled three to six patients. Intrapatient dose escalation was not allowed. The maximum-administered dose (MAD) was defined when a dose-limiting toxicity (DLT) was observed in at least two of the three to six patients. In the next cohort, the dose was decreased to midway between the previous dose and the MTD. The MTD was defined as the highest dose level less than the MAD at which a DLT was experienced in less than 33% of patients. DLTs were defined as any of the following first-cycle AEs considered causally related to CP-868,596: grade 3 or greater nonhematologic toxicity, except grade 3 nausea, vomiting, diarrhea or hypertension that could be improved to grade 1 or better with optimal supportive care; grade 2 nonhematologic toxicity, other than alopecia, that persisted for more than 14 days despite optimal supportive care; confirmed QTc prolongation of grade 3 or greater; any grade 4 hematologic toxicity and grade 3 thrombocytopenia that persisted for more than 14 days or that was associated with bleeding. Details of protocols for dosing with and without food are available (Data Supplement, online only).
This study was conducted in compliance with the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practice Guidelines protocol. It was also approved by the institutional review boards at each participating center. All patients provided written informed consent before study participation.
Evaluation of Safety and Tolerability
Patients who received at least one dose of study medication were evaluable for safety analyses. Safety and tolerability data were collected throughout the study. ECGs and laboratory evaluations were performed at specified time points throughout the study. The safety evaluation period extended through 30 days from the last dose of study drug or through recovery to grade 1 or better or to baseline from all acute toxicities associated with drug administration.
Pharmacokinetic Methods and Analyses
Single-dose and steady-state pharmacokinetics were evaluated by using noncompartmental analysis. Additional details are available (Data Supplement, online only).
Evaluation of Antitumor Activity
A maximum of 10 target lesions, representative of all involved organs, were selected per patient on the basis of size and suitability for accurate repeated measurements. Baseline tumor evaluation was conducted within 4 weeks of the lead-in dose. Subsequent tumor measurements were conducted at the end of cycle 2 and every two cycles thereafter. Tumor response was assessed by using RECIST (Response Evaluation Criteria in Solid Tumors Group) guidelines.9
Statistical Analyses
Sample size was selected empirically; it was estimated that 30 to 50 patients were required to determine the MTDs (ie, MTDUES, MTDUFED, and MTDFC) and the recommended phase II dose. No specific statistical hypothesis tests were planned; descriptive statistics were used for the analysis of pharmacokinetic, safety, and tumor response data.
RESULTS
Patient Characteristics and Drug Exposure
Fifty-nine patients were enrolled; all received at least one dose of study medication. Patient baseline characteristics and disease status are listed in Table 1. The most common tumor locations were colon, connective/soft tissue, bronchus/lung and ovary. Most patients (96.6%) had received at least one prior chemotherapy regimen (median, 3.4 regimens; range, 0 to 9 regimens), and nearly half (44%) had received radiotherapy. Patients received a total of 133 cycles of therapy. Of these, 83 cycles (61%) were delivered without therapy interruption, 37 cycles (27.2%) were delivered with one cycle, and 13 cycles (9.6%) were delivered with at least two therapy interruptions. Study drug administration details are listed in Tables 2, 3, and 4.
Table 1.
Patient Baseline Demographic and Clinical Characteristics
| Characteristic | Patients | |
|---|---|---|
| No. | % | |
| Sex | ||
| Male | 29 | 49.2 |
| Female | 30 | 50.8 |
| Age, years | ||
| Median | 58.6 | |
| Range | 18-80 | |
| ECOG score | ||
| 0 | 14 | 23.7 |
| 1 | 42 | 71.2 |
| 2 | 3 | 5.1 |
| Measurability of baseline disease | ||
| Measurable | 58 | 98.3 |
| Nonmeasurable | 1 | 1.7 |
| Primary tumor site | ||
| Colon | 10 | |
| Connective/soft tissue | 7 | |
| Bronchus/lung | 5 | |
| Ovary | 5 | |
| Other | 32 |
Table 2.
Daily Dosing and DLT Summary in the Without-Food Treatment Cohort
| Variable | Treatment Cohort Dosage (mg) | |||||
|---|---|---|---|---|---|---|
| 100 Daily | 200 Daily | 340 Daily | 280 Daily | 200 Daily | 60 Twice Daily | |
| No. of patients | 3 | 3 | 5 | 7 | 3 | 4 |
| Cycles | ||||||
| Total No. started | 16 | 8 | 17 | 11 | 9 | 5 |
| Median | 2.0 | 2.0 | 1.0 | 2.0 | 2 | 1.0 |
| Minimum-maximum range | 2-12 | 1-5 | 1-12 | 1-2 | 1-6 | 1-2 |
| Longest treatment duration without interruption, days | ||||||
| Median | 56.0 | 36.0 | 11.0 | 23.0 | 36 | 25.0 |
| Minimum-maximum range | 50-309 | 30-113 | 6-308 | 1-56 | 7-99 | 15-50 |
| No. of patients with DLTs | 0 | 0 | 2 | 2 | 1 | 0 |
| No. of MTD-evaluable patients per cohort | 3 | 3* | 4 | 6 | 3† | 3 |
| DLTs | None | None | Nausea/vomiting, increased ALT | Increased ALT, increased γ-GT, hypermagnesemia | Hematuria | None |
| Conclusion | Dose escalate | Dose escalate | MAD without food | Dose de-escalate | MTD without food | Subsequent escalation conducted in DEPUFED |
Table 3.
Daily Dosing and DLT Summary in the With-Food Treatment Cohort
| Variable | Treatment Cohort Dosage (mg) | |
|---|---|---|
| 60 Twice Daily | 100 Twice Daily | |
| No. of patients | 8 | 12 |
| Cycles | ||
| Total No. started | 15 | 27 |
| Median | 2.0 | 2.0 |
| Minimum-maximum range | 1-3 | 1-8 |
| Longest treatment duration without interruption, days | ||
| Median | 37.5 | 37.5 |
| Minimum-maximum range | 6-85 | 1-112 |
| No. of patients with DLTs | 1 | 1 |
| No. of MTD-evaluable patients per cohort | 6 | 9 |
| DLTs | Increased γ-GT | Insomnia |
| Conclusion | Dose escalate | MAD, RP2D |
Table 4.
Daily Dosing and DLT Summary in the Film-Coated Treatment Cohort
| Variable | Treatment Cohort Dosage (mg) | |||
|---|---|---|---|---|
| 100 Daily | 200 Daily | 140 Twice Daily | 100 Twice Daily | |
| No. of patients | 3 | 3 | 3 | 5 |
| Cycles | ||||
| Total No. started | 7 | 6 | 5 | 10 |
| Median | 2.0 | 2.0 | 2.0 | 2.0 |
| Minimum-maximum range | 2-3 | 2-2 | 1-2 | 1-3 |
| Longest treatment duration without interruption, days | ||||
| Median | 56.0 | 47.0 | 47.0 | 49.0 |
| Minimum-maximum range | 55-85 | 33-51 | 7-54 | 16-81 |
| No. of patients with DLTs | 0 | 0 | 1 | 0 |
| No. of MTD-evaluable patients per cohort | 3 | 3 | 3 | 4 |
| DLTs | None | None | Nausea/vomiting | None |
| Conclusion | Dose escalate | Proceed with twice-daily dosing because no DLTs occurred | MAD film coated; dose de-escalate | MTD film coated |
MTDs and DLTs
The dosing steps employed to determine the MTD for each of the DEPs, and the DLTs, are summarized in Tables 2 through 4. The MTD determined for DEPUES cohorts was 200 mg daily, and hematuria was observed as a DLT in one of six evaluable patients. In the DEPUFED group, there was one DLT (ie, increased γ-glutamyltransferase [γ-GT]) in the 60-mg twice-daily cohort and one (ie, insomnia) in the 100-mg twice-daily cohort. Only one DLT (ie, nausea and vomiting) at the 140-mg twice-daily dosage group was observed in the group of patients who received the film-coated formulation.
Safety and Tolerability
A total of 210 of 558 AEs reported by 59 patients were treatment related. Treatment-related AEs that occurred in 5% or more of the study population are listed in Table 5. Most treatment-related AEs were of grades 1 or 2 severity, and nausea, vomiting, and diarrhea were most commonly reported; there was no evidence of cumulative toxicity. Treatment-related, grades 3 or 4 events were infrequent and included nausea and vomiting. A total of 59 severe AEs were reported in 21 patients. Most hematologic laboratory abnormalities were of grades 1 or 2 severity. Grade 4 laboratory abnormalities reported across the treatment cohorts included hyponatremia, hyperglycemia, hyperkalemia, elevation in creatinine, and elevation in γ-GT.
Table 5.
Incidence of Treatment-Related AEs Occurring in ≥ 5% of the Study Population
| AE | NCI CTC Severity Grade | |||||
|---|---|---|---|---|---|---|
| 1 to 2 | 3 to 4 | Total | ||||
| No. | % | No. | % | No. | % | |
| Nausea | 39 | 66.1 | 1 | 1.7 | 40 | 67.8 |
| Vomiting | 32 | 54.3 | 1 | 1.7 | 33 | 55.9 |
| Diarrhea | 15 | 25.4 | 0 | 0 | 15 | 25.4 |
| Asthenia | 9 | 15.3 | 0 | 0 | 9 | 15.3 |
| AST increased | 7 | 11.9 | 0 | 0 | 7 | 11.9 |
| γ-GT increased | 3 | 5.1 | 3 | 5.1 | 6 | 10.2 |
| Headache | 5 | 8.5 | 0 | 0 | 5 | 8.5 |
| ALT increased | 3 | 5.1 | 2 | 3.4 | 5 | 8.5 |
| Dysgeusia | 5 | 8.5 | 0 | 0 | 5 | 8.5 |
| Anorexia | 3 | 5.1 | 1 | 1.7 | 4 | 6.8 |
| Dyspepsia | 4 | 6.8 | 0 | 0 | 4 | 6.8 |
| Periorbital edema | 4 | 6.8 | 0 | 0 | 4 | 6.8 |
| Peripheral edema | 4 | 6.8 | 0 | 0 | 4 | 6.8 |
| Abdominal pain | 2 | 3.4 | 1 | 1.7 | 3 | 5.1 |
| Alkaline phosphatase increased | 2 | 3.4 | 1 | 1.7 | 3 | 5.1 |
| Dehydration | 3 | 5.1 | 0 | 0 | 3 | 5.1 |
| Dizziness | 3 | 5.1 | 0 | 0 | 3 | 5.1 |
| Oral candidiasis | 3 | 5.1 | 0 | 0 | 3 | 5.1 |
| Rash | 3 | 5.1 | 0 | 0 | 3 | 5.1 |
There were eight deaths on the study. One of the deaths occurred before enrollment. Four of the on-study deaths were due to disease progression, and two were due to disease-related respiratory compromise/failure. The remaining death was secondary to myocardial infarction. No deaths were reported as treatment related. Twenty-four patients discontinued the study; 10 discontinuations were treatment related. The most frequent treatment-related events that resulted in permanent discontinuation were nausea and/or vomiting (n = 4) and reversible elevations in hepatic enzymes (ie, alkaline phosphatase, γ-GT, AST, ALT; n = 2). Ten patients interrupted treatment; nine of these occurrences were treatment related. The most frequent treatment-related AEs that resulted in therapy interruption were nausea and/or vomiting (n = 3) and increases in hepatic enzymes (n = 4).
Pharmacokinetic Evaluation
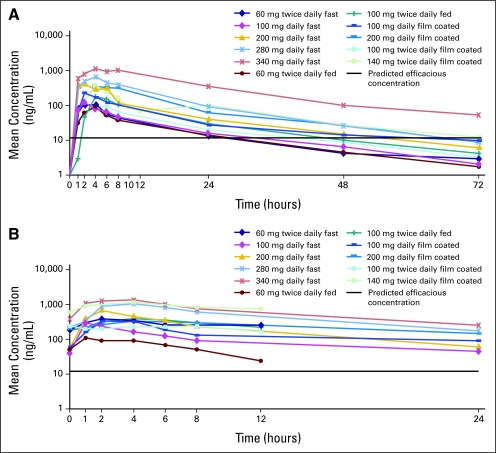
Pharmacokinetic parameters determined for CP-868,596 are presented in Table 6. Mean systemic exposure parameters after a single dose and at steady-state generally increased with dose in patients who received the uncoated formulation (with or without food) or who received the film-coated formulation on an empty stomach. After a single lead-in dose, the mean (±standard deviation) terminal half-life (t1/2; 14.0 ± 4.18 hours) was similar across all dose levels, independent of formulation or food (Appendix, online only; Fig 2A) and was consistent with the mean AUC accumulation of CP-868,596 when given once daily or twice daily. Mean steady-state serum concentrations in all dose cohorts exceeded the preclinically predicted minimal efficacious concentration (ie, 16 ng/mL; Appendix; Fig 2B). Absorption of CP-868,596 was slow for both formulations; the median time to maximum observed serum concentration (tmax) was 4 hours for the uncoated (with or without food) formulation and was 6 hours for the film-coated formulations (Table 6). For the uncoated formulation that was administered on an empty stomach, interpatient variability in Cmax and AUC demonstrated percentage coefficients of variability (CV%s) of 22% to 43% at dosages of 200 mg or less once daily and 74% to 87% at higher doses (ie, 280 and 340 mg) after a single administration of CP-868,596. At steady-state, these values were 7% to 75% at dosages of 200 mg or less once daily and 68% to 92% at higher doses. Interpatient variability was higher after administration of CP-868,596 with food or as a film-coated formulation (CV%, 42.5% to 109% and 24% to 130%, respectively). On the basis of the single lead-in dose data, the effect of food or film coating on Cmax and AUCinf (AUC from time 0 to infinity) appeared to vary at different doses. For example, the mean AUC from time 0 to the last time with quantifiable concentration (ie, AUClast) after administration with food was decreased by approximately 60% at 60 mg (n = 4 to 7) but increased by approximately 100% at 100 mg (n = 3 to 10) when compared with administration on an empty stomach.
Table 6.
Pharmacokinetic Parameters of CP-868,596 After a Single, Lead-in Dose at Day −2 and at Steady-State on Day 15
| Dosage, mg | Pharmacokinetic Parameters by Evaluation Time* | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead-in Dose | Day 15 | |||||||||||||||
| No. of Patients | Median tmax (hours) | Cmax (ng/mL) | AUClast (h × ng/mL) | t1/2 (hours) | No. of Patients | Median tmax (hours) | Cmax (ng/mL) | AUClast (h × ng/mL) | Ro† | |||||||
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | |||||
| Fasting | ||||||||||||||||
| 100 daily | 3 | 1 | 169 | 42 | 1,390 | 22 | 16.8 | 33 | 3 | 1 | 302 | 40 | 2,330 | 27 | 2.16 | 16 |
| 200 daily | 6 | 1.5 | 579 | 42 | 4,610 | 43 | 13.4 | 18 | 4 | 2 | 691 | 7 | 5,180 | 19 | 1.52 | 36 |
| 280 daily | 7 | 2 | 729 | 87 | 8.230 | 74 | 12.3 | 32 | 4 | 3 | 1.090 | 68 | 11,500 | 92 | 2.09 | 19 |
| 340 daily | 5 | 4 | 1,280 | 74 | 22,600 | 78 | 15.1 | 21 | 2 | 3 | 1,580 | 64 | 15,300 | 84 | 1.35 | 73 |
| 60 twice daily | 4 | 3 | 160 | 22 | 1,240 | 23 | 12.8 | 22 | 4 | 2 | 459 | 64 | 3,220 | 75 | 3.96 | 59 |
| Fed | ||||||||||||||||
| 60 twice daily | 7 | 4 | 50.9 | 42.5 | 501 | 62.1 | 13.7 | 40.1 | 7 | 2 | 134 | 109 | 1,570 | 92 | 2.62 | 67 |
| 100 twice daily | 10 | 4 | 225 | 68.1 | 2,830 | 80.6 | 14.3 | 30 | 10 | 4 | 467 | 80 | 70,980 | 90 | 2.75 | 52 |
| With film-coated tablets | ||||||||||||||||
| 100 daily | 3 | 2 | 250 | 82 | 2,510 | 62 | 18.5 | 13 | 3 | 2 | 419 | 85 | 3,130 | 78 | 1.81 | 38 |
| 100 twice daily | 5 | 4 | 131 | 24 | 1,600 | 79 | 13.3 | 48 | 5 | 2 | 307 | 66 | 5,140 | 85 | 2.91 | 43 |
| 200 daily | 3 | 6 | 402 | 38 | 5,600 | 57 | 14.4 | 22 | 3 | 4 | 400 | 45 | 5,680 | 66 | 1.36 | 37 |
| 140 twice daily | 3 | 4 | 373 | 86 | 5.250 | 112 | 12.9 | 40 | 3 | 2 | 1,260 | 114 | 22,000 | 130 | 4.46 | 70 |
Fig 2.
Mean serum CP-868,596 concentrations after (A) a single, oral dose (ie, day −2) and (B) continuous dosing at steady-state (ie, day 15).
Antitumor Activity
All 59 patients were evaluable for time-to-progression analysis, and 48 patients were evaluable for tumor response. No objective responses (ie, complete or partial responses) were reported. Stable disease was noted in eight patients (17%; 95% CI, 7.5% to 30%) and had a mean duration of 5.7 months. Progressive disease was reported for 38 patients, and there were two early, disease-related deaths (before the first on-study assessment). Four patients—one each with bronchoalveolar carcinoma, spindle cell breast cancer, intra-abdominal spindle cell tumor and retroperitoneal liposarcoma—remained on study and had prolonged stable disease that lasted from 173 to 338 days.
DISCUSSION
CP-868,596 is a small-molecule tyrosine kinase inhibitor selective for PDGFR and is the only drug of this type to enter clinical trials to date. This report describes the safety and tolerability profile of CP-868,596 and its pharmacokinetic parameters and MTDs on three different dosing regimens (ie, empty stomach, with food, and with a film-coated formulation). Nausea and vomiting, which were prominent toxicities after dosing without food, were reported for the initial three patients treated on the study (with 100 mg once daily without food). These events occurred typically 30 to 40 minutes after ingestion of CP-868,596 and were self limiting. As a result, concomitant antiemetic prophylaxis with a 5-hydroxytryptamine3 receptor antagonist was allowed, and the prophylaxis nearly eliminated emesis during the without-food DEP. Other antiemetics appeared less effective. The MTD determined for once-daily dosing on an empty stomach was 200 mg, and the only significant toxicity was hematuria. It is notable, however, that the DLTs associated with the MAD of 340 mg once daily for dosing on an empty stomach were nausea, vomiting, and increased ALT. In an effort to mitigate nausea and vomiting without the need for daily 5-hydroxytryptamine3 receptor antagonist, the original protocol was amended to investigate the effects of food and a film-coated formulation on the adverse effect profile and pharmacokinetics of CP-869,596. The MAD for the film-coated formulation (ie, 140 mg twice daily) was associated with a DLT of nausea and vomiting, although this formulation was generally well tolerated. The maximum dosage administered with food (ie, 100 mg twice daily) was not associated with DLTs and was the best-tolerated dose and administration condition overall. It should be noted, however, that this dosage was associated with the highest AUC; for targeted agents, such as CP-868,596, it is possible that a concentration greater than the predicted effective concentration may be a more relevant pharmacokinetic parameter for establishing an effective dose and schedule. This hypothesis requires validation in trials intended to evaluate efficacy, which could possibly be designed to randomly assign patients across dose levels.10
Nausea and vomiting were the most frequently reported treatment-related AEs of any severity across the study. It was evident that food mitigated nausea and vomiting, which occurred at a severity of grade 1 or greater in only one patient of nine in the cohort who received CP-868,596 at a dosage of 100 mg twice daily with food. This suggests that nausea and vomiting probably result from local irritation of the gastrointestinal tract rather than from centrally mediated effects. This hypothesis is also supported by the effectiveness of 5-HT3 antagonists, which specifically target abdominal vagal afferent receptors, in mitigating this AE.11 A total of 110 treatment-related AEs were reported in 24 of 25 patients dosed without food, and 63 AEs occurred in 17 of 20 patients dosed with food. Furthermore, treatment-related permanent discontinuations, dose interruptions, and severe AEs were less frequent in patients who received CP-868,596 with food than in patients who received the drug without food. These data indicate that administration of CP-868,596 with food results in generally improved tolerability. The film-coated formula was associated with the lowest incidence of treatment-related AEs (n = 37; 11 of 14 patients) and dose reductions, but the film-coated cohort had a similar incidence of discontinuations to the cohort of dosing without food. Although additional development of the film-coated formulation remains a viable option, the uncoated formulation administered with food is preferred for phase II studies.
Other tyrosine kinase inhibitors, such as sunitinib, imatinib, sorafenib, motesanib and XL999, target PDGFR in tandem with other receptors, such as VEGFR, c-KIT, RET, FGFR, src, and ABL tyrosine kinases. Nausea, vomiting, and diarrhea also have been seen with many of these tyrosine kinase inhibitors,12–14 but the prominence of nausea and vomiting in our study is distinctive. Other than nausea and vomiting, however, we did not detect significant overlap with the AE profile of these other agents and the pattern of AEs seen with CP-868,596. Although it has been suggested previously that PDGFR inhibition results in AEs related to fluid retention,15 edema was not a prominent AE on this study.
Pharmacokinetic parameters of CP-868,596 were characterized. Although the number of patients was small (n = 3 to 7) and interpatient variability was high (CV%, 7% to 92%), systemic exposure parameters (Cmax and AUC) appeared to increase more than proportionally with dose (after a single dose and at steady-state), particularly at higher doses. Mean steady-state serum concentrations in all dose cohorts exceeded the preclinically predicted minimal efficacious concentration (ie, 16 ng/mL). At the recommended phase II dosage (ie, 100 mg twice daily with food), the steady-state serum concentrations were greater than 16 ng/mL for the entire dosing interval in all patients (n = 10). The mean terminal t1/2 (range, 12.3 to 18.5 hours) was similar across all dose levels and was independent of food or film coating. The mean AUC accumulation of CP-868,596 was 1.35-fold to 2.16-fold when given once daily, and it was 2.62-fold to 4.46-fold when given twice daily, which is consistent with the mean terminal t1/2. Food and film coating did not appear to affect the terminal t1/2, but they did delay tmax and increase interpatient variability in Cmax and AUC. The effect of food or film coating on Cmax and AUC was variable. However, the interpretation of both food and film-coated pharmacokinetic findings warrants caution, given the parallel cohort design and the small number of patients (n = three to 10) relative to the moderate-to-large interpatient variability (CV%, 24% to 130%). Reversible AST and ALT elevations appeared to be positively correlated to pharmacokinetic exposures of CP-868,596 (data not shown).
No objective responses (ie, complete or partial responses) were reported on this study. The population was, as expected, heavily pretreated, and the study was not designed with the primary objective of assessing efficacy. Nonetheless, eight patients had stable disease, and there were indications of antitumor activity. Of particular interest are the four patients with bronchoalveolar carcinoma (n = 1), spindle cell neoplasms (n = 1) and sarcoma (n = 2) who remained on study for longer than 170 days, which suggests a possible driving role for PDGFR signaling in these tumors. However, as bronchoalveolar carcinoma and well-differentiated retroperitoneal liposarcoma can be indolent tumors, it is difficult to draw conclusions from these data. Nonetheless, it is notable that the patient with liposarcoma had extensive disease that progressed through six prior treatment regimens, including imatinib. The patient with bronchoalveolar carcinoma had received three prior regimens and had actively progressive disease on gefitinib at the time of study entry. It will also be of interest to assess the activity of CP-868,596 in combination with cytotoxic agents, particularly as agents that target PDGFR may elevate intratumor concentrations of cytotoxic agents.
In summary, CP-868,596 demonstrated generally increasing pharmacokinetic exposures with dose. Twice-daily administration with an uncoated formulation was well tolerated, particularly at a recommended phase II dosage of 100 mg twice daily with food.
Supplementary Material
[Data Supplement]
Acknowledgment
We thank ACUMED (Tytherington, United Kingdom) for editorial assistance; we also thank the patients and their families; the study coordinators; and the physicians who referred their patients to this study.
Appendix
Gene details.
A proportion of patients with gastrointestinal stromal tumors have activating point mutations and small deletions in the PDGFRα gene. Dermatofibrosarcoma protuberans, a rare, recurrent infiltrative tumor of the skin, is generally associated with translocations that fuse the collagen 1A1 and platelet-derived growth factor receptor (PDGFR) β genes to result in overexpression of PDGFR β.1,3
Inclusion criteria.
Inclusion criteria were as follows: Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; resolution of prior chemotherapy, radiation therapy, biologic therapy, or hormonal therapy toxicity to National Cancer Institute Common Toxicity Criteria for Adverse Events (version 3.0) grade 1 or better; adequate bone marrow (absolute neutrophil count ≥ 1,500 cells/mm3; platelets ≥ 100,000 cells/mm3; and hemoglobin ≥ 8.5 g/dL); adequate hepatic function (bilirubin ≤ 1.5 mg/dL and AST, ALT, and alkaline phosphatase ≤ 2.5× upper limit of normal range [ULN]); and adequate renal function (serum creatinine ≤ 1.5× ULN or an estimated creatinine clearance of ≥ 60 mL/min and urine protein < 1+ in urinalysis [ie, either 0 or trace urine protein, or 24-hour urine protein < 500 mg if urine protein was ≥ 1+]). Patients enrolled after determination of the maximum-administered dose (MAD) were required to have at least one measurable lesion previously untreated with radiotherapy. Patients were excluded if they had a surgical procedure within 4 weeks of study entry; had chemotherapy, radiotherapy, immunotherapy, or any investigational therapy within 3 weeks of study entry; had a requirement for therapeutic anticoagulant therapy (other than a low dose for maintenance of central venous access); or had a history of hemorrhagic or thrombotic disorders in the previous 12 months or clinically significant cardiac or vascular disease (including a myocardial infarction within the last 6 months or a requirement for antiarrhythmics).
CP-868,596 administration.
In the dose-escalation progression (DEP) group with an uncoated formulation administered on an empty stomach (ie, DEPUES group), no food was ingested for 2 hours before or 1 hour after CP-868,596 administration. In the DEP group with an uncoated formulation administered with food (ie, DEPUFED group), CP-868,596 was administered 5 minutes after ingestion of a regular meal (of 200 to 800 calories). In both of these groups, CP-868,596 was administered as uncoated tablets with at least 170 mL of water. In the DEP group with a film-coated formulation (ie, DEPFC group), CP-868,596 was administered as a pH-sensitive, film-coated tablet given with a maximum of 170 mL of water after a fast of at least 12 hours. Patients were not allowed to drink water during the 2 hours after drug administration.
Pharmacokinetics analyses.
Serial venous blood samples were obtained after a single lead-in dose was administered (on day −2 at 0 hours [ie, predose]) and at 0.5, 1, 2, 4, 6, 8, 24, 48, and 72 hours postdose for evaluation of single-dose pharmacokinetics. Continuous daily dosing was initiated 72 hours after the single lead-in dose. Steady-state kinetics were evaluated on day 15 of continuous daily dosing; serial venous blood samples were obtained at 0 hours (ie, predose); at 0.5, 1, 2, 4, 6, 8, and 24 hours postdose in the once-daily cohorts; and at 0, 0.5, 1, 2, 4, 6, 8, and 12 hours postdose in the twice-daily cohorts. Noncompartmental pharmacokinetic analysis of serum concentration-time data was performed by using WINNonlin (Scientific Consultant, Apex, NC) version 3.2 software (Pharsight, Mountain View, CA). Maximum observed serum concentration (Cmax) and time of first occurrence of Cmax (tmax) were calculated from the observed data; estimated parameters included the apparent terminal half-life (t1/2), area under the concentration-time curve (AUC) from time 0 to the last time with quantifiable concentration (AUClast), and AUC during the dosing interval (AUCτ). The accumulation ratio (Ro) of CP-868,596 was calculated as the ratio of AUCτ at day 15 to AUCτ after the lead-in dose. Arithmetic means (and standard deviations) were reported for all parameters except for the time of first occurrence of Cmax, which was expressed as the median and range.
Footnotes
Supported by Pfizer Oncology and by Grant No. CA06927 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Feng Guo, Pfizer (C); Kristen J. Pierce, Pfizer (C); Anthony J. Olszanski, Pfizer (C) Consultant or Advisory Role: None Stock Ownership: Feng Guo, Pfizer; Kristen J. Pierce, Pfizer; Anthony J. Olszanski, Pfizer Honoraria: None Research Funding: Lionel D. Lewis, Pfizer; Roger B. Cohen, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Nancy L. Lewis, Nandi J. Reddy, Anthony J. Olszanski, Roger B. Cohen
Provision of study materials or patients: Nancy L. Lewis, Lionel D. Lewis, Joseph P. Eder, Feng Guo, Roger B. Cohen
Collection and assembly of data: Nancy L. Lewis, Lionel D. Lewis, Joseph P. Eder, Kristen J. Pierce, Anthony J. Olszanski, Roger B. Cohen
Data analysis and interpretation: Nancy L. Lewis, Lionel D. Lewis, Joseph P. Eder, Nandi J. Reddy, Kristen J. Pierce, Anthony J. Olszanski, Roger B. Cohen
Manuscript writing: Nancy L. Lewis, Lionel D. Lewis, Joseph P. Eder, Nandi J. Reddy, Feng Guo, Kristen J. Pierce, Anthony J. Olszanski, Roger B. Cohen
Final approval of manuscript: Nancy L. Lewis, Lionel D. Lewis, Joseph P. Eder, Nandi J. Reddy, Feng Guo, Kristen J. Pierce, Anthony J. Olszanski, Roger B. Cohen
REFERENCES
- 1.Pietras K, Sjoblom T, Rubin K, et al. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–443. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y. Opinion: Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 3.Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 4.Lewis NL. The platelet-derived growth factor receptor as a therapeutic target. Curr Oncol Rep. 2007;9:89–95. doi: 10.1007/s11912-007-0003-6. [DOI] [PubMed] [Google Scholar]
- 5.Pietras K, Ostman A, Sjoquist M, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–2934. [PubMed] [Google Scholar]
- 6.Pietras K, Rubin K, Sjoblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- 7.Pietras K, Stumm M, Hubert M, et al. STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin Cancer Res. 2003;9:3779–3787. [PubMed] [Google Scholar]
- 8.Lewis N, Eder JP, Guo F, et al. Phase 1 study of CP-868,596 an oral, highly specific PDGFR inhibitor. J Clin Oncol. 2007;25(suppl):144s. abstr 3524. [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: If you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22:4442–4445. doi: 10.1200/JCO.2004.07.960. [DOI] [PubMed] [Google Scholar]
- 11.Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: An important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- 12.Demetri GD, von MM, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 13.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 14.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 15.Jayson GC, Parker GJ, Mullamitha S, et al. Blockade of platelet-derived growth factor receptor-beta by CDP860, a humanized, PEGylated di-Fab, leads to fluid accumulation and is associated with increased tumor vascularized volume. J Clin Oncol. 2005;23:973–981. doi: 10.1200/JCO.2005.01.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Data Supplement]