Prefrontal Structural and Functional Brain Imaging findings in Antisocial, Violent, and Psychopathic Individuals: A Meta-Analysis (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 30.
Abstract
Brain imaging studies suggest that antisocial and violent behavior is associated with structural and functional deficits in the prefrontal cortex, but there is heterogeneity in findings and it is unclear whether findings apply to psychopaths, non-violent offenders, community-based samples, and studies employing psychiatric controls. A meta-analysis was conducted on 43 structural and functional imaging studies and results show significantly reduced prefrontal structure and function in antisocial individuals. Effect sizes were significant for both structural and functional studies. With minor exceptions, no statistically significant moderating effects of sample characteristics and methodological variables were observed. Findings were localized to the right orbitofrontal cortex, right anterior cingulate cortex, and left dorsolateral prefrontal cortex. Findings confirm the replicability of prefrontal structural and functional impairments in antisocial populations and highlight the involvement of orbitofrontal, dorsolateral frontal, and anterior cingulate cortex in antisocial behavior.
Keywords: antisocial, violent, psychopathy, prefrontal
1. Introduction
In the past decade, research on antisocial behavior (aggression, psychopathy, and conduct problems) has been able to identify several environmental, psychological, and social pathways that potentially lead to these behaviors (Holmes, Slaughter, and Kashani, 2001; Raine, 2002; Vermeiren et al., 2002). In addition, mounting evidence has shown structural and functional abnormalities in antisocial individuals and hypotheses have been presented linking antisocial behavior to deficits in the prefrontal cortex, temporal cortex, insula, amygdala, hippocampus/parahippocampus, and anterior/posterior cingulate gyrus (Blair, 2001; Kiehl, 2006; Raine and Yang, 2006). Among these brain regions, the prefrontal cortex has been most commonly recognized as the most crucial (although not only) brain structure to be compromised in violent and antisocial populations (Davidson, Putnam, and Larson, 2000; Henry and Moffitt, 1997; Raine, 1993; Raine and Buchsbaum, 1996). However, clear interpretation of the literature has proved elusive due to some failures to replicate and some complex findings (e.g. significantly increased rather than decreased activation).
One problem in drawing conclusions from these disparate studies is that most studies treat the prefrontal cortex as one unitary structure based on the fact that it is rich in inter-cortical connectivity, and many areas overlapped in their functions (Dum and Strick, 1991; Ongur, Ferry, and Price, 2003; Petrides and Pandya, 1999, 2001). However, based on anatomical landmarks, studies have suggested that the prefrontal cortex can be broadly subdivided into the orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), and the medial prefrontal cortex (MPFC) (Ongur, Ferry, and Price, 2003; Petrides and Pandya, 1999, 2001). Functional studies have also supported such delineation by showing functional specificity of these prefrontal sub-regions (Bechara, 2004; Campbell, 2007; Volz, Schubotz, and von Cramon, 2006; Duncan & Own, 2000; Stuss et al., 2001). Therefore, it is of value to investigate the specificity of any abnormality to prefrontal sub-regions (Raine & Yang, 2006).
Another important issue concerns whether there are both structural and functional abnormalities in antisocial populations. Despite the fact that studies have shown a correlation between volumetric reduction and decreased brain activation (Johnson et al., 2000; Thomsen et al., 2004), very few if any imaging studies examine both structure and function in the same population. Additional issues that might contribute to variability in findings include heterogeneity in antisocial samples and variation in imaging methodology. Violence, psychopathy, and comorbid psychiatric disorders may moderate study outcomes (Mena et al., 2005; Raine and Yang, 2004; Spampinato et al., 2005; Yang and Raine, 2006). Several imaging methodology variables have been shown to influence quality, including the magnet strength, repetition time (TR), full-width-at-half-maximum (FHWM), and uptake time (Levin and Hoffman, 1999; McCarley et al., 1999), and differences in findings on antisocial behavior could be attributable to these factors.
In order to address these problems, the present meta-analytic review was undertaken to: (a) aggregate the outcomes of all imaging studies on the prefrontal cortex in antisocial individuals, (b) examine the association between antisocial behavior and sub-regions of the prefrontal cortex, (c) evaluate whether such an association is more prominent in functional or structural imaging studies, and (d) delineate reasons for variability in previous findings.
2. Method
2.1. Study Selection
The search for candidate studies to be included in the meta-analysis was conducted using 35 keywords relevant to antisocial behavior and brain imaging (i.e. Antisocial personality disorder / APD, antisocial behavior, conduct disorder / CD, oppositional defiant disorder / ODD, disruptive behavior disorder / DBD, psychopath, psychopathy, psychopathic, violent, violence, aggressive, aggression, offender, criminal, anatomical magnetic resonance imaging / aMRI, volumetric magnetic resonance imaging / vMRI, diffusion tensor imaging / DTI, structural imaging, functional magnetic resonance imaging / fMRI, magnetic resonance spectroscopy / MRS, perfusion emission tomography / PET, single photon emission computerized tomography / SPECT, functional imaging, prefrontal cortex / PFC) in three electronic indices (PubMed, PsycINFO, ISI Web of Science) for English language studies published between January 1965 and September 2007. In addition, all of the reference lists of the studies included for analysis, as well as several review articles on the relation of brain imaging with aggression and antisocial behavior were reviewed (e.g. Anckarsater, 2006; Brower and Price, 2001; Raine, 2002; Raine and Yang, 2004, 2006; Yang, Glenn, and Raine, 2008; Yang and Raine, 2008).
To be included in this meta-analysis, the study had to meet all criteria listed below. First, if a group comparison was used, a study had to include at least one antisocial group (defined as a group that contains individuals with APD, antisocial behavior, conduct disorder, oppositional defiant disorder or disruptive behavior disorder, psychopaths, criminals, violent offenders, or aggressive individuals), and one control group of either appropriate psychiatric controls or healthy normal subjects. If correlational analysis was used, a study must have had at least one assessment of antisocial behavior (defined as above). Second, studies needed to include one or more of the following imaging methods: aMRI, DTI, fMRI, MRS, PET, or SPECT. Third, the imaging method the study used had to include assessment of either the structure (e.g. volume, neural connectivity) or function (e.g. hemodynamic response, regional cerebral blood flow) of the prefrontal cortex. The prefrontal cortex was defined as the frontal region anterior to the precentral sulcus (primary and association motor areas were excluded). Results found in the following prefrontal sub-regions were also included for region of interest (ROI) analyses: OFC (Brodmann area (BA) 11, 12, 47), DLPFC (BA 8, 9, 10, 46), VLPFC (BA 44, 45), MPFC (medial section of BA 8, 9, 10, 11, 12), and ACC (BA 24, 32) (see Figure 1). For papers that used a different nomenclature for anatomical regions (e.g. inferior frontal cortex instead of VLPFC), their findings were classified into the four ROIs examined in this review using the information provided by the authors (i.e. BA location, anatomical landmarks). For studies reporting findings in the MPFC, further examination of the Talairach coordinates or delineation methods was conducted to determine whether they actually localized in the ACC to minimize overlapping between these two ROIs. For aMRI studies, if prefrontal tissue classification was applied, only findings on gray matter were included to maintain comparability with other imaging methodologies on cortical blood flow and glucose metabolism. Lastly, studies had to report sufficient statistical details to permit the calculation of effect size. Prefrontal abnormalities reported from interaction effects that were specific to a particular study design (e.g. 3 phase × 2 conditioned-stimulus type × 3 group interaction in Veit et al., 2002) were also excluded from this review due to the difficulty in evaluating the compatibility of these indirect results to the results from other studies and/or the lack of sufficient statistical results in follow-up pairwise group comparisons for calculating effect sizes.
Figure 1.
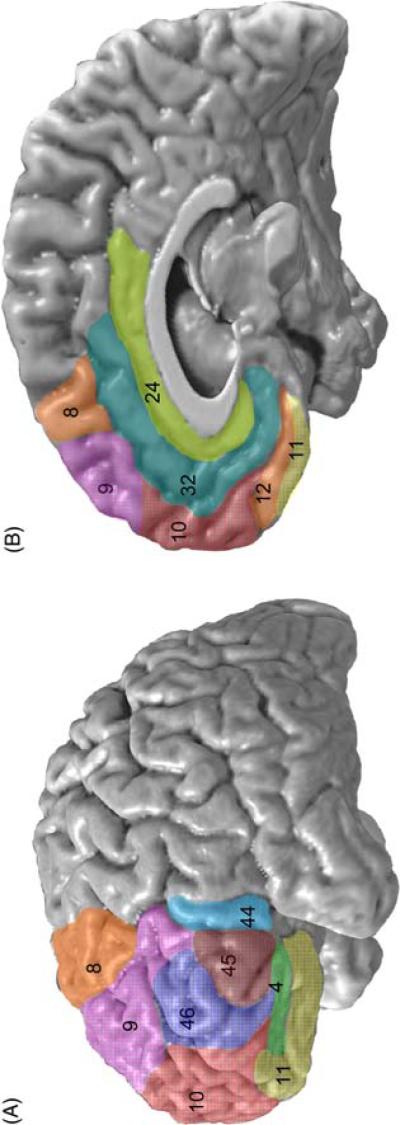
Lateral (A) and medial (B) illustration of the Brodmann Areas (BA) in the orbitofrontal, dorsolateral prefrontal, ventrolateral prefrontal, medial prefrontal, and anterior cingulate cortices. The orbitofrontal cortex included BA 11, 12, and 47. The dorsolateral prefrontal cortex included BA 8, 9, 10, and 46. The ventrolateral prefrontal cortex included BA 44 and 45. The medial prefrontal cortex included BA 8, 9, 10, 11, and 12. The anterior cingulate cortex included BA 24 and 32.
Studies of animals, articles written in languages other than English, studies in which antisocial behavior was manipulated experimentally (e.g. showing images that provoke anger), pharmacological studies, and case reports or observations on patients with antisocial symptoms were excluded. In addition, only studies published in peer-reviewed journals were included to assure the quality of the study and that sufficient information would be provided to allow the calculation of the effect sizes as well as the conduction of moderator analyses. When a sample was used in more than one publication, the one with the largest sample size was selected to be included in the analysis.
As a result of the systematic search of the databases, a total of 54 publications were initially found and among them 11 studies were excluded due to insufficient statistical results for calculating effect sizes. The demographic information and antisocial sample characteristics of the remaining 43 studies included in this meta-analysis are presented in Table 1. There were a total of 789 antisocial individuals and 473 control subjects. Close to half of the studies used only male participants and the percentage of males in the antisocial sample was 83.9 % across studies (see Table 1). Diagnostic criteria were broadly comparable, with studies using DSM-III-R or DSM-IV criteria for APD diagnosis and Psychopathy Checklist - Revised (PCL-R) or Psychopathic Personality Inventory (PPI) for psychopathy (Hare, 2003; Lilienfeld and Andrews, 1996).
Table 1.
Demographics information and sample characteristics of the 43 studies.
| Antisocial Sample | Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | ImagingType | No. of Subject | Male(%) | Mean age(years) | violent | institutional-based | psychiatriccontrol | comorbidity | psychopathy | effectsize (d) |
| patient | control | |||||||||
| Amen et al, 1996 | SPECT | 40 | 40 | 75 | 30 | Yes | Yes | Others | .36 | |
| Antonucci et al, 2006 | aMRI | 15 | 73.3 | 39 | Yes | Yes | Others | -.94 | ||
| Barkataki et al, 2006 | aMRI | 13 | 15 | 100 | 31.6 | Yes | Yes | Others | -.06 | |
| Barkataki et al, 2006 | aMRI | 13 | 15 | 100 | 34.5 | Yes | Yes | Yes | Schizophrenia | -.77 |
| Birbaumer et al, 2005 | fMRI | 10 | 10 | 100 | 35.3 | Yes | -.98 | |||
| Coccaro et al, 2007 | fMRI | 10 | 10 | 50 | 34.3 | Yes | Others | -1.77 | ||
| Critchley et al, 2000 | MRS | 10 | 8 | 90 | 23 | Yes | Yes | Yes | Others | -1.57 |
| Dolan et al, 2002 | aMRI | 18 | 19 | 100 | 30.3 | Yes | Yes | Others | Yes | -.15 |
| Frankle et al, 2005 | aMRI | 10 | 10 | 50 | 35 | Yes | Others | -.25 | ||
| Frankle et al, 2005 | PET | 10 | 10 | 50 | 35 | Yes | Others | -1.06 | ||
| George et al, 2004 | PET | 8 | 11 | 100 | 32.9 | Yes | Yes | Alcoholism | -.37 | |
| Gordon et al, 2004 | fMRI | 10 | 10 | 100 | 23.5 | -.98 | ||||
| Goyer et al, 1994 | PET | 17 | 70.6 | 24.6 | Yes | Yes | Others | -1.17 | ||
| Hirono et al, 2000 | SPECT | 10 | 10 | 40 | 75.3 | Yes | Yes | Dementia | -2.22 | |
| Hoptman et al, 2005 | aMRI | 49 | 87.8 | 41.5 | Yes | Yes | Yes | SA | .82 | |
| Hoptman et al, 2002 | DTI | 14 | 100 | 40.5 | Yes | Yes | Schizophrenia | .26 | ||
| Intrator et al, 1997 | SPECT | 8 | 9 | 100 | 36.8 | Yes | Yes | SA | Yes | .37 |
| Joyal et al, 2007 | fMRI | 12 | 12 | 100 | 42 | Yes | Yes | Yes | Schizophrenia/SA | -.33 |
| Juhasz et al, 2001 | PET | 6 | 7 | 50 | 9.9 | Yes | Yes | Epilepsy | -1.90 | |
| Kiehl et al, 2001 | fMRI | 8 | 8 | n/a | 33.9 | Yes | Yes | Yes | -2.59 | |
| Kiehl et al, 2004 | fMRI | 8 | 8 | 100 | 33.9 | Yes | Yes | -2.68 | ||
| Kruesi et al, 2004 | aMRI | 10 | 10 | 90 | 16.1 | ADHD | -.61 | |||
| Kumari et al, 2006 | fMRI | 10 | 13 | 100 | 31.3 | Yes | Yes | -1.67 | ||
| Kumari et al, 2006 | fMRI | 12 | 13 | 100 | 34 | Yes | Yes | Yes | Schizophrenia | -.77 |
| Kuruoglu et al, 1996 | SPECT | 50 | 100 | 37.5 | Yes | Yes | Alcoholism | -.77 | ||
| Laakso et al, 2002 | aMRI | 24 | 33 | 100 | 31 | Yes | Yes | Alcoholism | Yes | -.11 |
| Li et al, 2005 | DTI | 36 | 40 | 60.5 | 14 | Yes | -.78 | |||
| Li et al, 2006 | fMRI | 27 | 63 | 36.2 | Yes | Yes | SA | .44 | ||
| Mathews et al, 2005 | fMRI | 19 | 19 | 74 | 14.1 | Yes | -.22 | |||
| Müller et al, 2003 | fMRI | 6 | 6 | 100 | 33 | Yes | Yes | -.01 | ||
| Nakano et al, 2006 | SPECT | 22 | 63.6 | 62.9 | Yes | FTD | .51 | |||
| Oder et al, 1992 | SPECT | 36 | 86.1 | 30.2 | Yes | Yes | Yes | CHI | -.02 | |
| Parsey et al, 2002 | PET | 25 | 52 | 40.3 | Yes | Yes | -1.04 | |||
| Raine et al, 1997 | PET | 41 | 41 | 95.1 | 34.3 | Yes | Yes | Yes | Others | -.56 |
| Raine et al, 2000 | aMRI | 21 | 26 | 100 | 31.9 | Yes | SA | Yes | -.79 | |
| Rilling et al, 2006 | fMRI | 30 | 50 | 21.2 | Yes | -.20 | ||||
| Schneider et al, 2000 | fMRI | 12 | 12 | 100 | 31.5 | Yes | Yes | .95 | ||
| Soderstrom et al, 2000 | SPECT | 21 | 11 | 95.2 | 27 | Yes | Yes | SA | -.41 | |
| Soderstrom et al, 2002 | SPECT | 32 | 90.6 | 31.5 | Yes | Yes | Yes | Others | -.11 | |
| Stadler et al, 2007 | fMRI | 27 | 100 | 12.9 | Yes | Yes | ADHD | -.70 | ||
| Sterzer et al, 2005 | fMRI | 13 | 14 | 100 | 12.9 | Yes | ADHD | -2.08 | ||
| Volkow et al, 1995 | PET | 8 | 8 | 100 | 34 | Yes | Yes | -1.09 | ||
| Woermann et al, 2000 | aMRI | 25 | 25 | 68 | 27 | Yes | Yes | Temporal lobe epilepsy | -1.16 |
2.2. Meta-Analysis Procedure
Meta-analyses were performed using Comprehensive Meta-Analysis, Version 2, Biostat, Englewood NJ (Borenstein et al., 2005). For each study included in the meta-analyses, the effect size was calculated using Cohen's method as the difference between means divided by the pooled standard deviation and expressed as Cohen's d (Hedges and Olkin, 1985; Cohen, 1988). If more than one probability (P) was presented for a sub-region, results were combined following the method proposed by Rosenthal (Rosenthal, 1978). If multiple independent samples were reported separately in one study (e.g. violent schizophrenia and violent APD, men and women), these samples were treated as separate. According to the classification adopted by Cohen, small, medium and large effect sizes were defined by Cohen's d values of 0.2, 0.5, and 0.8, respectively (Cohen, 1988). Negative effect sizes in the present meta-analysis reflect reduced / smaller prefrontal activation/volume associated with increased antisocial behavior. The 95% confidence interval around the composite effect size was also calculated (Hedges and Olkin, 1985).
For each meta-analysis, a homogeneity (Q) test was performed to determine whether the studies can reasonably be described as sharing a common effect size (Hedges and Olkin, 1985). Publication bias was assessed using both Egger's regression (Egger et al., 1997) and Orwin's fail-safe N (Orwin, 1983) to evaluate whether the available literature was biased toward excluding non-significant studies. Egger's method regresses the effect size against the precision of the d, and bias is likely when the P value is significant (less than 0.05). Orwin's fail-safe N addresses the “file drawer problem” (Rosenthal, 1979, 1991) by computing the number of studies (with an effect size of 0) required to reduce the mean effect size to non-significance (P > 0.05).
The meta-analyses were based on the more conservative random effects model (Hedges and Olkin, 1985). Under this model, both the within-study variances (e.g. sample size of each group) and the between-study variances (e.g. the number of studies, the Q tests and the weight for each study) are considered. Studies were weighted by the precision of their d estimate, which is proportional to the study sample size. For the overall effect size of the prefrontal impairment, a meta-analysis was performed combining all sub-regions in all of the studies. In addition, for each of the sub-regions, a meta-analysis was conducted for all studies combined and also for each hemisphere separately.
2.3. Potential Moderators
Coding of antisocial sample moderators
Several potential moderators were coded in order to address the issue of heterogeneity among antisocial populations. Studies were coded for each of the five moderators: violent vs. non-violent, institutional-based vs. community-based, with comorbidity vs. without comorbidity, psychiatric control vs. healthy control, and psychopathy vs. non-psychopathy. The violent code was assigned to studies which the majority of the antisocial individuals (i.e. more than half) have a history of aggressive behavior, have displayed clinically significant aggressive behavior, have been convicted or charged with violent crimes, or have displayed physical aggression toward family members (e.g. spouse abuse). Studies that did not specify their antisocial samples as violent were coded as non-violent. Studies were coded as institutional-based if their antisocial individuals were recruited from controlled environments such as hospitals and prisons. Studies that recruited antisocial samples from non-confined environmental settings such as outpatient clinics and temporary employment agencies were coded as community-based. Studies that had participants from both sources were excluded for the analysis. The comorbidity code was assigned to studies reporting that antisocial patients had comorbid psychiatric disorders (e.g. alcohol/substance abuse), while the others were coded as without comorbidity. The code for psychiatric control was assigned to studies that either a psychiatric comparison group was used to match any comorbid psychiatric disorder in the antisocial group (e.g. alcoholics with APD compared with alcoholics without APD) or a correlational analysis was used (e.g. correlation between psychopathy score and aggression). The code for healthy control was assigned to studies that used a healthy comparison group that was clear of any neurological and psychological illness. The studies were coded as psychopathy if their antisocial samples also fulfilled criteria for psychopathy. The mean age (or median age if mean age was not available), the male proportion, and the total PCL-R score of the antisocial sample were also recorded as potential moderators.
Coding of imaging methodology moderators
First, aMRI and DTI studies were coded as structural while fMRI, PET, SPECT, and MRS studies were coded as functional. For MRI studies (aMRI/DTI/MRS/fMRI), four imaging methodology moderators were coded: magnet strength (Tesla), slice thickness (mm), TR (ms), and field-of-view (FOV; cm2). In addition, task type (i.e. emotional, cognitive) was also coded for fMRI studies. As for PET and SPECT studies, two imaging methodology moderators were coded including FWHM (mm), and uptake period (min). In addition, PET studies were also coded on whether the subject was cognitively engaged in a task versus resting.
Statistical analyses for moderators
Moderators reported in each study are listed in Table 1. The influence of each moderator effect was individually tested using analysis of variance for categorical moderators and fixed effect regression for continuous moderators. For analysis of the moderator effect significance, the minimum level of significance was set at p < 0.05.
3. Results
3.1. Meta-Analyses
Results of the meta-analyses across all 43 structural and functional studies are detailed in Table 2. A meta-analysis including all prefrontal and prefrontal sub-regional findings indicated antisocial individuals showed reduced structure / function in the prefrontal cortex, Cohen's d = - 0.60, P < 0.001. The association between antisocial behavior and prefrontal reduction was somewhat stronger in the 31 functional imaging studies (d = - 0.72, P < 0.001) than the 12 structural imaging studies (d = - 0.37, P = 0.038), however the difference was non-significant (P = 0.15). Analyses on the region of interests showed the prefrontal abnormality to be localized in the right OFC (d = - 0.48, P < 0.001), left DLPFC (d = - 0.83, P = 0.009), and right ACC (d = - 1.12, P = 0.006). The assessments of publication bias confirmed that there was no publication bias for the right OFC (Egger's t = 0.98, P = 0.36; Fail-safe N = 25), left DLPFC (Egger's t = 2.36, P = 0.05; Fail-safe N = 63), and right ACC (Egger's t = .72, P = 0.51; Fail-safe N = 35) (see Table 2). In contrast, no significant abnormality was found in the left OFC, right DLPFC, left ACC, VLPFC, or MPFC.
Table 2.
Mean effect sizes for the 5 regions of interest across all 43 structural and functional imaging studies.
| Region of Interest | No. of studies | Random Effect Model | Heterogeneity | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cohen's d | 95% Confidence Interval | P | Q | P | Egger's Regression | Fail-safe | |||
| t | P | N | |||||||
| OFC (combined) | 16 | -0.43 | [-0.79, -0.07] | 0.019 | 40.3 | < 0.001 | .94 | .36 | 44 |
| Left | 8 | -0.20 | [-0.66, 0.26] | 0.38 | 15.8 | 0.027 | |||
| Right | 9 | -0.48 | [-0.74, -0.22] | < 0.001 | 8.1 | 0.42 | .98 | .36 | 25 |
| DLPFC (combined) | 15 | -0.29 | [-0.78, 0.20] | 0.24 | 96.5 | < 0.001 | |||
| Left | 9 | -0.83 | [-1.46, -0.21] | 0.009 | 53.4 | < 0.001 | 2.36 | 0.05 | 63 |
| Right | 8 | -0.49 | [-1.17, 0.19] | 0.16 | 40.2 | < 0.001 | |||
| VLPFC (combined) | 8 | -0.30 | [-1.09, 0.49] | 0.46 | 33.2 | < 0.001 | |||
| Left | 5 | -0.25 | [-1.11, 0.62] | 0.58 | 37.6 | < 0.001 | |||
| Right | 6 | 0.31 | [-0.70, 1.32] | 0.55 | 24.3 | < 0.001 | |||
| MPFC (combined) | 13 | -0.24 | [-0.93, 0.46] | 0.51 | 81.3 | < 0.001 | |||
| Left | 4 | -1.0 | [-2.11, 0.05] | 0.061 | 14.6 | 0.002 | |||
| Right | 7 | -0.28 | [-1.30, 0.74] | 0.59 | 40.4 | < 0.001 | |||
| ACC (combined) | 17 | -0.82 | [-1.28, -0.35] | 0.001 | 61.8 | < 0.001 | 1.0 | 0.33 | 170 |
| Left | 6 | -0.60 | [-1.80, 0.61] | 0.34 | 41.23 | < 0.001 | |||
| Right | 6 | -1.12 | [-1.93, -0.32] | 0.006 | 17.5 | 0.004 | 0.72 | 0.51 | 35 |
Across the 31 functional imaging studies, antisocial individuals showed a significant decrease in prefrontal functioning, again in the right OFC (d = - 0.57, P < 0.001), left DLPFC (d = - 0.89, P = 0.031), and right ACC (d = -1.35, P = 0.002) (see Table 3). The assessments of publication bias again confirmed that there was no publication bias for the right OFC (Egger's t = 1.51, P = 0.19; Fail-safe N = 26), left DLPFC (Egger's t = 2.28, P = 0.07; Fail-safe N = 33), and right ACC (Egger's t = 0.31, P = 0.78; Fail-safe N = 34) (see Table 3). However, the number of structural imaging studies (12 in total) was insufficient to conduct meaningful region of interest analyses.
Table 3.
Mean effect sizes for the 5 regions of interest across the 31 functional imaging studies.
| Region of Interest | No. of studies | Random Effect Model | Heterogeneity | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cohen's d | 95% Confidence Interval | P | Q | P | Egger's Regression | Fail-safe N | |||
| t | P | ||||||||
| OFC (combined) | 12 | -0.54 | [-0.90, -0.17] | 0.004 | 21.7 | 0.03 | 0.58 | 0.58 | 41 |
| Left | 5 | -0.37 | [-0.76, 0.02] | 0.06 | 4.3 | 0.37 | |||
| Right | 7 | -0.57 | [-0.84, -0.29] | <0.001 | 5.4 | 0.50 | 1.51 | 0.19 | 26 |
| DLPFC (combined) | 12 | -0.36 | [-0.91, 0.20] | 0.21 | 72.5 | < 0.001 | |||
| Left | 7 | -0.89 | [-1.69, -0.08] | 0.031 | 48.1 | < 0.001 | 2.28 | 0.07 | 33 |
| Right | 7 | -0.56 | [-1.35, 0.23] | 0.17 | 39.6 | < 0.001 | |||
| VLPFC (combined) | 7 | -0.37 | [-1.25, 0.51] | 0.41 | 32.0 | < 0.001 | |||
| Left | 4 | -0.28 | [-1.25, 0.70] | 0.58 | 37.1 | < 0.001 | |||
| Right | 5 | -0.28 | [-0.90, 1.46] | 0.64 | 23.7 | < 0.001 | |||
| MPFC (combined) | 11 | -0.17 | [-1.03, 0.68] | 0.69 | 73.2 | < 0.001 | |||
| Left | 3 | -1.45 | [-3.11, 0.21] | 0.087 | 12.6 | 0.002 | |||
| Right | 6 | -0.35 | [-1.64, 0.95] | 0.60 | 40.3 | < 0.001 | |||
| ACC (combined) | 16 | -0.86 | [-1.35, -0.36] | 0.001 | 60.5 | < 0.001 | 0.94 | 0.36 | 163 |
| Left | 5 | -0.65 | [-2.18, 0.89] | 0.41 | 41.2 | < 0.001 | |||
| Right | 5 | -1.35 | [-2.20, -0.51] | 0.002 | 11.8 | 0.019 | 0.31 | 0.78 | 34 |
3.2. Moderator Analysis
Results of the meta-analyses on the moderators are detailed in Table 4. For the antisocial sample characteristic moderators, the ANOVAs showed that effect sizes did not differ significantly between studies using samples that were violent or non-violent (d = - 0.62, - 0.57, respectively; P = 0.87), institutional-based or community-based (d = - 0.47, - 0.82, respectively; P = 0.12), compared to healthy or psychiatric controls (d = - 0.76, - 0.42, respectively; P = 0.14), with or without comorbidity (d = - 0.49, - 0.77, respectively; P = 0.24), and psychopathic or non-psychopathic (d = - 0.56, - 0.62, respectively; P = 0.87). The analyses of fixed-effect regression also showed that the effect size was not moderated by male proportion (b = - 0.1, P = 0.79), mean age (b = 0.01, P = 0.08) or the mean PCL-R score (b = - 0.03, P = 0.21) of the antisocial samples.
Table 4.
Moderator analyses.
| No. of studies | Random Effect Model | Heterogeneity | Moderator Analysis | ||||
|---|---|---|---|---|---|---|---|
| Cohen's d | 95% C.I. | p | Q | p | p | ||
| Antisocial Sample Moderators | |||||||
| Violent | 29 | -0.62 | [-.88, -.35] | < 0.001 | 87.1 | < 0.001 | |
| Non-violent | 14 | -0.57 | [-1.06, -.08] | 0.023 | 44.9 | < 0.001 | 0.87 |
| Institutional-based | 22* | -0.47 | [-.80, -.14] | 0.005 | 68.7 | < 0.001 | |
| Community-based | 19* | -0.82 | [-1.12, -0.53] | < 0.001 | 37.8 | 0.004 | 0.12 |
| Psychiatric control | 21 | -0.42 | [-0.76, -0.08] | 0.015 | 63.5 | < 0.001 | |
| Healthy control | 22 | -0.76 | [-1.07, -0.46] | < 0.001 | 61.1 | < 0.001 | 0.14 |
| With comorbidity | 26 | -0.49 | [-0.79, -0.20] | 0.001 | 79.8 | < 0.001 | |
| Without comorbidity | 17 | -0.77 | [-1.13, -0.41] | < 0.001 | 44.9 | < 0.001 | 0.24 |
| Psychopathy | 9 | -0.56 | [-1.32, 0.21] | 0.16 | 35.2 | < 0.001 | |
| Non-psychopathy | 34 | -0.62 | [-0.86, -0.38] | < 0.001 | 95.4 | < 0.001 | 0.87 |
| Methodology Moderators | |||||||
| Functional | 31 | -0.72 | [-1.02, -0.42] | < 0.001 | 102.3 | < 0.001 | |
| Structural | 12 | -0.37 | [-0.73, -0.02] | 0.038 | 29.0 | 0.002 | 0.15 |
| aMRI | 10 | -0.36 | [-0.76, 0.05] | 0.085 | 25.1 | 0.003 | |
| fMRI | 15 | -0.89 | [-1.39, -0.38] | 0.001 | 53.0 | < 0.001 | |
| PET | 7 | -0.76 | [-1.08, -0.44] | < 0.001 | 4.7 | 0.58 | 0.13 |
| SPECT | 8 | -0.23 | [-0.73, 0.26] | 0.36 | 26.0 | 0.001 | |
| Emotional task (fMRI) | 9 | -0.87 | [-1.63, -0.10] | 0.026 | 37.3 | < 0.001 | |
| Cognitive task (fMRI) | 6 | -0.90 | [-1.58, -0.21] | 0.01 | 15.6 | 0.008 | 0.95 |
| Task (PET) | 3 | -0.59 | [-0.97, -0.21] | 0.002 | 1.2 | 0.56 | |
| Resting (PET) | 4 | -1.17 | [-1.76, -0.59] | < 0.001 | 0.88 | 0.83 | 0.10 |
For the imaging methodology moderators, the effect size was strongest for fMRI studies (d = - 0.89, P = 0.001), followed by PET studies (d = - 0.76, P < 0.001), aMRI studies (d = - 0.36, P = 0.085), and SPECT studies (d = - 0.23, P = 0.36). However, group comparison was non-significant (P = 0.13). Studies using DTI (2 studies) and MRS (1 study) were excluded due to insufficient numbers of studies for conducting meaningful comparisons. Moderator analyses were also conducted separately for each of the four imaging methods. For fMRI studies, larger effect sizes were associated with increased TR (b = 0.0003, P < 0.001), decreased slice thickness (b = - 0.28, P = 0.01), and decreased FOV (b = - 0.008, P < .001). However, no significant association was found for scanner strength (b = - 0.27, P = 0.35). Comparable effect sizes were obtained for emotional tasks (d = - 0.87, P = 0.026) and cognitive tasks (d = - 0.90, P = 0.01) used in fMRI studies. For PET studies, no moderator effect was found for FHWM (b = - 0.022, P = 0.79), uptake time (b = - 0.059, P = 0.14), or the use of a challenge task (P = 0.10). For SPECT studies, a significant positive correlation was found between smaller FHWM and larger effect size (b = 0.34, P < 0.001). However, no moderator effect was found for the uptake time across SPECT studies (b = 0.003, P = 0.53). For aMRI studies, a significant positive correlation was found between FOV and the effect size (b = 0.003, P = 0.048). However, no such moderator effect was found for the scanner strength (b = - 0.305, P = 0.38), TR (b = 0.00003, P = 0.76), or slice thickness (b = 0.014, P = 0.87) across the aMRI studies.
4. Discussion
This is the first brain imaging meta-analysis of antisocial behavior, evaluating the relationship between prefrontal impairment and antisocial / violent / psychopathic behavior across 43 independent studies. Results demonstrated that antisocial behavior was significantly associated with reduced prefrontal structure and function. Specifically, increased antisocial behavior was particularly associated with structural and functional reductions in the right OFC, left DLPFC, and right ACC. Results were not moderated by the antisocial characteristics such as age, gender, psychiatric control, comorbid psychiatric disorder, or psychopathy. Imaging methodology moderated results, depending upon the type of imaging methods. Overall, findings establish fairly robust and significant prefrontal structural and functional impairments in antisocial populations as assessed by brain imaging.
4.1. Localization and Lateralization of the Prefrontal Reductions
The findings of this meta-analysis review are consistent with the prefrontal sub-regions hypothesized to be impaired in antisocial individuals in several previous reviews, which include the OFC, DLPFC and ACC (Blair, 2001; Kiehl, 2006; Raine and Yang, 2006; Yang, Glenn, and Raine, 2008). When study findings were analyzed separately for each hemisphere, the association between DLPFC reduction and antisocial behavior was found to be limited to the left hemisphere, while reductions in the ACC and OFC was more prominent in the right hemisphere. These findings echo evidence that antisocial behavior is more associated with right-sided prefrontal pathology, particularly in the OFC and ACC. For example, Tranel, Bechara and Denburg (2002) showed patients with unilateral lesion to the right OFC to be impaired in social conduct, decision-making, emotional processing, and personality, whereas the left OFC patients had normal social and interpersonal behavior. This notion is supported by several other studies on patients with antisocial / psychopathic features showing damage predominantly limited to their right OFC (e.g. Angrilli et al., 1999; Erlinger and Damasio, 1985).
Similarly, unilateral lesions to the right ACC, but not the left ACC, were found to cause impairments in inhibitory control as well as emotional processing (e.g. Danckert et al., 2000; Hornak et al., 2003). On the other hand, damage to the DLPFC, particularly the left DLPFC, has been associated with impairments in higher cognitive and self-regulatory processes such as attention, cognitive flexibility, and impulse control as revealed by the Stroop task and Iowa Gambling task (e.g. Grattan and Eslinger, 1992; Hornak et al., 2004; Stuss et al., 2001). The failure in patients with left DLPFC deficits in performing these tasks has been attributed to attention deficits and poor goal-directed behavior (e.g. Colvin, Dunbar and Grafman, 2001; Hornak et al., 2004; Stuss et al., 2001). Overall, as suggested by the lesion studies, it may be hypothesized that the reduction in right prefrontal cortex, including the OFC and ACC, is associated with emotional deficits and poor decision-making in antisocial individuals, while reduction in the left DLPFC is more linked to antisocial features of impulsivity and poor behavioral control.
Findings of this meta-analysis review are in line with several biological theories on antisocial behavior and psychopathy. For example, the results support the Frontal Lobe Dysfunction Theory (Gorenstein & Newman, 1980) and Somatic Marker Hypothesis (Damasio, 1994) in suggesting that antisocial behavior in humans might be a consequence of inherited or acquired deficits in the frontal brain areas, especially the OFC. However, the implication of the findings may be less direct for theories such as the Left Hemisphere Activation Hypothesis of psychopathy (Kosson, 1998). Based on the findings that psychopaths made more errors following cues presented in the right visual field (processed initially by left hemisphere), Kosson (1998) proposed that difficulty in processing information in the left hemisphere and shifting attention from left to right hemisphere may contribute to attentional abnormalities observed in psychopathic individuals (Kosson, 1998). Findings in this meta-analysis support the hypothesis and suggest that structural and functional deficits in the left DLPFC impair the allocation and sustaining of attention in antisocial, psychopathic individuals. The additional deficits in the right OFC and ACC may also indirectly support the hypothesis because these regions are key in processing secondary cues such as emotional contents, thus if damaged may fail to effectively direct attention to important information in the right hemisphere when needed. Nevertheless, future development of neurobiological theory on antisocial behavior incorporating neuroimaging, neuropsychological and behavioral data is needed to understand the complex mechanism underlying antisocial personality disorder and psychopathy.
Although the VLPFC and MPFC have generated a great deal of interest in antisocial research, non-significant results were found for both regions in this meta-analysis. However, there were trend associations between antisocial behavior and prefrontal reduction in the left MPFC (p = 0.061). It is notable that, for both regions, some studies included in the meta-analysis demonstrate effects in opposing directions. We caution however against firm conclusions on null results because effects sizes were quite substantial for some subregions, and small sample sizes reduce statistical power. For example a d of - 1.0 was obtained from the four studies assessing left MPFC, an effect which may be significant with more studies. Similarly, the overall non-significant effect size for right DLPFC from 8 studies was non-trivial (- 0.49). Confirmation or refutation of these null results and the possible lateralization and localization of the prefrontal deficits in antisocial individuals constitutes important issues for future studies.
Although no significant moderator effect for sample characteristics was found, the null findings may be contributed in part by the method of study classification for moderator analyses, specifically for the violent and comorbidity nature of the samples, which depends solely on information reported by the investigators. This approach did not allow us to draw conclusions with full confidence that the results are truly reflective of the confounding effect that violent behavior and comorbid psychiatric disorders has on the frontal structure and function. Another limitation of this meta-analysis is that, although we were able to assess the frontal structural and functional correlates of global psychopathy scores, the small number of studies providing separate results for sub-factors of psychopathy (one sMRI and one fMRI) prevents us from conducting meaningful subsidiary analyses to further assess the effect of sub-features of psychopathy. Therefore, despite that the mean PCL-R score was found to show no moderator effect on the results, it remains a possibility that the prefrontal findings may be moderated by sub-factors of PCL-R, particularly the antisocial-lifestyle sub-factor, which is associated more closely with frontal deficits such as impulsivity and poor behavioral control.
Effect of Imaging Methodology
Several imaging methodology variables were found to moderate the association between antisocial behavior and the prefrontal cortex. For example, larger effect sizes were associated with an increase in TR, but a decrease in both FOV and slice thickness in fMRI studies. These findings are somewhat surprising due to the fact that studies have found shorter TR to be associated with better BOLD contrast sensitivity (e.g. Menon, Thomas, Gati, 1997). However, the higher signal-to-noise ratio permitted by the use of longer TR improves the quality of the fMRI scans which are known to be sensitive to motion and image-to-image fluctuation. On the other hand, smaller FOV and thinner slice improve the spatial resolution of the images, thus increased the chance of localizing activation differences between groups (Creasy, Partain, and Price, 1995). However, when the matrix size is fixed, a decrease in FOV results in a drop in the signal-to-noise ratio. These factors may contribute to the ability of an fMRI study to better detect brain activity changes associated with antisocial behavior.
Conclusions
This meta-analytic review highlights the significance of prefrontal structural and functional impairments in antisocial individuals. More specifically, reductions in the prefrontal cortex were particularly marked in the right OFC, right ACC, and left DLPFC. This meta-analysis underscores the critical need for longitudinal imaging studies as well as studies that include female antisocial individuals and which assess potential mediating variables (e.g.. impulsivity, emotional regulation). We emphasize that multiple regions other than the prefrontal cortex are likely to be significantly implicated in antisocial and violent behavior (Raine and Yang, 2006). Consequently, although additional research on the prefrontal cortex is warranted, future brain imaging research on antisocial populations could usefully focus on other regions of interest (amygdala, hippocampus, insula, angular gyrus) which have been much less studied to date.
Acknowledgments
This study was supported by a grant to the first author from the National Institute of Mental Health (National Research Service Award 1F31MH079592) and a grant to the second author from the National Institute of Child Health and Development (I RO1 HD42259). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amen DG, Stubblefield M, Carmicheal B, Thisted R. Brain SPECT findings and aggressiveness. Annals of Clinical Psychiatry. 1996;8(3):129–137. doi: 10.3109/10401239609147750. [DOI] [PubMed] [Google Scholar]
- Anckarsater H. Central nervous changes in social dysfunction: autism, aggression, and psychopathy. Brain Research Bulletin. 2006;69(3):259–265. doi: 10.1016/j.brainresbull.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Angrilli A, Palomba D, Cantagallo A, Maietti A, Stegagno L. Emotional impairment after right orbitofrontal lesion in a patient without cognitive deficits. Neuroreport. 1999;10(8):1741–1746. doi: 10.1097/00001756-199906030-00021. [DOI] [PubMed] [Google Scholar]
- Antonucci AS, Gansler DA, Tan S, Bhadelia R, Patz S, Fulwiler C. Orbitofrontal correlates of aggression and impulsivity in psychiatric patients. Psychiatry Research: Neuroimaging. 2006;147(23):213–220. doi: 10.1016/j.pscychresns.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Barkataki I, Kumari V, Das M, Taylor P, Sharma T. Volumetric structural brain abnormalities in men with schizophrenia or antisocial personality disorder. Behavioural Brain Research. 2006;169(2):239–47. doi: 10.1016/j.bbr.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision making: evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery, & Psychiatry. 2001;71(6):727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archive General Psychiatry. 2005;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, McCurry S, Larson EB. Developmental and vascular risk factors for Alzheimer's disease. Neurobiology of Aging. 2005;26(3):325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Brower MC, Price BH. Neuropsychiatry of frontal lobe dysfunction in violent and criminal behavior: A critical review. Journal of Neurology, Neurosurgery, & Psychiatry. 2001;71(6):720–726. doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TG. The best of a bas bunch: the ventromedial prefrontal cortex and dorsal anterior cingulate cortex in decision-making. Journal of Neuroscience. 2007;27(3):447–448. doi: 10.1523/JNEUROSCI.4967-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62(2):168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. Academic Press; New York: 1988. [Google Scholar]
- Colvin MK, Dunbar K, Grafman J. The effects of frontal lobe lesions on goal achievement in the water jug task. Journal of Cognitive Neuroscience. 2001;13(8):1129–47. doi: 10.1162/089892901753294419. [DOI] [PubMed] [Google Scholar]
- Creasy JL, Partain CL, Price RP. Quality of clinical MR images and the use of contrast agents. Radiographics. 1995;15(3):683–696. doi: 10.1148/radiographics.15.3.7624572. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Simmons A, Daly EM, Russell A, van Amelsvoort T, Robertson DM, Glover A, Murphy DG. Prefrontal and medial temporal correlates of repetitive violence to self and others. Biological Psychiatry. 2000;47(10):928–34. doi: 10.1016/s0006-3223(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: emotion, reason, and the human brain. Grosset/Putnam; New York: 1994. [Google Scholar]
- Danckert J, Maruff P, Ymer C, Kinsella G, Yucel M, de Graaff S, Currie J. Goal-directed selective attention and response competition monitoring: evidence from unilateral parietal and anterior cingulated lesions. Neuropsychology. 2000;14(1):16–28. doi: 10.1037//0894-4105.14.1.16. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation - a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Deakin JF, Roberts N, Anderson IM. Quantitative frontal and temporal structural MRI studies in personality-disordered offenders and control subjects. Psychiatry Research: NeuroImaging. 2002;116(3):133–149. doi: 10.1016/s0925-4927(02)00085-9. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. Journal of Neuroscience. 1991;11(6):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, Hwang D, Slifstein M, Curry S, Abi-Dargham A, Laruelle M, Siever LJ. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. American Journal of Psychiatry. 162(5):915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- George DT, Rawlings RR, Williams WA, Phillips MJ, Fong G, Kerich M, Momenan R, Umhau JC, Hommer D. A select group of perpetrators of domestic violence: evidence of decreased metabolism in the right hypothalamus and reduced relationships between cortical/subcortical brain structures in position emission tomography. Psychiatry Research: NeuroImaging. 2004;130(1):11–25. doi: 10.1016/S0925-4927(03)00105-7. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56(7):516–21. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, Clayton AH. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10(1):21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Grattan LM, Eslinger PJ. Long-term psychological consequences of childhood frontal lobe lesion in patient DT. Brain and Cognition. 1992;20(1):185–195. doi: 10.1016/0278-2626(92)90068-w. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychological Review. 1980;87(2):301–315. [PubMed] [Google Scholar]
- Hare RD. Manual for the Revised Psychopathy Checklist. 2nd ed. Multi-Health Systems; Toronto, ON, Canada: 2003. [Google Scholar]
- Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Academic Press; San Diego: 1985. [Google Scholar]
- Henry B, Moffitt TE. Handbook of Antisocial Behavior. 1997. Neuropsychological and neuroimaging studies of juvenile delinquency and adult criminal behavior; pp. 280–288. [Google Scholar]
- Hirono N, Mega MS, Dinov ID, Mishkin F, Cummings JL. Left frontaltemporal hypoperfusion is associate with aggression in patients with dementia. Archives of Neurology. 2000;57(6):861–6. doi: 10.1001/archneur.57.6.861. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Slaughter JR, Kashani J. Risk factors in childhood that lead to the development of conduct disorder and antisocial personality disorder. Child Psychiatry and Human Development. 2001;31(3):183–193. doi: 10.1023/a:1026425304480. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Weiss EM, Czobor P, Szeszko PR, Gerig G, Chakos M, Blocher J, Citrome LL, Lindenmayer J, Sheitman B, Lieberman JA, Bilder RM. Quantitative MRI measures of orbitofrontal cortex in patients with chronic schizophrenia or schizoaffective disorder. Psychiatry Research: NeuroImaging. 140(2):133–145. doi: 10.1016/j.pscychresns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biological Psychiatry. 2002;52(1):9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulated cortices. Brain. 2003;126(7):1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Intrator J, Hare R, Stritzke P, Brichtswein K, Dorfman D, Harpur T, Bernstein D, Handelsman L, Schaefer C, Keilp J, Rosen J, Machac J. A brain imaging (Single Photon Emission Computerized Tomography) study of semantic and affective processing in psychopaths. Biological Psychiatry. 1997;42(2):96–103. doi: 10.1016/S0006-3223(96)00290-9. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Cioffi GA, Liemann JR, Sample PA, Zangwill LM, Weinreb RN. The relationship between structural and functional alternations in glaucoma: a review. Seminars in Ophthalmology. 2000;15(4):221–233. doi: 10.3109/08820530009037873. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Putkonen A, Mancini-Marie A, Hodgins S, Kononen M, Boulay L, Pihlajamaki M, Soininen H, Stip E, Tiihonen J, Aronen HJ. Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophrenia Research. 2007;91(13):97–102. doi: 10.1016/j.schres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Behen ME, Muzik O, Chugani DC, Chugani HT. Bilateral medial prefrontal and temporal neocortical hypometabolism in children with epilepsy and aggression. Epilepsia. 2001;42(8):991–1001. doi: 10.1046/j.1528-1157.2001.042008991.x. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142(23):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50(9):677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Mendrek A, Forster BB, Hare RD, Liddle PF. Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Research. 2004;130(3):27–42. doi: 10.1016/S0925-4927(03)00106-9. [DOI] [PubMed] [Google Scholar]
- Kosson DS. Psychopathy and dual-task performance under focusing conditions. Journal of Abnormal Psychology. 1998;105(3):391–400. doi: 10.1037//0021-843x.105.3.391. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research. 2004;132(1):1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kuruoglu AC, Arikan Z, Vural G, Karatas M, Arac M, Isik E. Single photon emission computerized tomography in chronic alcoholism: Antisocial personality disorder may be associated with decreased frontal perfusion. British Journal of Psychiatry. 1996;169(3):348–354. doi: 10.1192/bjp.169.3.348. [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Hodgins S, Zachariah E, Barkataki I, Howlett M, Sharma T. Association between violent behavior and impaired prepulse inhibition of the startle response in antisocial personality disorder and schizophrenia. Behavioural Brain Research. 2005;158(1):159–66. doi: 10.1016/j.bbr.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volume in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Research. 2002;114(2):95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Levin CS, Hoffman EJ. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Physics in Medicine and Biology. 1999;44(3):781–799. doi: 10.1088/0031-9155/44/3/019. [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Antisocial personality and stress-induced brain activation in cocaine-dependent patients. NeuroReport. 2006;17(3):243–247. doi: 10.1097/01.wnr.0000199471.06487.a2. [DOI] [PubMed] [Google Scholar]
- Li TQ, Mathews VP, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Annals of the New York Academy of Sciences. 2005;1064:184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Developmental and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66(3):488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Mathews VP, Kronenberger WG, Wang Y, Lurito JT, Lowe MJ, Dunn DW. Media violence exposure and frontal lobe activation measured by functional magnetic resonance imaging in aggressive and nonaggressive adolescents. Journal of Computer Assisted Tomography. 2005;29(3):287–292. doi: 10.1097/01.rct.0000162822.46958.33. [DOI] [PubMed] [Google Scholar]
- Mena JC, Cuellar H, Vargas D, Riascos R. PET and SPECT in drug and substance abuse. Topics in Magnetic Resonance Imaging. 2005;16(3):253–256. doi: 10.1097/01.rmr.0000192177.01789.c0. [DOI] [PubMed] [Google Scholar]
- Menon RS, Thomas CG, Gati JS. Investigation of BOLD contrast in fMRI using multi-shot EPI. NMR in Biomedicine. 1997;10(45):179–182. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<179::aid-nbm463>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biological Psychiatry. 1999;45(9):1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, Schuierer G, Klein HE, Hajak G. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: Evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54(2):152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Nakano S, Asada T, Yamashita F, Kitamura N, Matsuda H, Hirai S, Yamada T. Relationship between antisocial behavior and regional cerebral blood flow in frontotemporal dementia. Neuroimage. 2006;32(1):301–306. doi: 10.1016/j.neuroimage.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Oder W, Goldenberg G, Spatt J, Podreka I, Binder H, Deecke L. Behavioural and psychosocial and regional cerebral blood flow: A SPECT study. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(6):475–80. doi: 10.1136/jnnp.55.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Orwin RG. A fail safe N for effect size in meta-analysis. Journal of Educational Statistics. 1983;8(2):157–159. [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100625. Brain Research. 2002;954(2):173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience. 2001;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30(5):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A. The psychopathology of crime: Criminal behavior as a clinical disorder. Academic Press; San Diego: 1993. [Google Scholar]
- Raine A, Buchsbaum MS. Aggression and violence: Genetic, neurobiological, and biosocial perspectives. 1996. Violence, brain imaging, and neuropsychology; pp. 195–217. [Google Scholar]
- Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biological Psychiatry. 1997;42(6):495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc Cognitive and Affective Neuroscience. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Yang Y. The neuroanatomical bases of psychopathy: A review of brain imaging findings. In: Patrick CJ, editor. Handbook of Psychopathy. Guilford; New York: 2004. [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61(11):1260–1270. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Combining results of independent studies. Psychological Bulletin. 1978;85(1):185–193. [Google Scholar]
- Rosenthal R. The “file drawer problem” and tolerance for null results. Psychological Bulletin. 1979;86(3):638–641. [Google Scholar]
- Rosenthal R. Meta-analysis: A review. Psychosomatic Medicine. 1991;53(3):247–271. doi: 10.1097/00006842-199105000-00001. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Posse S, Grodd W, Muller-Gartner H. Functional imaging of conditioned aversive emotional responses in antisocial personality disorder. Neuropsychobiology. 2000;42(4):192–201. doi: 10.1159/000026693. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Hultin L, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced frontotemporal perfusion in psychopathic personality. Psychiatry Research. 2002;114(2):81–94. doi: 10.1016/s0925-4927(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced regional cerebral blood flow in non-psychotic violent offenders. Psychiatry Research. 2000;98(1):29–41. doi: 10.1016/s0925-4927(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Spampinato MV, Castillo M, Rojas R, Palacios E, Frascheri L, Descartes F. Magnetic resonance imaging findings in substance abuse: alcohol and alcoholism and syndromes associated with alcohol abuse. Topics in Magnetic Resonance Imaging. 2005;16(3):223–230. doi: 10.1097/01.rmr.0000192175.26243.a7. [DOI] [PubMed] [Google Scholar]
- Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: association with temperament traits. Journal of Psychiatric Research. 2007;41(5):410–417. doi: 10.1016/j.jpsychires.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57(1):7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39(8):771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Thomsen T, Specht K, Rimol LM, Hammer A, Nyttingnes J, Ersland L, Hugdahl K. Brain localization of attentional control in different age groups by combining functional and structural MRI. Neuroimage. 2004;22(2):912–919. doi: 10.1016/j.neuroimage.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38(4):589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;238:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Vermeiren R, Deboutte D, Ruchkin V, Schwab-Stone M. Antisocial behaviour and mental health. Findings from three communities. European Child & Adolescent Psychiatry. 2002;11(4):168–175. doi: 10.1007/s00787-002-0275-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tancredi LR, Grant C, Gillespie H, Valentine A, Mullani N, Wang GJ, Hollister L. Brain glucose metabolism in violent psychiatric patients: A preliminary study. Psychiatry Research. 1995;61(4):243–253. doi: 10.1016/0925-4927(95)02671-j. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Decision-making and the frontal lobes. Current Opinion in Neurology. 2006;19(4):401–406. doi: 10.1097/01.wco.0000236621.83872.71. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Van Elst LT, Koepp MJ, Free SL, Thompson PJ, Trimble MR, Duncan JS. Reduction of frontal neocortical grey matter associated with affective aggression in patients with temporal lobe epilepsy: an objective voxel by voxel analysis of automatically segmented MRI. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;68(2):162–169. doi: 10.1136/jnnp.68.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Functional and structural brain imaging research on psychopathy. In: Felthous AH, Sass H, editors. International Handbook on Psychopathic Disorders and the Law. Wiley; New Jersey: 2006. [Google Scholar]
- Yang Y, Glenn A, Raine A. Brain abnormalities in antisocial individuals: Implications for the law. Behavioral Science and the Law. 2008;26(1):65–83. doi: 10.1002/bsl.788. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A. Brain abnormalities in antisocial, psychopathic Individuals. Netherlands's J Psychiatry. In press. [Google Scholar]
- Yang Y, Raine A. Functional neuroanatomy of psychopathy. Psychiatry. 2008;7(3):133–136. [Google Scholar]