Ancient DNA reveals late survival of mammoth and horse in interior Alaska (original) (raw)
Abstract
Causes of late Quaternary extinctions of large mammals (“megafauna”) continue to be debated, especially for continental losses, because spatial and temporal patterns of extinction are poorly known. Accurate latest appearance dates (LADs) for such taxa are critical for interpreting the process of extinction. The extinction of woolly mammoth and horse in northwestern North America is currently placed at 15,000–13,000 calendar years before present (yr BP), based on LADs from dating surveys of macrofossils (bones and teeth). Advantages of using macrofossils to estimate when a species became extinct are offset, however, by the improbability of finding and dating the remains of the last-surviving members of populations that were restricted in numbers or confined to refugia. Here we report an alternative approach to detect ‘ghost ranges’ of dwindling populations, based on recovery of ancient DNA from perennially frozen and securely dated sediments (_sed_aDNA). In such contexts, _sed_aDNA can reveal the molecular presence of species that appear absent in the macrofossil record. We show that woolly mammoth and horse persisted in interior Alaska until at least 10,500 yr BP, several thousands of years later than indicated from macrofossil surveys. These results contradict claims that Holocene survival of mammoths in Beringia was restricted to ecologically isolated high-latitude islands. More importantly, our finding that mammoth and horse overlapped with humans for several millennia in the region where people initially entered the Americas challenges theories that megafaunal extinction occurred within centuries of human arrival or were due to an extraterrestrial impact in the late Pleistocene.
Keywords: extinction, permafrost, megafauna, Beringia
Around the time of the Pleistocene/Holocene transition, the Americas experienced a wave of faunal extinctions, culminating in the loss of more than half of its large mammals (the “megafauna”). The woolly mammoth and horse disappeared during this event (1). The underlying cause of these extinctions has been the subject of a lengthy but unresolved debate, with proposed mechanisms including rapid overkill (“blitzkrieg”) by human hunters, changes in climate and vegetation, or a combination of these (2–4). Others have suggested that hyperdisease (5) or an extraterrestrial impact 12,900 ± 100 years before present (yr BP)* (6, 7) were contributory factors. Discriminating between these alternative explanations for megafauna extinction in the Americas requires accurate age estimates for the presumed “last” occurrence of particular species.
The youngest reliably dated macrofossil (usually a bone or tooth) of an extinct species is commonly taken to represent the approximate time of its disappearance. In practice, however, there is a very low probability of discovering fossil remains of the last members of any species, so ages for extinction based on dated macrofossil finds will likely be older than the true ages (raising the possibility of “ghost ranges” of unknown duration). Known as the Signor–Lipps effect (SLE), such sampling bias is an inevitable feature of the structure of any paleontological data set (8). The extent of this bias depends on fossil abundances and sampling intensity over time and can be estimated statistically under particular assumptions of constant or declining rates of fossil deposition and preservation (9). In regions where megafauna persisted over extended time spans, extensive macrofossil-dating surveys are required to detect small, late-surviving populations (10, 11). Estimating extinction times is a common problem in paleontology (1, 5), so alternative and complementary approaches to the direct dating of faunal remains are needed.
Previous studies have shown that mitochondrial DNA (mtDNA) putatively derived from the feces, urine, epidermal cells, and hair of a diverse range of vertebrates may be preserved for long periods in suitable sedimentary environments, such as those in the Arctic, even in the absence of identified macrofossils (12–16). This so-called “sedimentary” ancient DNA (_sed_aDNA) has been shown to be of local origin (12, 13, 17), requiring an animal to have been physically present at the site for its DNA to be deposited (13). Although leaching of DNA may occur between layers in nonfrozen depositional settings (13), several studies have demonstrated that this problem does not appear to affect either perennially frozen sediments (17–20) or sediments frozen recently (15). Furthermore, in cases where strata have remained undisturbed, DNA extracted from modern surface sediments at localities in the Arctic and temperate regions has yielded the genetic signatures of extant fauna only (12, 16), which suggests that DNA is not readily reworked from older deposits and incorporated into younger deposits. As each animal deposits large quantities of DNA into the environment during its lifetime, but only a single skeleton after death, the analysis of _sed_aDNA offers the potential to detect small, late-surviving populations of now-extinct species, which macrofossil surveys would likely miss due to severe limitations on sampling and/or dating. That such environmentally preserved DNA can be used to establish the presence of restricted animal populations that are otherwise hardly detectable in the landscape has been demonstrated for extant species (21), but remains to be tested for extinct taxa.
We used the _sed_aDNA approach to investigate how long woolly mammoths and horses may have persisted in Alaska, widely accepted as a possible point of entry for humans into the Americas (22). The duration of any overlap between these species and people in this region should, in principle, provide a test of alternative theories of megafaunal extinction.
Results and Discussion
The Study Site.
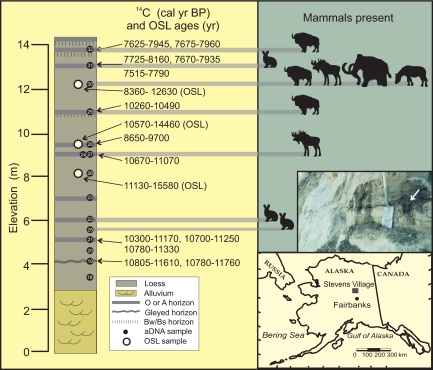
The study site is located near Stevens Village in the Yukon Flats, on the south bank of the braided Yukon River in interior Alaska (65° 59'N, 148° 57'W; Fig. 1). The floodplain is 4–6 km wide and capped by locally derived aeolian sediments deposited in the late Pleistocene and early Holocene (23). The site consists of 2 m of fluvial sand overlain by 12 m of calcareous loess, interbedded with at least seven buried soils (paleosols), each in the early stages of soil development (Inceptisols). Permafrost at the site is “dry” with no visible ice present. All samples were obtained from frozen material by excavating horizontally to a depth of 1–2 m into the exposure. We completed detailed descriptions of the soils and interbedded sediments, including fine laminae, to ensure that no vertical structures suggestive of movement by water were present. We then collected duplicates of 15 permafrost core samples for _sed_aDNA analysis and three sediment samples for dating by optically stimulated luminescence (OSL); see SI Text, Dating of Exposure. The OSL ages are compatible with the radiocarbon (14C) chronology, based on 13 ages for plant macrofossils recovered from the paleosols. The chronology indicates that the earliest paleosol developed ≈11,000 yr BP and that deposition of loess did not cease until shortly after 8,000 yr BP (Tables S1 and S2).
Fig. 1.
Stratigraphic profile and location (see inset map) of the Stevens Village site. Elevation is height in meters above river level, and age ranges (in calendar years) are shown at the 95 and 68% confidence intervals for radiocarbon (14C) and OSL, respectively. OSL ages were obtained from quartz sediments and 14C ages from plant macrofossils. Inset photo shows detail of buried vegetation (with arrow at shrub root) and lateral continuity of paleosol at 5 m elevation. The mammalian taxa identified from _sed_aDNA sequences are shown by symbols, with the scientific names given in Table 1.
We also collected 10 control samples from the surface of the modern soil at the Stevens Village exposure, 17 samples of Yukon River water from near bluffs composed of late Pleistocene/early Holocene sediments, and 12 samples of surface sediment from river bars (Table S5 and Fig. S4). These control samples were used to test the possibility that _sed_aDNA derived from older Pleistocene deposits is present in modern river water or in modern floodplain sediments, from where it could have been transported by wind on to the surface of the Stevens Village site and incorporated into the early Holocene deposits.
Sequence Retrieval and Determination.
Established procedures were applied to guard against contamination of the _sed_aDNA samples during the collection and subsequent DNA retrieval from the permafrost cores (12, 24). Mitochondrial DNA (mtDNA) sequences were obtained through the use of both generic mammalian, and species-specific, primers, using either conventional PCR and cloning methods, coupled with Sanger sequencing, or high-throughput sequencing-by-synthesis (GS FLX) of PCR products (SI Text, DNA Methodology). Sequence identification followed a statistical approach for taxon assignment (25, 26) and a BLAST search of GenBank (SI Text, Taxonomic Assignments). Both identification methods yielded congruent results and reveal that mtDNA of woolly mammoth, bison, moose, horse, and snowshoe hare are preserved in the frozen sediments (Fig. 1, Table 1, and Fig. S3 for sequence alignment). _sed_aDNA from moose and hare were restricted to two and three layers, respectively, of the 15 total permafrost layers, despite these species being present throughout the entire time range covered by the site (Fig. 1). This supports previous observations that an animal has to be physically present at a site to leave its DNA traces behind in the sediments, and thus why its DNA is not expected to be found in each layer (12, 13). Interestingly, mtDNA of mammoth and horse could be obtained from a single layer dated to between ≈10,500 and 7,600 yr BP (Fig. 1). The identified mammoth control region mtDNA sequences derive from at least two individuals, belonging to clade I and subclades C10 or D (Table 1 and Fig. S3). These clades have been previously identified in DNA studies of fossil remains as late-surviving members of woolly mammoth clades in northern North America. Furthermore, subclade C has, so far, been found only among New World mammoths (27–29). This finding provides support for a local origin of the _sed_aDNA sequences at Stevens Village, which is compatible with previous reports that _seda_DNA is not readily transported over long distances (12, 13).
Table 1.
Information for _sed_aDNA sequences obtained from seven permafrost layers (age estimates shown in Fig. 1)
| Permafrost layers | SAP sequence ID (genus) | SAP probability support, % | SAP sequence ID (species) | SAP probability support, % | Highest BLAST hit | BLAST similarity, % | Molecular marker(s) |
|---|---|---|---|---|---|---|---|
| DNA32 | Bison | 85 | B. bison | 85 | B. bison | 98 | 16S3-4† |
| DNA31 | Lepus | 100 | L. americanus | 97 | L. americanus | 100 | 16S3-4† |
| DNA30 | Equus | 97 | E. caballus | 98, 100 | 16S1-2*, 16S3-4* | ||
| DNA30 | Mammuthus | 100 | M. primigenius | 100 | M. primigenius | 100, 100 | 16S1-2*,†, control region* |
| DNA30 | Bison | 88 | B. bison | 88 | B. bison | 98 | 16S3-4*,† |
| DNA30 | Alces | 95 | A. alces | 95 | A. alces | 100 | 16S1-2† |
| DNA29 | Bison | 86 | B. bison | 86 | B. bison | 98 | 16S3-4† |
| DNA27 | Alces | 100 | A. alces | 100 | A. alces | 100 | 16S1-2*,† |
| DNA25 | Lepus | 100 | L. americanus | 98 | L. americanus | 100, 100 | 16S1-2*, 16S3-4*,F |
Importantly, we obtained PCR products of the expected length from six of the 39 modern control samples using the same primers and amplification conditions as were applied to the ancient samples; the remaining control samples produced no PCR products of the expected length. In-depth FLX sequencing of the products generated a total of 32,874 sequence reads, all of which derive from common laboratory contaminants—cow, pig, or mouse (30) or, in one case, bacterial DNA—and not from megafauna taxa such as mammoth or horse. Amplification of low-level laboratory contaminants is expected when no other target DNA is present (31). Our findings, therefore, confirm previous observations that _sed_aDNA is not widely distributed in the environment (12, 16), probably because it largely exists as extracellular free molecules (18) and decays quickly when exposed to UV irradiation, water, and oxygen free radicals (20, 24). Given the results obtained from the modern control samples, we consider it highly unlikely that the mammoth and horse _sed_aDNA preserved in the early Holocene sediments at the Stevens Village exposure are derived from remobilized older deposits. The absence of putative mammalian DNA in the modern, surface samples is probably because, in contrast to the ancient samples, they represent only 1 or 2 years of sediment accumulation. There is little chance, therefore, of an animal leaving its DNA in any particular locality within this short time span.
Comparison with the Fossil Record.
Currently, the youngest macrofossil ages for mammoth and horse north of the Cordilleran and Laurentide ice sheets in northwestern North America are 11,500 ± 160 and 12,480 ± 80 14C yr BP, respectively (4). These correspond to calibrated (calendar year) age ranges of 13,100–13,710 and 14,180–14,960 yr BP at the 95% confidence interval. The recovery of mammoth and horse DNA from the 10,500- to 7,600-year-old sediments suggests that both species survived in interior Alaska for at least 2,600 and 3,700 years longer, respectively, than established from macrofossil surveys north of the Cordilleran and Laurentide ice sheets.
We are confident that DNA leaching cannot explain the presence of mammoth and horse _sed_aDNA at the Stevens Village exposure. No evidence of DNA leaching been found under permafrost settings, despite several investigations (15, 18–20), and for mammoth and horse DNA to be recovered from sediments several millennia younger than the youngest macrofossil remains from mainland Alaska/Yukon would require the DNA to have migrated more than 8 m upward through frozen sediments, without leaving any traces behind in the intervening strata (Fig. 1).
Our _sed_aDNA ages for mammoth and horse are also notably younger, by ≈1,300 and 2,300 years respectively, than the youngest fossil remains found to the south of these ice sheets (mammoth: 10,350 ± 130 14C yr BP or 11,770–12,650 yr BP; horse: 10,870 ± 45 14C yr BP or 12,815–12,910 yr BP) (32). The latter macrofossils are unlikely to include the last members of either species, which underlines the critical significance of the _sed_aDNA approach for detecting rare, late-surviving populations of mammoth and horse in different parts of North America.
It may be significant that these late surviving populations were found in the Yukon Flats area of central Alaska. The wide braidplain that characterizes this low-relief landscape in interior Alaska favors a disturbance regime, with the margins of shifting river bars supporting colonizing plants that would have provided high-quality forage, and aeolian inputs maintaining soil nutrients. This type of disturbance landscape has been hypothesized as a means to maintain mammoth-steppe habitat (33).
Probability of Missing Late Survival in the Fossil Record.
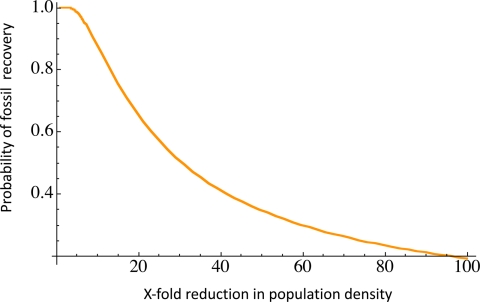
We can estimate the reduction in population density required to fail to detect the last of the woolly mammoths from macrofossil surveys by assuming that the number of fossil remains is directly proportional to population density and that fossil preservation has not changed over time. This is a reasonable assumption, given that macrofossils are exceptionally well preserved in the perennially frozen deposits in these regions. Because the number of individual mammoths would have decreased as the species approached extinction, so too would the number of macrofossils. The probability of not recovering fossils of the last-surviving mammoths is as high as 5% for a modest 7-fold reduction in population density; if the population size had decreased 31-fold, then there is a 50% probability of failing to detect any late-persisting mammoths among dated fossils (Fig. 2). Nearly identical results are obtained using a standard parametric analysis (9) to estimate the 95% confidence interval for the latest appearance date (LAD). Both methods of estimating the extent of the SLE are described in SI Text, Statistical Modeling of Macrofossil Ages. Hence, to confidently rule out the possibility that small numbers of mammoths persisted into the Holocene in northwestern North America, macrofossil surveys would need to be intensified significantly.
Fig. 2.
Probability of recovery of mammoth fossils in mainland Alaska and Yukon, plotted as a function of the magnitude of population decline. The modeled time interval is 13,100 yr BP (the youngest age for a dated macrofossil at the 95% confidence interval) to 10,260 yr BP (conservatively the youngest age for mammoth DNA at the Stevens Village site, also at the 95% confidence interval). For model details, see SI Text Statistical Modeling of Macrofossil Ages.
Implications for Extinction Theories.
Our results have implications for theories of megafaunal extinction. The classical human overkill (“blitzkrieg”) hypothesis for the Americas asserts that extinction took place rapidly, within 1,000 years of human arrival (3). The oldest reliable evidence of human presence in Alaska [≈14,000 yr BP at Swan Point (22)] and the youngest macrofossil age for mammoth in this region (13,100–13,710 yr BP) are consistent with the blitzkrieg model. But the _sed_aDNA evidence for mammoth and horse persisting into the Holocene in interior Alaska is incompatible with such rapid extinction and indicates that late-surviving mammoths in the New World were not confined to islands in the Bering Sea that might have afforded protection from human hunters (10, 34). The protracted survival of mammoth and horse is also inconsistent with the hyperdisease hypothesis (5) (which requires their swift demise following human contact) and with megafaunal extinction due to end-Pleistocene environmental changes associated with abrupt climatic events (35), altered vegetation patterns (2), or intense wildfires sparked by a presumed extraterrestrial impact (6, 7).
We cannot exclude the possibility that the drastic decline in the number of mammoths surviving into the Holocene was originally triggered by human overkill, hyperdisease, climate and vegetation changes, and/or an extraterrestrial impact in the late Pleistocene. But our findings suggest that these events, if they occurred as classically conceived, did not deliver the deathblow. Given recent reports of human presence elsewhere in the Americas by ≈14,000 yr BP (36, 37) and the small likelihood of discovering evidence of Homo sapiens or the most recent traces of mammoth and horse in the New World, the duration of human/megafaunal overlap was probably even greater than suggested by our _sed_aDNA results, raising questions about the mode and tempo of extinction.
Concluding Remarks.
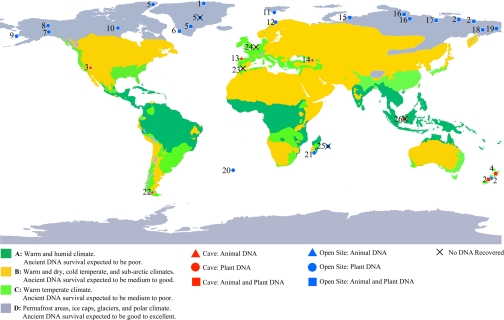
Animal DNA from modern environmental samples has been used to detect secretive organisms present at low densities, without requiring their direct observation (Fig. 3) (21). Our study shows that the same approach can be extended to extinct animals, to detect their former geographic ranges and lastest appearance dates. For this purpose, we chose the woolly mammoth and horse, finding that small populations of these megafaunal species persisted well into the Holocene in northwestern North America. The prolonged overlap of humans, mammoth and horse, revealed here by the application of the _sed_aDNA approach, suggests that the timing and process of extinction of other species in the Americas and on other continents should be reassessed using a similar strategy. Furthermore, recognition of their late survival from traces of _sed_aDNA is of more general importance to the field of Quaternary paleontology, because it demonstrates that ancient DNA preserved in certain sedimentary environments can be used to detect dwindling populations of taxa that will likely be missed using the more traditional approach of finding, and subsequently dating, macrofossils.
Fig. 3.
World map showing the suitability of different regions for _sed_aDNA recovery. Sites are marked where recovery of plant and/or animal _sed_aDNA has been attempted: 1. Hans Tausen Glacier, Greenland, up to ca. 4,000 yr BP (40); 2. permafrost up to ca. 400,000 yr BP and temperate cave sediments up to ca. 4,000 yr BP; negative findings at permafrost sites ca. 1.5 million yr BP (12); 3. hot and dry cave sediments, Rampart Cave, Arizona, ca. 10,800 yr BP (41); 4. temperate cave sediments, Hukanui, New Zealand, up to ca. 3,200 yr BP (13); 5. basal silty ice, Greenland and Canada, up to ca. 500,000 yr BP; negative findings at GRIP ca. 2.5 million yr BP (17); 6. “GUS” Norse site, Greenland ca. 1,000 yr BP (15); 7. permafrost, Stevens Village, Alaska up to ca. 9,800 yr BP (present study); 8. permafrost, Canada and Alaska: Irish Gulch, Thistle Creek, Goldbottom, Lost Chicken, Christie Mine, Ross Mine, Thanglat, Dominion Creek, Desdeash Patch, up to ca. 800,000 yr BP; 9. Lake core, St. Michael Island, Alaska; 10. Permafrost, Thelon River, Canada; 11. permafrost, Advendalen and Collesdalen, Svalbard, Norway, late Pleistocene/Holocene; 12. cold but not frozen sediments, Varanger, Norway, modern surface samples; 13. hot and dry cave sediments, El Sidron, Spain ca. 43,000 yr BP; 14. cold cave sediments, Mezmaiskaya, Russia, ca. 36,000 yr BP; 15. cold but not frozen sediments, Yuribei River, Marita River, Russia; 16. permafrost, Taimyr, Russia: Cape Sabler, Upper Taimyr River, Baskura Peninsula, Ovragzny Peninsula, Taimyr Lake Holocene, Drainage Lake, Bolshaya Balakhnaya, Khatanga River, up to ca. 45,000 yr BP; 17. permafrost, Buor Khaya, Russia, up to ca. 800 yr BP; 18. permafrost, Ice Bluff, Main River, Russia up to ca. 13,500 yr BP; 19. permafrost, Anadyr, Russia, ca. 3,000 yr BP; 20. hot humid climate, Tristan da Cunha, modern; 21. hot humid climate, Talaky, Anovao, Itampolo, Madagascar, Holocene; 22. cold but not frozen sediments, Ultima Esperanza, Chile, late Pleistocene; 23. temperate climate, Gorham's Cave, Gibraltar, late Pleistocene; 24. temperate climate, Trou Al'Wesse, Belgium, late Pleistocene; 25. hot humid climate, Mauritius: Fort Frederik Hendrik, ca. 400 yr BP, Mare aux Songes, ca. 4,000 yr BP; 26. hot humid climate, Niah Cave, Sarawak, late Pleistocene. Results for sites 8 to 26 are from unpublished data.
Importantly, as _sed_aDNA cannot be dated directly, it is crucial to acknowledge that the fidelity of the DNA record depends on it being contemporaneous with the associated dated material. This will generally require that the sediments at the study site have remained undisturbed since deposition and that the DNA has not migrated between strata; these constraints apply in addition to other controls on long-term DNA preservation (see SI Text). We recommend, therefore, that _sed_aDNA analyses should be conducted concurrently with direct dating of macrofossils, rather than as a replacement for it, especially in settings where postdepositional reworking of sediments and fossils cannot be discounted. Nevertheless, coupling the _sed_aDNA approach with that of recent climatic niche modeling, which can identify likely target areas for late survival of extinct species (38), offers a powerful tool to reexamine when, where, and why various late Quaternary species went extinct.
Materials and Methods
Frozen sediment samples were collected using established protocols to guard against sample-based contamination (12, 24). All ancient DNA work was confined to dedicated laboratories, following rigorous, accepted ancient DNA standards (39) (SI Text, DNA Methodology). DNA extractions and amplifications followed established protocols for _sed_aDNA (12), with minor modifications (Table S3). To confirm authenticity, selected mammoth sequences were reproduced independently. Sequences were assigned to hierarchical taxonomic levels using BLAST and a phylogenetic approach (25, 26) (SI Text, Taxonomic Assignments). Radiocarbon ages were obtained for plant materials collected in growth position and for plant macrofossils extracted from bulk samples of the buried soils. Samples were prepared using established procedures, and the 14C content measured by decay- or atom-counting techniques (Table S1). OSL dating provides an estimate of the time elapsed since luminescent minerals, such as quartz, were last exposed to sunlight. OSL ages for buried quartz grains were calculated from the burial dose (estimated using the OSL signal) divided by the dose rate due to ionizing radiation (SI Text, Dating of Exposure and Table S2).
Supplementary Material
Supporting Information
Acknowledgments.
The work was supported by the Danish National Research Foundation; the Natural Science and Engineering Research Council of Canada; the Alberta Ingenuity Foundation New Faculty Program; the Australian Research Council (Grant DP0558446); the University of Wollongong; Discovery Communications, Inc.; the Arts and Humanities Research Board; and the Arts and Humanities Research Council.
Footnotes
The authors declare no conflict of interest.
*
In this paper, calibrated radiocarbon ages (and OSL ages) are reported in calendar years (yr BP), whereas uncalibrated radiocarbon ages are reported as 14C yr BP.
References
- 1.Grayson DK. Deciphering North American Pleistocene extinctions. J Anth Res. 2007;63:185–214. [Google Scholar]
- 2.Guthrie RD. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature. 2006;441:207–209. doi: 10.1038/nature04604. [DOI] [PubMed] [Google Scholar]
- 3.Burney DA, Flannery TF. Fifty millennia of catastrophic extinctions after human contact. Trend Ecol Evol. 2005;20:395–401. doi: 10.1016/j.tree.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Ann Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 5.MacPhee R, Marx P. In: Natural Change and Human Impact in Madagascar. Goodman SM, Patterson B, editors. Washington, DC: Smithsonian Inst Press; 1997. [Google Scholar]
- 6.Firestone R, et al. Evidence for an extraterrestrial impact 12,900 years ago that contributed to the megafaunal extinctions and the Younger Dryas cooling. Proc Natl Acad Sci USA. 2007;104:16016–16021. doi: 10.1073/pnas.0706977104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennett DJ, et al. Nanodiamonds in the Younger Dryas boundary sediment layer. Science. 2009;323:94. doi: 10.1126/science.1162819. [DOI] [PubMed] [Google Scholar]
- 8.Signor PW, Lipps JH. In: Geological Society of America Special Paper. Silver LT, Schultz PH, editors. Boulder, CO: Geol Soc Am; 1982. pp. 291–296. [Google Scholar]
- 9.Solow AR, et al. On the Pleistocene extinctions of Alaskan mammoths and horses. Proc Natl Acad Sci USA. 2006;103:7351–7353. doi: 10.1073/pnas.0509480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guthrie R. Radiocarbon evidence of mid-Holocene mammoths stranded on an Alaskan Bering Sea island. Nature. 2004;441:746–749. doi: 10.1038/nature02612. [DOI] [PubMed] [Google Scholar]
- 11.Stuart AJ, et al. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature. 2004;431:684–689. doi: 10.1038/nature02890. [DOI] [PubMed] [Google Scholar]
- 12.Willerslev E, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300:791–795. doi: 10.1126/science.1084114. [DOI] [PubMed] [Google Scholar]
- 13.Haile J, et al. Ancient DNA chronology within sediment deposits: Are paleobiological reconstructions possible and is DNA leaching a factor? Mol Biol Evol. 2007;24:982–989. doi: 10.1093/molbev/msm016. [DOI] [PubMed] [Google Scholar]
- 14.Lydolph M, et al. Beringian paleoecology inferred from permafrost-preserved fungal DNA. Appl Environ Microbiol. 2005;71:1012–1017. doi: 10.1128/AEM.71.2.1012-1017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebsgaard MB, et al. The farm beneath the sand—An archaeological case study on ancient ‘dirt’ DNA. Antiquity. 2009;83:430–444. [Google Scholar]
- 16.Haile J. Ancient DNA from Sediments and Associated Remains. Oxford, UK: Oxford Univ Thesis; 2009. [Google Scholar]
- 17.Willerslev E, et al. Ancient biomolecules from deep ice cores reveal a forested southern Greenland. Science. 2007;317:111–114. doi: 10.1126/science.1141758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willerslev E, et al. Long-term persistence of bacterial DNA. Curr Biol. 2004;14:R9–R10. doi: 10.1016/j.cub.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Hansen AJ, et al. Crosslinks rather than strand breaks determine access to ancient DNA sequences from frozen sediments. Genetics. 2006;173:1175–1179. doi: 10.1534/genetics.106.057349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SS, et al. Ancient bacteria show evidence of DNA repair. Proc Natl Acad Sci USA. 2007;104:14401–14405. doi: 10.1073/pnas.0706787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ficetola GF, et al. Species detection using environmental DNA from water samples. Biol Lett. 2008;4:423–425. doi: 10.1098/rsbl.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goebel T, et al. The Late Pleistocene dispersal of modern humans in the Americas. Science. 2008;319:1497–1502. doi: 10.1126/science.1153569. [DOI] [PubMed] [Google Scholar]
- 23.Froese DG, et al. Characterizing large river history with shallow geophysics: Middle Yukon River, Yukon Territory and Alaska. Geomorphology. 2005;67:391–406. [Google Scholar]
- 24.Willerslev E, et al. Isolation of nucleic acids and cultures from fossil ice and permafrost. Trend Ecol Evol. 2004;19:141–147. doi: 10.1016/j.tree.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Munch K, et al. Fast phylogenetic DNA barcoding. Philos Trans R Soc London Ser B. 2008;363:3997–4002. doi: 10.1098/rstb.2008.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munch K, et al. Statistical assignment of DNA sequences using Bayesian phylogenetics. Syst Biol. 2008;57:750–757. doi: 10.1080/10635150802422316. [DOI] [PubMed] [Google Scholar]
- 27.Barnes I, et al. Genetic structure and extinction of the woolly mammoth, Mammuthus primigenius. Curr Biol. 2007;17:1072–1075. doi: 10.1016/j.cub.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert MTP, et al. Whole-genome shotgun sequencing of mitochondria from ancient hair shafts. Science. 2007;317:1927–1930. doi: 10.1126/science.1146971. [DOI] [PubMed] [Google Scholar]
- 29.Debruyne R, et al. Out of America: Ancient DNA evidence for a New World origin of Late Quaternary woolly mammoths. Curr Biol. 2008;18:1320–1326. doi: 10.1016/j.cub.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 30.Leonard JA, et al. Animal DNA in PCR reagents plagues ancient DNA research. J Arch Sci. 2007;34:1361. [Google Scholar]
- 31.Rasmussen M, et al. Response to Comment by Goldberg, et al. on “DNA from pre-Clovis human coprolites in Oregon, North America”. Science. 2009;325:5937. doi: 10.1126/science.1167531. [DOI] [PubMed] [Google Scholar]
- 32.Harington CR. Annotated Bibliography of Quaternary Vertebrates of Northern North America. Toronto, Canada: Univ of Toronto Press; 2003. [Google Scholar]
- 33.Laxton NF, et al. Productivity of loessal grassland in the Kluane Lake region, Yukon Territory, and the Beringian “Productivity Paradox”. Arctic. 1996;49:129–140. [Google Scholar]
- 34.Vartanyan SL, et al. Collection of radiocarbon dates on the mammoths (Mammuthus primigenius) and other genera of Wrangel Island, northeast Siberia, Russia. Quat Res. 2008;70:51–59. [Google Scholar]
- 35.Steffensen JP, et al. High-resolution Greenland ice core data show abrupt climate change happens in a few years. Science. 2008;321:680–684. doi: 10.1126/science.1157707. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert MTP, et al. DNA from pre-Clovis human coprolites in Oregon, North America. Science. 2008;320:786–789. doi: 10.1126/science.1154116. [DOI] [PubMed] [Google Scholar]
- 37.Dillehay TD, et al. Monte Verde: Seaweed, food, medicine, and the peopling of South America. Science. 2008;320:784–786. doi: 10.1126/science.1156533. [DOI] [PubMed] [Google Scholar]
- 38.Nogués-Bravo D, et al. Climate change, humans, and the extinction of the woolly mammoth. PLoS Biol. 2008;6:e79. doi: 10.1371/journal.pbio.0060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willerslev E, Cooper A. Ancient DNA. Proc R Soc Lond Ser B. 2005;272:3–16. doi: 10.1098/rspb.2004.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willerslev E, et al. Diversity of Holocene life forms in fossil glacier ice. Proc Natl Acad Sci USA. 1999;96:8017–8021. doi: 10.1073/pnas.96.14.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofreiter M, et al. Molecular caving. Curr Biol. 2003;13:R693–R695. doi: 10.1016/j.cub.2003.08.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information