The Late Permian herbivore Suminia and the early evolution of arboreality in terrestrial vertebrate ecosystems (original) (raw)
Abstract
Vertebrates have repeatedly filled and partitioned the terrestrial ecosystem, and have been able to occupy new, previously unexplored habitats throughout their history on land. The arboreal ecospace is particularly important in vertebrate evolution because it provides new food resources and protection from large ground-dwelling predators. We investigated the skeletal anatomy of the Late Permian (approx. 260 Ma) herbivorous synapsid Suminia getmanovi and performed a morphometric analysis of the phalangeal proportions of a great variety of extant and extinct terrestrial and arboreal tetrapods to discern locomotor function and habitat preference in fossil taxa, with special reference to Suminia. The postcranial anatomy of Suminia provides the earliest skeletal evidence for prehensile abilities and arboreality in vertebrates, as indicated by its elongate limbs, intrinsic phalangeal proportions, a divergent first digit and potentially prehensile tail. The morphometric analysis further suggests a differentiation between grasping and clinging morphotypes among arboreal vertebrates, the former displaying elongated proximal phalanges and the latter showing an elongation of the penultimate phalanges. The fossil assemblage that includes Suminia demonstrates that arboreality and resource partitioning occurred shortly after the initial establishment of the modern type of terrestrial vertebrate ecosystems, with a large number of primary consumers and few top predators.
Keywords: Synapsida, Anomodontia, arboreality, terrestrial ecosystem, evolution
1. Introduction
Arboreal vertebrates are a major component of terrestrial ecosystems. Small and medium-sized vertebrates, among them several lineages of reptiles, including birds, and several lineages of synapsids, including mammals, have repeatedly and independently invaded this ecospace. Although adaptations to life in trees evolved through convergent evolution, most vertebrates share similar mechanisms, including grasping, clinging, hooking, arm swinging and adhering (Hildebrand & Goslow 2001). As a result, there are readily recognizable ecomorphological adaptations for similar arboreal behaviours. While some of these mechanisms are restricted to certain groups (e.g. arm swinging being limited to primates and adhering being unique to animals of very small body size), grasping is much more widespread and well known in extant primates, other placental mammals, marsupials, chameleons and perching birds (Hildebrand & Goslow 2001). Grasping and clinging can also be readily recognized in the fossil record because it has clear osteological correlates (e.g. Hopson 2001; Bloch & Boyer 2002; Luo et al. 2003).
New material of the basal anomodont Suminia getmanovi (Ivakhnenko 1994) that was recently excavated at the Upper Permian Kotel'nich locality in the Kirov region of Russia consists of more than a dozen mostly complete and articulated skeletons on a single large block (figure 1). The skeletal anatomy of Suminia displays numerous features that are associated with an arboreal lifestyle, documenting the earliest evidence for arboreality in the fossil record of vertebrates. Anomodontia is an extinct clade of non-mammalian synapsids from the Permian and Triassic, and possibly the Cretaceous (Thulborn & Turner 2003), representing the dominant herbivores of particularly Late Permian terrestrial vertebrate ecosystems (Hotton 1986; DiMichele et al. 1992). As a clade, anomodonts obtained a cosmopolitan distribution and, in terms of number of taxa, as well as variety of body forms, they are one of the most diverse groups of terrestrial tetrapods of their time (e.g. Fröbisch 2008, 2009). The new material of Suminia comprises an ontogenetic span ranging from mostly subadult (femur length 59.20 mm) to adult (femur length 80.87 mm) individuals, providing one of the most complete pictures of the postcranial anatomy of any Palaeozoic synapsid. The well-preserved skeletons show no evidence of weathering, predation or scavenging, indicating rapid burial, possibly as a result of a catastrophic event. Discrete disarticulation of some skeletons suggests that minor transportation might also have occurred.
Figure 1.
Suminia getmanovi, large block with articulated skeletons. (a) Photograph and (b) colour-coded outline drawing of PIN 2212/116, showing the presence of at least 15 specimens.
The current study presents a preliminary account on the postcranial anatomy of the basal anomodont S. getmanovi, which will be described in more detail elsewhere. This study further investigates the variety of phalangeal proportions in extant as well as extinct tetrapods and aims to enable the identification of locomotor behaviour in fossil tetrapods, with special reference to Suminia, based on the phalangeal proportions. Moreover, the vertebrate fauna of the Kotel'nich locality represents an excellent example of Late Permian terrestrial vertebrate communities and provides new insights into the evolution of arboreality in terrestrial vertebrate ecosystems.
2. Material and methods
The length of the metacarpal, proximal phalanx and penultimate phalanx of the third digit was measured in selected fossil synapsids, as well as in arboreal and non-arboreal members of a great variety of extant tetrapods, using digital callipers (table S1 in the electronic supplementary material). Individuals were measured only once. The sampled fossil synapsids include members of every major clade of basal synapsids and non-mammalian therapsids. Measurements of extant tetrapods were taken from chamaeleonids, agamids and iguanids among reptiles, and several groups of mammals, including Primates, Dermoptera, Chiroptera and didelphimorphian Marsupialia. In addition, data for diprotodontian marsupials, rodents and selected primates and lacertids were obtained from the literature (Arnold 1998; Hamrick 2001; Weisbecker & Warton 2006; Weisbecker & Schmid 2007). All measurements were plotted on ternary diagrams using the software package PAST v. 1.78 (Hammer et al. 2001).
3. Results
(a). Discrete evidence for an arboreal lifestyle in Suminia
The newly available material of the small anomondont Suminia (body length approx. 50 cm), known for its unique dentition (Rybczynski & Reisz 2001), reveals numerous autapomorphic features in its postcranium, most of which are associated with an arboreal lifestyle. These include an elongated neck with unusually broad cervical vertebrae, a long tail, a slender, tall scapular blade and elongated limbs (figures 1 and 2). In particular, the proportions of the manus are unusual, measuring 40 per cent of the length of the entire forelimb. Additional autapomorphic characters are displayed in the manus and pes, and comprise an enlarged, phalangiform distal carpal 1 and tarsal 1, elongated penultimate phalangeal elements, as well as a plesiomorphic phalangeal formula for amniotes: 2-3-4-5-3 in the hand (manus) and 2-3-4-5-4 in the foot (pes). Moreover, the penultimate phalanges possess a robust proximal end, which becomes progressively more slender towards the distal end of the element. A round distal articular surface for the terminal (ungual) phalanx, not seen in other Palaeozoic vertebrates, permits a greater mobility of the terminal phalanx. The terminal phalanges of Suminia are strongly curved and laterally compressed, resulting in a claw-like morphology, which is known to be indicative of clinging abilities in arboreal tetrapods (e.g. Feduccia 1993). These features are particularly conspicuous when compared with other Palaeozoic anomodonts (figure 3a,c). Overall, the skeleton of S. getmanovi is characterized by its distinct slenderness and a flexible vertebral column, indicated by the elongated cervical region and the lack of fusion between the vertebral centra and neural arches in the dorsal region. The latter feature does not appear to be related to body size and thus ontogenetic age, as some large individuals (e.g. specimens 2 and 3 on the block, Paleontological Institute, Russian Academy of Science, Moscow, Russia—PIN 2212/116) show this lack of fusion, while other smaller individuals display well co-ossified centra and neural arches in this region (e.g. specimen 1 of PIN 2212/116 and Kotel'nich Paleontological Museum, Kotel'nich, Russia—KPM 173).
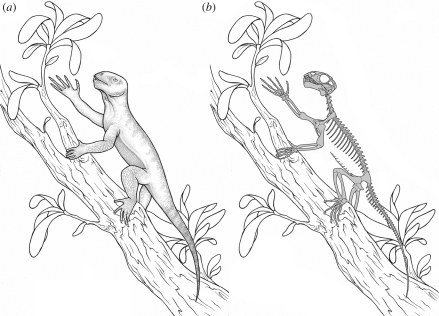
Figure 2.
Reconstruction of the basal anomodont Suminia getmanovi. (a) Flesh and (b) skeletal reconstruction.
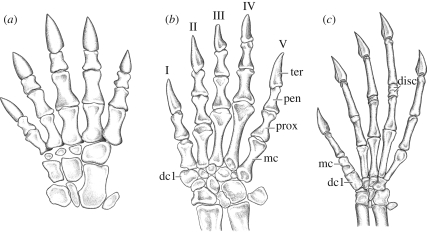
Figure 3.
Reconstructions of the manus in anomodonts. (a) The dicynodont anomodont Robertia (terrestrial, redrawn from Hopson 1995), and the basal anomodonts (b) Galechirus (terrestrial) and (c) Suminia (inferred arboreal, morphotype (ii)). dc, distal carpal; disc, disc-like phalangeal element; mc, metcarpal; pen, penultimate phalanx; prox, proximal phalanx; ter, terminal phalanx. Not to scale.
The most striking features of the postcranial skeleton of Suminia are its extremely elongated penultimate phalanges on the large hand and long forelimb, indicating an arboreal lifestyle (see below). There are two ways in which strong elongation of the penultimate phalanx is achieved in Suminia: the obvious increase in length, and the addition of phalangeal elements to the penultimate phalanx in digits III and IV, and digit V in the pes. The latter is unexpected because reduction of the phalangeal count from the primitive amniote condition of 2-3-4-5-3 in the manus and 2-3-4-5-4 in the pes to the mammalian phalangeal formula of 2-3-3-3-3 in manus and pes is a general, well-documented evolutionary trend within non-mammalian synapsids (Hopson 1995). This reduction is achieved by a loss in both manus and pes of one or more elements, preceded by a shortening of the respective elements to disc-like bones. None of the known anomodonts show the primitive condition but rather the derived mammalian phalangeal count, including the basal taxa Patranomodon, Galeops and Eodicynodon (Brinkman 1981; Rubidge et al. 1994; Rubidge & Hopson 1996). Only Suminia shows the intermediate condition of having one disc-like phalangeal element in digit III, two in digit IV and one in the pedal digit V. Optimization of this character on current cladograms of anomodonts results in divergent patterns. Previous phylogenetic analyses suggest that the condition in Suminia represents an evolutionary reversal (e.g. Fröbisch 2007), whereas a recently published phylogeny of basal anomodonts implies a more basal position of Suminia than Patranomodon and Galeops (Liu et al. in press), which would explain the phalangeal formula in Suminia as intermediate condition. It is these disc-shaped elements that contribute further to the elongation by being tightly attached to the penultimate phalanx in subadults, and co-ossified in the manus of the largest known specimens. Thus, the fusion of these elements combined with the elongated penultimate phalanges has provided a means to greatly enlarge the phalangeal proportion of the autopodium of Suminia.
Additional and diagnostic features for arboreality, unseen in any other Palaeozoic vertebrate, include the widely divergent first digit with an angle of approximately 30–40° to the remaining digits of the manus and pes (figures 2 and 3). Distal carpal 1 in the manus and tarsal 1 in the pes are enlarged and phalangiform, and metapodial I is massive and short, and does not contact the other metapodials. The articular surfaces of the carpal and tarsal of the first digit indicate an ability to flex ventrally as well as to abduct and adduct relative to the rest of the manus and pes. This indicates that the first digit may have been used as an opposable, proximally robust ‘thumb’. At the same time, metapodials II–V have very narrow proximal ends that contact each other, forming a very tight complex that allows for little more than flexion and extension along the distal edge of the carpus and tarsus.
A further potential specialization for arboreality is the modified tail. Although Suminia shows the plesiomorphic condition of having a very long tail, at least 120 per cent of the precaudal region, the proximal 22 caudal vertebrae bear prominent transverse processes that are fused to comparably long caudal ribs, and the spinous processes retain much of the length of those in the posterior dorsal region. Among anomodonts, these features are seen only in Suminia. These characteristics indicate a relative expansion of the anterior region of the tail and are probably related to a hypertrophy of the musculoskeletal system along the proximal part of the tail in order to enable balancing and potentially prehensile abilities in this small synapsid (German 1982; Jenkins & Krause 1983; Rose 1987; Youlatos 2003).
(b). Morphometric evidence for an arboreal lifestyle in Suminia
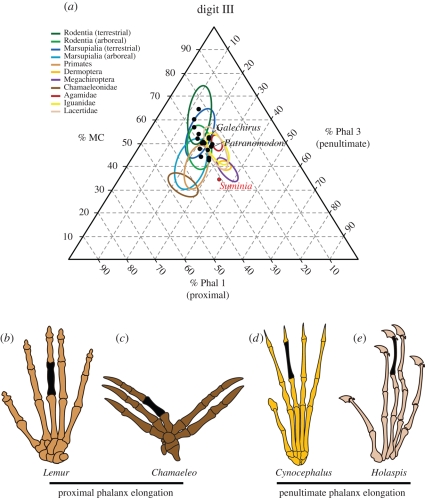
Phalangeal proportions have previously been used to describe and infer an arboreal lifestyle in other tetrapods, including various mammals (Van Valkenburgh 1985; Lemelin 1999; Hamrick 2001; Bloch & Boyer 2002; Luo et al. 2003; Weisbecker & Warton 2006; Weisbecker & Schmid 2007; Kirk et al. 2008), lizards (Arnold 1998), pterosaurs (Clark et al. 1998) and birds (Hopson 2001). We investigated the phalangeal proportions in the manus of extinct non-mammalian synapsids and various clades of extant tetrapods, including members of reptilian and synapsid amniotes, in a comparative morphometric analysis (figure 4; table S1 in the electronic supplementary material). The results strongly support a general trend in arboreal tetrapods towards an increased phalangeal index, which is the combined length of the proximal and penultimate phalanges as a percentage of their respective metapodial element. Thus, a typical terrestrial tetrapod is characterized by a long metapodium and short phalanges, whereas a typical arboreal tetrapod shows a short metapodium and long phalanges. In addition, we recognized for the first time two distinct patterns of skeletal specialization within arboreal tetrapods: (i) an elongate proximal phalanx and (ii) an elongate penultimate phalanx. The first morphotype (i) is typically found in primates, including ‘plesiadapiforms’ (Hamrick 2001; Bloch & Boyer 2002; Kirk et al. 2008), and other mammals, including diprotodontian marsupials (e.g. possums, kangaroos, wallabies, wombats; Weisbecker & Warton 2006) and didelphid marsupials (opossums; Lemelin 1999; Luo et al. 2003), carnivorans (Van Valkenburgh 1985) and rodents (Weisbecker & Schmid 2007), as well as chameleons (J. Fröbisch 2008, personal observation), and probably represents grasping adaptations using opposable digits. The second morphotype (ii) is known from dermopterans (colugos or ‘flying lemurs’; Hamrick 2001; Luo et al. 2003) and megachiropterans (megabats; Hamrick 2001) among mammals, as well as iguanid and lacertid lizards (J. Fröbisch 2008, personal observation; Arnold 1998), and the pes of pterosaurs (Clark et al. 1998) and birds (Hopson 2001), and appears to be related to clinging behaviour. This latter morphotype (ii) is also clearly developed in the basal therapsid Suminia, demonstrating the early evolution of this ecomorphological adaptation for clinging in tetrapods. The position of Suminia in the ternary diagram (figure 4) reflects similar phalangeal proportions to extant tetrapods that display not only an arboreal lifestyle but are further capable of gliding (e.g. Dermoptera, Megachiroptera and the lacertid lizard Holaspis). However, the skeletal anatomy of Suminia shows no further indication that this taxon was a glider, and is therefore conservatively considered to be arboreal only. In addition, Boyer & Bloch (2008) recently suggested that the elongation of penultimate phalanges in dermopterans, megachiropterans and sloths indicates suspensory behaviour rather than gliding adaptations, as has previously been proposed for dermopterans (Beard 1990). While this potentially holds true for mammals, this observation is not supported in reptilian tetrapods with elongated penultimate phalanges. Instead, the presence of such distinct phalangeal proportions as seen in Suminia seem to be associated with large and strongly recurved, claw-shaped terminal phalanges, indicating effective clinging behaviour such as is observable in modern lizards and birds (Arnold 1998; Hopson 2001).
Figure 4.
Digital proportions of selected extinct and extant tetrapods. (a) Ternary diagram depicting the digital proportions of digit III (MC, metacarpal; Phal 1, phalanx 1; Phal 3, phalanx 3) in selected extinct fossil synapsids and a variety of extant tetrapods. Data points of fossil synapsids are illustrated as black dots, including the basal anomodonts Galechirus and Patranomodon, with Suminia indicated in red. Various clades of extant tetrapods are shown as coloured ellipses. Note the distinct reduction of the metacarpal length in all arboreal taxa, and the clear distinction between the two morphotypes described with an elongated proximal phalanx ((i), bottom left) on the one hand and an elongated penultimate phalanx ((ii), bottom right) on the other hand (see text for details). Reconstructions of the manus in (b) the primate Lemur (arboreal, morphotype (i), after Böker 1935), (c) the chamaeleonid Chamaeleo (arboreal, morphotype (i), after Romer 1956), (d) the dermopteran Cynocephalus (arboreal, morphotype (ii), after Bloch et al. 2007) and (e) the lacertid Holaspis (arboreal, morphotype (ii), after Arnold 1998). Not to scale.
4. Discussion
Arboreal vertebrates are known from all major clades of terrestrial tetrapods, including several lineages among amphibians, reptiles (comprising birds) and mammals. As a result of parallel evolution, arboreal vertebrates independently evolved similar mechanisms as adaptations to an arboreal lifestyle, including grasping, clinging, hooking, arm swinging and adhering (Hildebrand & Goslow 2001). Very small vertebrates (less than 10 cm body length) primarily use adhesive mechanisms, such as capillary adhesion in tree-climbing frogs and salamanders versus dry adhesion in arboreal lizards, such as geckos, some anoline iguanids and skinks (Cartmill 1985). In contrast, larger vertebrates and in particular mammalian synapsids exploit rather different mechanisms, including skin friction, hooking, clinging and prehensile extremities for grasping. Among them, grasping is one of the most prominent mechanisms that is also clearly recognizable in the fossil record (e.g. Hopson 2001; Bloch & Boyer 2002; Luo et al. 2003). While prehensile tails are characterized by a number of characteristics (German 1982; Youlatos 2003), autopodial specializations are expressed in either one or two digits being markedly divergent from the remaining digits in the manus and pes. Such a pattern is well known in extant primates and other placental mammals, as well as marsupials, chameleons, certain tree frogs and perching birds (Cartmill 1985; Hildebrand & Goslow 2001). Moreover, arboreal vertebrates with grasping hands and feet display distinct phalangeal proportions when compared with non-arboreal relatives (Hopson 2001; Bloch & Boyer 2002; Weisbecker & Warton 2006; Weisbecker & Schmid 2007).
Arboreal vertebrates are frequently known from the fossil record and include taxa from the reptilian as well as synapsid lineages of amniotes. Among synapsids, prominent examples of inferred arboreal taxa comprise the Late Jurassic crown-group mammal Henkelotherium (Krebs 1991), selected Early Cretaceous crown-group mammals (e.g. Eomaia and Sinodelphis; Ji et al. 2002; Luo et al. 2003), members of the extinct mammalian clades Multituberculata and Apatemyidae (Jenkins & Krause 1983; Koenigswald & Schierning 1987), as well as fossil euprimates and ‘plesiadapiforms’ (Hamrick 2001; Bloch & Boyer 2002; Kirk et al. 2008). Among reptiles, specializations for an arboreal lifestyle have been described for a number of taxa, including the Triassic drepanosaurids (e.g. Megalancosaurus; Renesto 1994), the Late Triassic archosauromorph Trilophosaurus (Spielmann et al. 2005), at least selected members of pterosaurs (Wang et al. 2008), the Middle Triassic lepidosauromorph Megachirella (Renesto & Posenato 2003), the Early Cretaceous lepidosaur Scandensia (Evans & Barbadillo 1998) and the Early Eocene gecko Yantarogekko (Bauer et al. 2005). In addition, an arboreal lifestyle of primitive birds and closely related non-avian theropods has been extensively debated regarding the origin of avian flight (‘ground up’ versus ‘trees down’; for a critical review see Hutchinson & Allen 2009).
The combination of the morphologically distinct characters (discrete and morphometric) of Suminia and its small body size indicates that Suminia was an arboreal animal capable of using its manus and pes for grasping and clinging, thus representing the oldest evidence for arboreality in the vertebrate fossil record. This shows that the basal anomodont Suminia independently evolved grasping and clinging abilities long before (approx. 30 Ma) the evolution of these characters in any other tetrapod. In addition, the arboreality of Suminia further documents a broader pattern in synapsid evolution in which the same ecological role was iteratively occupied by successive bursts of synapsid diversification in non-mammalian and later mammalian therapsids (see Luo 2007). The interpretation of the lifestyle of Suminia as an arboreal animal is consistent with its highly specialized feeding behaviour, involving oral processing of high-fibre plant materials such as leaves and fine stems (Rybczynski & Reisz 2001).
The morphometric analysis presented in this study supports a general differentiation between grasping and clinging arboreal tetrapods, with the former showing relatively longer proximal than penultimate phalanges and the latter having elongated penultimate phalangeal elements, when compared with their terrestrial relatives. Thereby, Suminia displays the phalangeal proportions representative of the clinging morphotype. This results in the unique combination of morphological features in Suminia, showing clinging adaptations on the basis of phalangeal proportions and, in addition, prehensile abilities as a result of the divergent first digits in the manus and pes, as well as the likely prehensile tail.
Late Permian terrestrial vertebrate ecosystems provide the earliest evidence for a modern pattern of trophic interactions, with large numbers of herbivores supporting a relatively small number of top predators (Olson 1966; DiMichele et al. 1992; Sues & Reisz 1998). The Kotel'nich locality has yielded a rich, diverse assemblage of vertebrates, representing a prime example of Late Permian terrestrial vertebrate communities. The evidence for this assemblage has been accumulated through a systematic programme of excavations spanning two decades that has yielded more than 350 articulated and partially articulated skeletons. This large fauna is dominated numerically by herbivores, comprising approximately 83 per cent of the recorded specimens, and small numbers of insectivores (approx. 4%), as well as small (approx. 9%) and large (approx. 4%) carnivores (table S2 in the electronic supplementary material). Among herbivores, large-bodied vertebrates are represented by numerous articulated skeletons of dicynodont anomodonts and pareiasaurian reptiles (approx. 79%), as well as the small-bodied basal anomodont Suminia (approx. 21%). Although no stomach contents have been recovered yet, the sediments that have yielded the large block with the Suminia skeletons also contain several coprolites with extensive leaf fragments. The size of these coprolites (less than 10 mm) is consistent in their association with Suminia. The macroflora and palynology of the Kotel'nich locality is well known (Gomankov 1997; Gomankov et al. 1998), and in situ preservation of roots indicates the presence of large tree-like plants that exceeded 2.5 m in height, much taller than any contemporaneous ground-dwelling herbivores.
The highly diverse flora and fauna at this locality provides the first evidence of food partitioning between small arboreal and much larger ground-dwelling herbivores shortly after the establishment of the trophic structure of modern terrestrial ecosystems, with large numbers of primary consumers supporting a few top predators (DiMichele et al. 1992; Sues & Reisz 1998).
Acknowledgements
We thank K. D. Angielczyk, D. C. Evans, N. B. Fröbisch, J. J. Head, J. Hopson, H. C. E. Larsson and the Reisz (Toronto) and Larsson (Montreal) research laboratories for discussions. The manuscript benefited from reviews by Z.-X. Luo and an anonymous reviewer. M. T. Carrano, S. Kaal, M. Kearney, B. D. Patterson, M. Raath, O. Rieppel and K. Seymour provided access to collections under their care and are thanked at this point. We express thanks to D. Scott for photography, N. Wong Ken and C. Stoppa for selected illustrations in figures 2–4, and I. Novikov for the loan of PIN 2212/116. A Preparator's Grant from the Society of Vertebrate Paleontology to A. Khlupin, O. Patapova and K. Grekhov supported part of the preparation of PIN 2212/116. This work was supported by grants from the Government of Canada Awards Program (Full Scholarship), the German Academic Exchange Service (DAAD-Doktorandenstipendium), the University of Toronto, the Field Museum (Visiting Scholarship) and the Deutsche Forschungsgemeinschaft (FR 2457/3-1) to J.F., and from the Natural Sciences and Engineering Research Council of Canada and National Geographic Society to R.R.R.
References
- Arnold E. N.1998Structural niche, limb morphology and locomotion in lacertid lizards (Squamata, Lacertilia); a preliminary survey. Bull. Nat. Hist. Mus. London (Zool.) 64, 63–89 [Google Scholar]
- Bauer A. M., Böhme W., Weitschat W.2005An Early Eocene gecko from Baltic amber and its implications for the evolution of gecko adhesion. J. Zool. 265, 327–332 (doi:10.1017/s0952836904006259) [Google Scholar]
- Beard K. C.1990Gliding behavior and paleoecology of the alleged primate family Paromomyidae (Mammalia, Dermoptera). Nature 345, 340–341 (doi:10.1038/345340a0) [Google Scholar]
- Bloch J. I., Boyer D. M.2002Grasping primate origins. Science 298, 1606–1610 (doi:10.1126/science.1078249) [DOI] [PubMed] [Google Scholar]
- Bloch J. I., Silcox M. T., Boyer D. M., Sargis E. J.2007New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc. Natl Acad. Sci. USA 104, 1159–1164 (doi:10.1073/pnas.0610579104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böker H.1935Einführung in die vergleichende biologische Anatomie der Wirbeltiere. Erster Band Jena: Gustav Fischer [Google Scholar]
- Boyer D. M., Bloch J. I.2008Evaluating the mitten-gliding hypothesis for Paromomyidae and Micromomyidae (Mammalia, ‘Plesiadapiformes’) using comparative functional morphology of new Paleogene skeletons. In Mammalian evolutionary morphology: a tribute to Frederick S. Szalay (eds Sargis E. J., Dagosto M.), pp. 233–284 Dordrecht, The Netherlands: Springer [Google Scholar]
- Brinkman D.1981The structure and relationships of the dromasaurs (Reptilia: Therapsida). Breviora 465, 1–34 [Google Scholar]
- Cartmill M.1985Climbing. In Functional vertebrate morphology (eds Hildebrand M., Bramble D. M., Liem K. F., Wake D. B.), pp. 73–88 Cambridge, MA: Harvard University Press [Google Scholar]
- Clark J. M., Hopson J. A., Hernandez R., Fastovsky D. E., Montellano M.1998Foot posture in a primitive pterosaur. Nature 391, 886–889 (doi:10.1038/36092) [Google Scholar]
- DiMichele W. A., et al. 1992Paleozoic terrestrial ecosystems. In Terrestrial ecosystems through time–evolutionary paleoecology of terrestrial plants and animals (eds Behrensmeyer A. K., Damuth J. D., DiMichele W. A., Potts R., Sues H. D., Wing S. L.), pp. 205–325 Chicago, IL: University of Chicago Press [Google Scholar]
- Evans S. E., Barbadillo L. J.1998An unusual lizard (Reptilia: Squamata) from the Early Cretaceous of Las Hoyas, Spain. Zool. J. Linn. Soc. 124, 235–265 (doi:10.1111/j.1096-3642.1998.tb00576.x) [Google Scholar]
- Feduccia A.1993Evidence from claw geometry indicating arboreal habits of Archaeopteryx. Science 259, 790–793 (doi:10.1126/science.259.5096.790) [DOI] [PubMed] [Google Scholar]
- Fröbisch J.2007The cranial anatomy of Kombuisia frerensis Hotton (Synapsida, Dicynodontia) and a new phylogeny of anomodont therapsids. Zool. J. Linn. Soc. 150, 117–144 (doi:10.1111/j.1096-3642.2007.00285.x) [Google Scholar]
- Fröbisch J.2008Global taxonomic diversity of anomodonts (Tetrapoda, Therapsida) and the terrestrial rock record across the Permian-Triassic boundary. PLoS One 3, e3733 (doi:10.1371/journal.pone.0003733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröbisch J.2009Composition and similarity of global anomodont-bearing tetrapod faunas. Earth-Sci. Rev. 95, 119–157 (doi:10.1016/j.earscirev.2009.04.001) [Google Scholar]
- German R. Z.1982The functional-morphology of caudal vertebrae in new world monkeys. Am. J. Phys. Anthropol. 58, 453–459 (doi:10.1002/ajpa.1330580414) [DOI] [PubMed] [Google Scholar]
- Gomankov A. V.1997The Permian (Tatarian) flora from the Kotel'nich vertebrate locality (Kirov oblast). Strat. Geol. Correlation 5, 309–318 [Google Scholar]
- Gomankov A. V., Balme B. E., Foster C. B.1998Tatarian palynology of the Russian platform: a review. Proc. R. Soc. Victoria 110, 115–135 [Google Scholar]
- Hammer Ø., Harper D. A. T., Ryan P. D.2001PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9 [Google Scholar]
- Hamrick M. W.2001Primate origins: evolutionary change in digital ray patterning and segmentation. J. Hum. Evol. 40, 339–351 (doi:10.1006/jhev.2001.0467) [DOI] [PubMed] [Google Scholar]
- Hildebrand M., Goslow G. E. J.2001Analysis of vertebrate structure, 5th edn.New York, NY: John Wiley & Sons [Google Scholar]
- Hopson J. A.1995Patterns of evolution in the manus and pes of non-mammalian therapsids. J. Vertebr. Paleontol. 15, 615–639 [Google Scholar]
- Hopson J. A.2001Ecomorphology of avian and nonavian theropod phalangeal proportions: implications for the arboreal versus terrestrial origin of bird flight. In New perspectives on the origin and early evolution of birds. Proc. Int. Symp. in Honor of John H. Ostrom, New Haven, CT, 13–14 February 1999 (eds Gauthier J. A., Gall J. F.), pp. 211–235 New Haven, CT: Peabody Museum of Natural History, Yale University [Google Scholar]
- Hotton N.1986Dicynodonts and their role as primary consumers. In The ecology and biology of mammal-like reptiles (eds Hotton N., McLean P. D., Roth J. J., Roth E. C.), pp. 71–82 Washington, DC: Smithsonian Institution Press [Google Scholar]
- Hutchinson J. R., Allen V.2009The evolutionary continuum of limb function from early theropods to birds. Naturwissenschaften 96, 423–448 (doi:10.1007/s00114–008–0488–3) [DOI] [PubMed] [Google Scholar]
- Ivakhnenko M. F.1994A new Late Permian dromasaurian (Anomodontia) from Eastern Europe. Paleontol. J. 28, 96–103 [Google Scholar]
- Jenkins F. A. J., Krause D. W.1983Adaptations for climbing in North American multituberculates (Mammalia). Science 220, 712–715 (doi:10.1126/science.220.4598.712) [DOI] [PubMed] [Google Scholar]
- Ji Q., Luo Z.-X., Yuan C.-X., Wible J. R., Zhang J.-P., Georgi J. A.2002The earliest known Eutherian mammal. Nature 416, 816–822 (doi:10.1038/416816a) [DOI] [PubMed] [Google Scholar]
- Kirk E. C., Lemelin P., Hamrick M. W., Boyer D. M., Bloch J. I.2008Intrinsic hand proportions of euarchontans and other mammals: implications for the locomotor behavior of plesiadapiforms. J. Hum. Evol. 55, 278–299 (doi:10.1016/j.jhevol.2008.02.008) [DOI] [PubMed] [Google Scholar]
- Koenigswald W. v., Schierning H.-P.1987The ecological niche of an extinct group of mammals, the early Tertiary apatemyids. Nature 326, 595–597 (doi:10.1038/326595a0) [Google Scholar]
- Krebs B.1991Das Skelett von Henkelotherium guimarotae gen. et sp. nov. (Eupantotheria, Mammalia) aus dem Oberen Jura von Portugal. Berliner geowissenschaftl. Abh. A 133, 1–110 [Google Scholar]
- Lemelin P.1999Morphological correlates of substrate use in didelphid marsupials: implications for primate origins. J. Zool. 247, 165–175 (doi:10.1111/j.1469-7998.1999.tb00980.x) [Google Scholar]
- Liu J., Rubidge B., Li J.In press A new specimen of Biseridens qilianicus indicates its phylogenetic position as the most basal anomodont. Proc. R. Soc. B (doi:10.1098/rspb.2009.0883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.-X.2007Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 (doi:10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- Luo Z.-X., Ji Q., Wible J. R., Yuan C.2003An Early Cretaceous tribosphenic mammal and metatherian evolution. Science 302, 1934–1940 (doi:10.1126/science.1090718) [DOI] [PubMed] [Google Scholar]
- Olson E. C.1966Community evolution and the origin of mammals. Ecology 47, 291–302 (doi:10.2307/1933776) [Google Scholar]
- Renesto S.1994_Megalancosaurus_, a possibly arboreal archosauromorph (Reptilia) from the Upper Triassic of Northern Italy. J. Vertebr. Paleontol. 14, 38–52 [Google Scholar]
- Renesto S., Posenato R.2003A new lipodosauromorph reptile from the Middle Triassic of the Dolomites (Northern Italy). Riv. Ital. Paleontol. Strat. 109, 463–474 [Google Scholar]
- Romer A. S.1956. In Osteology of the reptiles Chicago, IL: University of Chicago Press [Google Scholar]
- Rose K. D.1987Climbing adaptations in the Early Eocene mammal Chriacus and the origin of Artiodactyla. Science 236, 314–316 (doi:10.1126/science.3426662) [DOI] [PubMed] [Google Scholar]
- Rubidge B. S., Hopson J. A.1996A primitive anomodont therapsid from the base of the Beaufort Group (Upper Permian) of South Africa. Zool. J. Linn. Soc. 117, 115–139 (doi:10.1111/j.1096-3642.1996.tb02152.x) [Google Scholar]
- Rubidge B. S., King G. M., Hancox P. J.1994The postcranial skeleton of the earliest dicynodont synapsid Eodicynodon from the Upper Permian of South Africa. Palaeontology 37, 397–408 [Google Scholar]
- Rybczynski N., Reisz R. R.2001Earliest evidence for efficient oral processing in a terrestrial herbivore. Nature 411, 684–687 (doi:10.1038/35079567) [DOI] [PubMed] [Google Scholar]
- Spielmann J. A., Heckert A. B., Lucas S. G.2005The Late Triassic archosauromorph Trilophosaurus as an arboreal climber. Riv. Ital. Paleontol. Strat. 111, 395–412 [Google Scholar]
- Sues H.-D., Reisz R. R.1998Origins and early evolution of herbivory in tetrapods. Trends Ecol. Evol. 13, 141–145 (doi:10.1016/S0169-5347(97)01257-3) [DOI] [PubMed] [Google Scholar]
- Thulborn T., Turner S.2003The last dicynodont: an Australian Cretaceous relict. Proc. R. Soc. Lond. B 270, 985–993 (doi:10.1098/rspb.2002.2296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valkenburgh B.1985Locomotor diversity within past and present guilds of large predatory mammals. Paleobiology 11, 406–428 [Google Scholar]
- Wang X. L., Kellner A. W. A., Zhou Z. H., Campos D. D.2008Discovery of a rare arboreal forest-dwelling flying reptile (Pterosauria, Pterodactyloidea) from China. Proc. Natl Acad. Sci. USA 105, 1983–1987 (doi:10.1073/p.nas.0707728105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbecker V., Schmid S.2007Autopodial skeletal diversity in hystricognath rodents: functional and phylogenetic aspects. Mamm. Biol. 72, 27–44 (doi:10.1016/j.mambio.2006.03.005) [Google Scholar]
- Weisbecker V., Warton D. I.2006Evidence at hand: diversity, functional implications, and locomotor prediction in intrinsic hand proportions of diprotodontian marsupials. J. Morphol. 267, 1469–1485 (doi:10.1002/jmor.10495) [DOI] [PubMed] [Google Scholar]
- Youlatos D.2003Osteological correlates of tail prehensility in carnivorans. J. Zool. 259, 423–430 (doi:10.1017/S0952836903003431) [Google Scholar]