Cardiorenal syndrome in heart failure: A cardiologist’s perspective (original) (raw)
Show available content in
Abstract
One of the most important comorbidities in heart failure is renal dysfunction. Diminished estimated glomerular filtration rate is a potent predictor of cardiovascular mortality and complications. On the other hand, worsening heart failure or acute decompensated heart failure can accelerate worsening of renal function – the so-called cardiorenal syndrome. Risk factors include hypertension, diabetes, elderly age, and prior history of heart or renal failure. The pathophysiology of the cardiorenal syndrome involves intrarenal hemodynamics, transrenal perfusion pressure and systemic neurohormonal factors. Clinical management of the patient with cardiorenal syndrome includes the challenge of diuretic resistance, which may involve correcting the underlying cause, combination diuretics or diuretic infusions. The key to improved outcome is the optimization of proven heart failure therapies. The use of vasodilator therapy is the current mainstay of treatment. Nesiritide, or recombinant B-type natriuretic peptide, has courted controversy regarding its role in cardiorenal syndrome. However, data are emerging that low doses appear to be renal-protective. Other more recent strategies include ultrafiltration, vasopressin antagonists and adenosine antagonists. All of these newer modalities promise more rapid volume removal, but their ultimate impact on survival or preservation of renal function is unknown at the present time.
Because of the complex nature of these patients, and the compromised outcome, it is important that cardiologists, nephrologists and internists all work together toward the common goal of protecting the patient with cardiorenal syndrome, and use the best available evidence for management.
Keywords: Adenosine antagonist, B-type natriuretic peptide, Cardiorenal syndrome, Diuretics, Heart failure, Renal dysfunction
OVERVIEW OF CARDIORENAL SYNDROME
As cardiovascular disease epidemiology changes from that of acute presentation to chronic disease in the community, such as coronary atherosclerosis or heart failure, the mortality and morbidity of cardiovascular disease will continue to escalate (1–3). However, heart failure as a chronic disease is accompanied by a number of major comorbidities that have a major impact on outcome (4,5). One of the most important comorbidities that impacts on outcome and clinical management is renal failure or renal insufficiency (6).
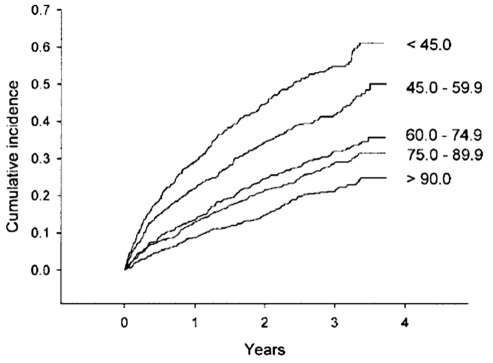
Heart performance and kidney function are closely interconnected physiologically and pathophysiologically, both in health and in disease. Renal function, as reflected by serum creatinine, or more precisely by estimated glomerular filtration rate (eGFR), is persistently the best predictor of cardiovascular outcomes in a broad spectrum of populations. This includes higher risk patients such as those postmyocardial infarction, and those with diabetes or congestive heart failure. In the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial (7), which included patients postmyocardial infarction receiving state-of-art therapy, those with eGFR below the median had 1.5 times the risk of major cardiovascular events such as death or myocardial infarction compared with those with eGFR above the median. A meta-analysis (8) of studies evaluating the relationship between renal dysfunction and heart failure revealed that 63% of patients had at least mild renal impairment, and 20% had moderate or severe renal dysfunction. There was a consistent risk relationship of a 7% increase in mortality for every 10 mL/min decrease in eGFR. This has further been confirmed in a broad spectrum of patients with heart failure (Figure 1) (9).
Figure 1).
Kaplan-Meier plot of cumulative incidence of cardiovascular death or unplanned admission to hospital for the management of worsening heart failure stratified by approximate quintiles of estimated glomerular filtration rate in mL/min/1.73 m2 (time in years). Reproduced with permission from reference 9
At least one in four patients hospitalized for acute decompensated heart failure has significant renal dysfunction (eGFR less than 60 mL/min/1.73 m2). Most patients admitted to hospital with heart failure will have some deterioration of renal function, and not surprisingly this generally occurs during the initial phase of hospitalization, temporally related to intense diuretic use. Not surprisingly, patients who develop worsening of renal function are associated with longer hospital stay, and an increase in both short- and long-term mortality. In the large-scale Acute Decompensated Heart Failure National Registry (ADHERE) consisting of 105,388 hospitalized visits of patients with acute decompensated heart failure in the United States, the best predictors of outcome included both serum blood-urea-nitrogen and creatinine (10), reflecting the powerful independent prerenal and renal components of the prognostic equation.
Data from an Ontario heart failure population derivation cohort of 2624 patients in the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study (2) identified that older age, low systolic blood pressure, high serum blood-urea-nitrogen and low serum sodium were independent predictors of outcome. By deriving a risk score based on the above factors, application in a validation cohort of 1407 patients showed that the area under the receiver operating characteristics curve was 0.80. The presence of renal dysfunction contributed at least 20% to the increase for heart failure mortality.
In a recent study of patients with diastolic heart failure (3), a syndrome of rapidly rising prevalence in the community, there was a similar risk relationship between the degree of renal dysfunction and heart failure mortality (3,11). The major predictors included renal function, age, serum sodium, anemia and dementia.
RISK FACTORS FOR CARDIORENAL SYNDROME
The most common underlying risk factors that account for renal dysfunction in the setting of heart failure or cardiac dysfunction include hypertension, diabetes and underlying severe atherosclerotic vascular disease (12).
Not surprisingly, the patients generally are older, and have a history of either heart failure, renal failure or both (Table 1). The risk factors for diuretic resistance or renal dysfunction in the setting of acute decompensated heart failure are not as well characterized, but likely are also dictated by a similar cluster of risk factor profiles.
TABLE 1.
Risk factors for renal dysfunction in heart failure
| Hypertension |
|---|
| Diabetes |
| Severe vascular disease |
| Elderly age |
| Past history of: |
| Heart failure |
| Renal dysfunction |
| Heart failure and renal dysfunction |
PATHOPHYSIOLOGY OF CARDIORENAL SYNDROME
The pathophysiology of cardiorenal syndrome likely varies according to the specific clinical circumstances. The general processes likely encompass hemodynamic factors, such as intrarenal hemodynamics, transrenal perfusion pressure and systemic neurohormonal factors.
Transrenal perfusion pressure is calculated as mean arterial pressure minus the central venous pressure. Therefore, for the patient with volume overload and heart failure, the combination of increased pulmonary artery or central venous pressure with low systemic pressure may lead to a severe compromise of the net renal perfusion pressure. Therefore, whenever there is an opportunity to reduce the central venous pressure, whether through vasodilation, improved oxygenation or volume reduction, this can result in significant improvements in renal blood flow and urine output.
In the setting of acute decompensated heart failure, there is generally inadequate cardiac output and decreased perfusion pressure. In the presence of risk factors such as diabetes and hypertension, there is further reduction of glomerular filtration. This will further worsen any pre-existing renal dysfunction.
However, a more important contributor is likely the neurohumoral activation mediated by activation of arterial baroreceptors and intrarenal sensors. These reflexes lead to the activation of the renin-angiotensin system, sympathoadrenal system and arginine-vasopressin system – an intrinsic self-defense system to maintain blood pressure and intravascular volume. All of these factors will lead to peripheral and intrarenal vasoconstriction, further decreasing renal blood flow and GFR, and leading to a decrease in renal function. The consequences also lead to renal hypoxia, inflammation, cytokine release, and progressive structural and functional loss. The net clinical consequences are sodium and fluid retention, and progressive reduction of renal function, initially reversible, but ultimately irreversible damage.
A recently identified contributing factor is adenosine and the related tubuloglomerular feedback. Adenosine can be locally released in the kidney under stress, and binds to receptors on the afferent arterioles and cause vasoconstriction, thereby reducing renal blood flow (13). Stimulation of the receptor also increases sodium resorption in the tubules, leading to further sodium and water retention. Acute delivery of sodium to the distal tubules by diuretic therapy in acute decompensated heart failure will in turn stimulate further adenosine release from macula densa, and further reduce glomerular filtration. This pathway is very attractive as a contemporary target for therapy, but it remains to be seen how effective adenosine blockade will be in preserving renal function in the setting of cardiorenal syndrome.
The aggressive use of diuretics may therefore cause further neuro-hormonal activation, and aggravate systemic and renal vasoconstriction, leading to additional reductions in renal function. The reduction in blood flow and filtration contribute actively to the clinical conundrum of diuretic resistance.
DIURETIC RESISTANCE
The definition of diuretic resistance is not entirely agreed upon. A practical definition is the condition in which there is persistent pulmonary congestion despite repeated doses of 80 mg furosemide, or greater than 240 mg furosemide per day (including continuous furosemide infusion), or combined diuretic therapy including loop diuretics with thiazide or aldosterone antagonist.
The consequence of diuretic resistance is that the expected hemodynamic improvements fail to occur, and the urgently anticipated relief of symptoms is minimal or incomplete, exposing the patient to longer hospital stay, higher risk for complications and increased probability of subsequent rehospitalization (14).
The predisposing factors for diuretic resistance again include elderly age, patients with prior history of heart failure, pre-existing renal dysfunction, diabetes and/or hypertension.
Furosemide is the most commonly used diuretic to treat volume overload state in heart failure, yet it is particularly prone to the problem of diuretic resistance because of its particular pharmacokinetics. Because of gut hypoperfusion and edema, oral furosemide is poorly absorbed in the volume-overloaded state. This usually is the situation in which patients are switched to intravenous boluses of furosemide. However, intravenous furosemide boluses release a large load of sodium down the tubules, leading to a rebound increase in sodium resorption, negating the very reason for diuresis in the first place. As a result, furosemide infusion has been shown to be potentially more efficacious than standard bolus administration alone.
Unfortunately, chronic diuretic use also induces hypertrophy in distal tubular cells, leading again to enhanced sodium reuptake, contributing further to diuretic resistance. Ultimately, most diuretics dependent on electrolyte removal as a means to remove fluid are prone to similar challenges. Combining a loop diuretic with another diuretic that can help to inhibit distal resorption of sodium, such as thiazides, may moderate this resistance, at the cost of significant salt losses. Alternatives to furosemide, such as torsemide, have been shown to have a slight advantage in selected studies because of somewhat more favourable pharmacokinetics.
CHALLENGES IN PATIENT MANAGEMENT WITH CARDIORENAL SYNDROME
Managing the patient with cardiorenal syndrome often involves making recommendations or choices of therapies that are mutually contradictory. Because one is attempting to treat volume overload and congestion, the aggressive use of diuretics and volume depletion directly worsens renal function. On the other hand, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, while cardiorenal protective, can lead to temporary worsening of renal function. To preserve renal function, it is preferable to replete intravascular volume and provide a salt load, but these measures directly worsen cardiac congestion. Cardiologists and nephrologists often provide contradictory recommendations that are mutually incompatible for the attending medical team, reflecting the difficult choices that have to be made.
Not surprisingly, many patients end up being discharged from hospital either still volume loaded or markedly worse in terms of renal function. Not surprisingly, there is a high readmission rate for patients recently discharged from hospital with heart failure or renal failure. This dilemma at least partially accounts for the worsening clinical outcomes for patients recently admitted with heart failure and the cardiorenal syndrome.
OPTIONS OF MANAGEMENT FOR CARDIORENAL SYNDROME
Optimize heart failure therapy
The acute management of the patient with symptomatic congestion often focuses on symptomatic relief and the rapid removal of volume. However, to date, no therapies focused mainly on symptomatic relief or volume removal have demonstrated any benefit on improving survival or attenuating disease progression. This refocuses the importance of instituting or optimizing disease-modifying therapy for the symptomatic patients as soon as possible. These include, where appropriate, optimal doses of angiotensin modulators, beta-blockers and/or aldosterone antagonists. All of these therapies, when used judiciously, will help to improve the patient’s survival and reduce hospitalization. However, their effect on renal function and blood pressure during acute decompensation will need to be monitored very closely, initially on a daily and later on a biweekly basis following discharge.
Much of this has been outlined in the Canadian Cardiovascular Society’s Heart Failure consensus guidelines updates (15,16). The specific recommendations related to patients with heart failure and renal dysfunction appear in the 2007 guidelines, and are outlined in Table 2.
TABLE 2.
Canadian Cardiovascular Society consensus recommendations on heart failure and concomitant renal dysfunction
| Recommendations |
|---|
| Heart failure patients with stable renal function (serum creatinine levels less than 200 μmol/L) should be monitored for serum potassium and creatinine if combination therapy is used or in the presence of potential dehydration (class I, level B). Patients with heart failure with increasing serum creatinine should be assessed for reversible causes such as concomitant medications (eg, nonsteroidal anti-inflammatory drugs), hypovolemia, hypotension, urinary tract obstruction or infection (class I, level C). In oliguric heart failure patients who are hemodynamically stable, diuretics, ACE inhibitors, ARBs, spironolactone and nonheart failure drugs that can impair renal function should be reviewed daily (class I, level C). In stable heart failure patients who are not oliguric but have increasing serum creatinine levels of more than 30% from a previous stable baseline, the dose of diuretics, ACE inhibitors, ARBs and spironolactone may be reduced until renal function stabilizes (class 1, level C). In heart failure patients not responding adequately to more than 240 mg intravenous furosemide daily, treatment options include: ○ More frequent or higher doses of intravenous boluses of diuretic (class IIb, level C); ○ Combination with thiazide diuretic, eg, hydrochlorothiazide or metolazone (class IIA, level B); or ○ Continuous intravenous furosemide infusion (class IIa, level B). |
Optimize diuretic therapy
Furosemide infusion, taking an estimated total daily dose and infusing it over 2 h to 4 h, may offer more effective diuresis and greater safety profile, in contrast to bolus injections. However, the studies to date (6,8) are generally small in size, and the design not optimal, thus precluding a definitive first-line recommendation.
Combination diuretics, where the loop diuretic (eg, furosemide) is preceded by an ascending tubular agent (eg, thiazides such as hydrochlorothiazide or metalazone), can be used to produce a more effective diuresis, overcome some diuretic resistance and increase fractional sodium excretion. However, patients on this combination need to be very carefully monitored for adverse events such as hypokalemia, worsening renal function and dehydration. The combination should be terminated as soon as the volume status has been restored to avoid long-term complications.
Vasodilator therapy
Vasodilators such as intravenous nitroglycerin or nesiritide (recombinant human B-type atrial natriuretic peptide) have been shown to be much less detrimental to renal function, particularly when used at low doses that do not decrease blood pressure. Vasodilators can rapidly reduce ventricular filling pressures and central venous pressures, and decrease myocardial oxygen consumption. They can also decrease systemic vascular resistance, decrease ventricular workload, increase stroke volume and improve cardiac output under the right circumstances. Intravenous nitroglycerin is a vasodilator commonly used to relieve pulmonary congestion in patients with decompensated heart failure. While it is an effective vasodilator, frequent dose titration of intravenous nitroglycerin is necessary to produce the desired hemodynamic effects and symptomatic relief. The reduction in venous pressure may be beneficial in reducing transrenal perfusion pressure. While intravenous nitroglycerin is effective in reducing symptoms and pulmonary congestion, it is not clear whether this has long-term benefits in terms of renal function or survival.
Recombinant human B-type natriuretic peptide (nesiritide)
Recombinant human B-type natriuretic peptide, or nesiritide, is an effective vasodilator with mild diuretic effects. It has been shown in moderate-sized controlled trials and registries to be effective in reducing symptoms when compared with placebo, with a reasonable safety profile (17,18). However, subsequent meta-analyses (19,20) suggested that administration of nesiritide at the variety of doses tested (0.01 μg/kg/mL to 0.03 μg/kg/mL) in the setting of acute heart failure may increase the risk of worsening renal function.
However, subsequent studies of nesiritide under somewhat different circumstances contradicted the earlier meta-analysis in terms of renal function. A substudy of the Follow-Up Serial Infusions of Nesiritide trial (FUSION I) (21) demonstrated that in a heart failure population at high risk for cardiorenal syndrome, infusion of nesiritide at two doses (0.005 μg/kg/mL or 0.01 μg/kg/mL) was well tolerated with no worsening of renal function.
Additional recent studies have also confirmed the safety of nonhypotensive low-dose nesiritide, such as 0.005 μg/kg/mL or 0.0025 μg/kg/mL, particularly without an initial bolus. In a case-control study (22), low-dose nesiritide was well tolerated without a significant decrease in systolic blood pressure, in contrast to the blood pressure-lowering effect with standard or high doses of nesiritide. The low-dose nesiritide group also showed improvement in renal function with efficacious diuresis despite lower doses of diuretics.
Therefore, data are emerging that low doses of nesiritide are potentially renal protective in the difficult clinical situation of managing patients with acute decompensated heart failure at risk for cardiorenal syndrome. However, additional outcome information on the efficacy of nesiritide in this setting awaits the larger ongoing outcome-driven clinical trials such as the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial.
Dialysis or ultrafiltration
Ultrafiltration is a mechanical process that directly removes plasma water across a semipermeable membrane that maintains the same osmolality as the plasma. On the other hand, hemodialysis aims to remove solutes from blood across the membrane down a concentration gradient, allowing customization of target electrolytes and solutes. Recent improvements in ultrafiltration devices allow flexible low-flow catheters to be inserted in the antecubital vein for venous-venous filtration. This is a relatively simple procedure, and obviates the need for intensive care admissions and monitoring.
Therefore, ultrafiltration can potentially address the clinical conundrum of worsening renal function, decreased urine output despite escalating doses of diuretics or diuretic resistance in severe heart failure. In the UltrafiltratioN versus Intravenous (IV) Diuretics for Patients HospitaLized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial (23), patients with acute decompensated heart failure were randomly assigned to ultrafiltration with flows of up to 500 mL/h versus standard intravenous diuretics. The ultrafiltration group showed a greater weight loss and greater volume removal at 48 h, although the change in dyspnea score was not different and both groups improved. The rates of rehospitalization and the total days of hospitalization were significantly lower in the ultrafiltration group at a three-month follow-up. However, the long-term impact in terms of renal function and clinical outcome is not clear, because preliminary data suggested that there was no significant protective effect of ultrafiltration on renal function. Surprisingly, there was no relationship between amounts of fluid removed versus changes in serum creatinine, suggesting that other factors not related to volume are responsible for the deterioration in renal function.
Vasopressin antagonist
Vasopressin or antidiuretic hormone is secreted by the posterior pituitary gland in response to hyperosmolality or volume depletion. The release of vasopressin engages the V1a (vascular) and V2 (renal) receptors, which leads to vasoconstriction and water reabsorption through aquaporin channels in the tubules. Selective V2 antagonists, such as tolvaptan, can effectively mobilize free water clearance, hence aquaresis, and would end up increasing the serum sodium in those who are hyponatremic.
In the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist (ACTIV) trial (24), patients with acute heart failure showed a greater reduction in body weight at 24 h receiving tolvaptan compared with those receiving placebo or standard therapy. There was also an increase in urine output at 24 h, and a slight increase in serum sodium in the group treated with tolvaptan.
The ACTIV trial paved the way for the much larger Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial (25). The study confirmed the efficacy of early vasopressin antagonist in producing weight loss in patients with acute heart failure. However, long-term outcomes of the patients were again not different between the vasopressin antagonist or the placebo groups (26). This suggests that vasopressin antagonist as used in the setting of acute heart failure can modify the renal response to water retention, but does not favourably influence remodelling the heart or kidneys over the long term toward recovery.
Adenosine antagonist
Adenosine antagonists are novel agents that have the effect of activating adenosine (A1) receptors and promoting diuresis. In the settingof impaired tubular glomerular filtration, adenosine is released and binds to A1 receptors to induce constriction of the afferent arterioles. This decreases renal blood flow and enhances sodium resorption by the proximal tubules. Adenosine antagonists have the potential to improve renal blood flow and increase sodium excretion.
Initial clinical studies of A1 antagonist (eg, BG9719) have been encouraging. Gottlieb et al (13) studied 63 patients with heart failure and volume overload. The addition of BG9719 to furosemide significantly increased the amount of diuresis. While furosemide alone caused a decline in renal function, the addition of BG9719 prevented the decline in renal function. Thus, adenosine antagonists may enhance diuresis with loop diuretics, while preserving renal function.
The safety and efficacy of adenosine antagonists are currently being evaluated in larger clinical trials to determine their net effect on renal function, and potential cardiovascular outcomes.
COMMON STRATEGIES FOR BOTH THE CARDIOVASCULAR AND NEPHROLOGY TEAMS
The cardiorenal syndrome is common in patients with cardiac dysfunction and risk factors, and is associated with adverse clinical outcome. The previous focus on symptomatic treatment with escalating doses of diuretics only met diuretic resistance.
The new focus should be to recognize the cardiorenal syndrome, and treat the whole patient, and treat for long term. The optimization of heart failure therapy also preserves renal function. Newer approaches to diuretic infusion or combination therapy may reduce the degree of renal dysfunction, while vasodilators such as nitroglycerin and particularly nesiritide at low doses may improve transrenal blood flow while protecting renal function. Newer approaches such as ultrafiltration, vasopressin antagonists and adenosine receptor blockade may offer additional opportunities to improve volume regulation while preserving renal and cardiac function.
Footnotes
Dr Liu holds the Heart & Stroke/Polo Chair Professor of Medicine and Physiology at the University Health Network, University of Toronto.
DISCLOSURE: Supported in part by grants from the Canadian Institutes of Health Research and the Heart & Stroke Foundation. Dr. Liu also receives grant support from Pfizer, Johnson & Johnson, Otsuka, Roche and Novartis.
REFERENCES
- 1.Johansen H, Strauss B, Arnold JM, Moe G, Liu P. On the rise: The current and projected future burden of congestive heart failure hospitalization in Canada. Can J Cardiol. 2003;19:430–5. [PubMed] [Google Scholar]
- 2.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA. 2003;290:2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Jong P, Gong Y, Liu PP, Austin PC, Lee DS, Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation. 2003;108:184–91. doi: 10.1161/01.CIR.0000080290.39027.48. [DOI] [PubMed] [Google Scholar]
- 5.Lee DS, Mamdani MM, Austin PC, et al. Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000. Am J Med. 2004;116:581–9. doi: 10.1016/j.amjmed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Secular trends in renal dysfunction and outcomes in hospitalized heart failure patients. J Card Fail. 2006;12:257–62. doi: 10.1016/j.cardfail.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 8.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–96. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 9.Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–8. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ, ADHERE Scientific Advisory Committee Study Group, and Investigators Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 11.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 12.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–7. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb SS, Brater DC, Thomas I, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348–53. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- 14.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–85. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 15.Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: Diagnosis and management Can J Cardiol 20062223–45.(Erratum in 2006;22:271). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold JM, Howlett JG, Dorian P, et al. Canadian Cardiovascular Society Consensus Conference recommendations on heart failure update 2007: Prevention, management during intercurrent illness or acute decompensation, and use of biomarkers. Can J Cardiol. 2007;23:21–45. doi: 10.1016/s0828-282x(07)70211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial JAMA 20022871531–40.(Erratum in 2002;288:577). [DOI] [PubMed] [Google Scholar]
- 18.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure Nesiritide Study Group N Engl J Med 2000343246–53.(Errata in 2000;343:1504 and 2000;343:896). [DOI] [PubMed] [Google Scholar]
- 19.Aaronson KD, Sackner-Bernstein J. Risk of death associated with nesiritide in patients with acutely decompensated heart failure. JAMA. 2006;296:1465–6. doi: 10.1001/jama.296.12.1465. [DOI] [PubMed] [Google Scholar]
- 20.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 21.Yancy CW, Singh A. Potential applications of outpatient nesiritide infusions in patients with advanced heart failure and concomitant renal insufficiency (from the Follow-Up Serial Infusions of Nesiritide [FUSION I] trial) Am J Cardiol. 2006;98:226–9. doi: 10.1016/j.amjcard.2006.01.081. [DOI] [PubMed] [Google Scholar]
- 22.Riter HG, Redfield MM, Burnett JC, et al. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol. 2006;47:2334–5. doi: 10.1016/j.jacc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–83. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 24.Gheorghiade M, Gattis WA, O’Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA. 2004;291:1963–71. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 25.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA. 2007;297:1332–43. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 26.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]