Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study (original) (raw)
Abstract
Objectives To evaluate the association between a simple lifestyle index based on the recommendations for five lifestyle factors and the incidence of colorectal cancer, and to estimate the proportion of colorectal cancer cases attributable to lack of adherence to the recommendations.
Design Prospective cohort study.
Setting General population of Copenhagen and Aarhus, Denmark.
Participants 55 487 men and women aged 50-64 years at baseline (1993-7), not previously diagnosed with cancer.
Main outcome measure Risk of colorectal cancer in relation to points achieved in the lifestyle index (based on physical activity, waist circumference, smoking, alcohol intake, and diet (dietary fibre, energy percentage from fat, red and processed meat, and fruits and vegetables)) modelled through Cox regression.
Results During a median follow-up of 9.9 years, 678 men and women had colorectal cancer diagnosed. After adjustment for potential confounders, each additional point achieved on the lifestyle index, corresponding to one additional recommendation that was met, was associated with a lower risk of colorectal cancer (incidence rate ratio 0.89 (95% confidence interval 0.82 to 0.96). In this population an estimated total of 13% (95% CI 4% to 22%) of the colorectal cancer cases were attributable to lack of adherence to merely one additional recommendation among all participants except the healthiest. If all participants had followed the five recommendations 23% (9% to 37%) of the colorectal cancer cases might have been prevented. Results were similar for colon and rectal cancer, but only statistically significant for colon cancer.
Conclusions Adherence to the recommendations for physical activity, waist circumference, smoking, alcohol intake, and diet may reduce colorectal cancer risk considerably, and in this population 23% of the cases might be attributable to lack of adherence to the five lifestyle recommendations. The simple structure of the lifestyle index facilitates its use in public health practice.
Introduction
Colorectal cancer is predominantly a disease of Westernised countries, indicating that components of a Western lifestyle may contribute to risk.1 In Denmark, colorectal cancer was the second most commonly occurring cancer in men and women in 2006.2 A huge body of evidence has implicated modifiable lifestyle factors,3 including smoking,4 5 physical activity,6 7 body composition,8 9 10 alcohol intake,11 12 and diet,13 14 15 16 17 in the causes of colorectal cancer. However, no single component seems likely to explain the large international variation in colorectal cancer incidence.18
Studies have shown the potential of healthy lifestyle factors combined to lower mortality and risk of chronic disease.19 20 21 22 23 24 25 26 From a public health perspective, a simple lifestyle assessment could readily be applied to motivate the population to modify lifestyles. A lifestyle index based on national and international, achievable public health recommendations would be a practical tool for counselling people on the effect of living in accordance with the recommendations in relation to the risk of certain diseases.
Until now, only one study has, in a sub-analysis, investigated generally good health behaviours in relation to colon cancer among men. In this population, the authors reported that 71% of the colon cancer cases could have been avoided if everyone had been unexposed to six risk factors, which would have been consistent with generally good health behaviours.27
The objective of the present study was to examine the association between a lifestyle index based on recommendations for physical activity, smoking, alcohol intake, waist circumference, and diet combined and risk of colorectal cancer in middle-aged Danish men and women, and, subsequently, to estimate the proportion of colorectal cancer cases that might be associated with lack of adherence to the recommendations. Our hypothesis was that extra points achieved in the lifestyle index were associated with a lower risk of colorectal cancer.
Methods
Study population
In 1993-7 a total of 160 725 Danish men and women living in Copenhagen and Aarhus were invited to participate in the Diet, Cancer and Health Cohort Study.28 They were identified from the computerised records of the Civil Registration System in Denmark to get population based participation. The criteria for invitation were age between 50 and 64 years, born in Denmark, and no diagnosis of cancer registered in the Danish Cancer Registry. A total of 57 053 (35%) accepted the invitation.
All participants filled in a lifestyle questionnaire including questions about social factors, health status, reproductive factors, and lifestyle habits, as well as a 192 item semi-quantitative food frequency questionnaire developed to assess the average intake of foods over the past 12 months. Both questionnaires were self administered and checked by an interviewer, and the food frequency questionnaire was validated.29 30 31 Professional staff members carried out anthropometrical measurements.
Exclusions
Of the 57 053 men and women enrolled into the study, 569 were excluded because of a recently recorded cancer diagnosis that had not been registered in the Danish Cancer Registry at the time of the invitation, and a further 37 were excluded because they did not fill in the lifestyle questionnaire. All participants with missing information on variables considered in the analyses where also excluded—a total of 960 men and women with missing data (343 for physical activity, 54 for diet, 23 for smoking, 44 for waist circumference, 26 for school education, 96 for first degree family history of cancer, 346 for use of non-steroidal anti-inflammatory drugs, and 26 women for use of hormone replacement therapy). Thus, 55 487 men and women were included in our analysis.
Assessment of modifiable lifestyle factors
Information on physical activity was based on validated questions covering the average number of hours per week spent in the past year on leisure time physical activity (sports, cycling, walking), and one question referred to level of occupational physical activity.32 33 Information on smoking and alcohol intake was based on questions covering current smoking status (smoker or non-smoker) and alcohol drinking patterns (number of drinks a week). At baseline all participants had waist circumference measured at the study centre by trained professionals.
Dietary information was obtained by a 192 item semi-quantitative food frequency questionnaire, and consumption was assessed in 12 categories of predefined responses, ranging from “never” to “eight or more times per day.” Daily intakes of foods and nutrients were calculated for each participant by means of the software program FoodCalc (www.ibt.ku.dk/jesper/foodcalc/) using population-specific standardised recipes and portion sizes. We used information on intake of fruit and vegetables, red and processed meat, dietary fibre, and energy percentage from fat.
Definition of lifestyle index
We generated a healthy lifestyle index based on a priori knowledge of risk factors for colorectal cancer and current national and international public health recommendations. The international recommendations were from the World Health Organization, World Cancer Research Fund, and the Nordic Nutrition Recommendations. Participants scored one point for each of the following recommendations they met at baseline: not smoking,34 physically active at least 30 minutes a day35 or had a job with light manual activity (such as postal delivery) or heavy manual activity (such as forestry), alcohol intake ≤7 drinks/week for women and ≤14 drinks/week for men,36 37 waist circumference <88 cm for women and <102 cm for men,37 38 and consumed a healthy diet. The fifth lifestyle factor, diet, was based on a dietary index including four dietary recommendations: ≥600 g fruit and vegetables a day,39 ≤500 g of red and processed meat a week,36 ≥3 g dietary fibre per MJ of dietary energy,37 and ≤30% of the total energy from fat,37 to reflect a healthy dietary pattern. Study participants who followed all four dietary recommendations received one point for the dietary factor in the lifestyle index.
Finally, we assigned a lifestyle index score for each participant by summing the scores for each of the five lifestyle factors; consequently, the lifestyle index ranged from zero (least healthy) to five points (most healthy).
Case ascertainment
All cohort members were followed from the date of visit to the study centre until the date of diagnosis of any cancer (except non-melanoma skin cancer), date of death, date of emigration, or 27 April 2006, whichever came first. Record linkage to the Central Population Registry gave information on vital status, date of death, or date of emigration. Up to 31 December 2003 each participant was linked to the Danish Cancer Registry, which receives notifications of all cancer cases in Denmark by use of the personal identification number.40 From 2004 to 27 April 2006 information on cancer occurrence was obtained through record linkage to the Danish Pathology Databank because of a delay in the update of the Danish Cancer Registry. The Danish Pathology Databank is a national databank that, via online computer systems, collects and stores all histological and cytological examinations that have taken place at pathology departments in Denmark (www.patobank.dk).
Statistical analysis
We used Cox proportional hazard regression models with age as the underlying time axis to estimate incidence rate ratios (and 95% confidence intervals) for colorectal cancer.41 The models were corrected for delayed entry. Time under study was included as a time dependent variable and was modelled as a linear spline with boundaries at one, two, and three years after entry into the cohort. The assumption of proportional hazards was evaluated graphically; no deviation from proportionality was found.
To reveal the possible combination of the two outcomes colon cancer and rectal cancer as colorectal cancer, we performed a competing risk test (all P values >0.16).42 Tests of heterogeneity were used to evaluate whether associations differed between women and men. All models where men and women were combined were stratified by sex, thus allowing for separate underlying hazards in men and women.
We adjusted all Cox models for variables that (based on the existing literature) could be potential confounders: first degree family history of cancer (yes or no), school education (≤7 years, 8-10 years, or >10 years), use of non-steroidal anti-inflammatory drugs (at least twice a month or not), and use of hormone replacement therapy in women (never, past use, or current use). All these variables were measured at baseline.
Because so few participants met none of the lifestyle recommendations, we pooled the groups with zero and one point. The group that achieved all five points on the lifestyle index was also small: we therefore treated it as a separate group in the analysis for men and women combined and with colorectal cancer as the output, but in the rest of the analyses we pooled the groups with four and five points. The lifestyle index was analysed both as a categorical variable with the least healthy group as the reference group (see figure) and as a continuous variable (table 3 and figure). The linearity of the lifestyle index was evaluated with a likelihood ratio test, and no deviation from linearity was found (all P values >0.57).
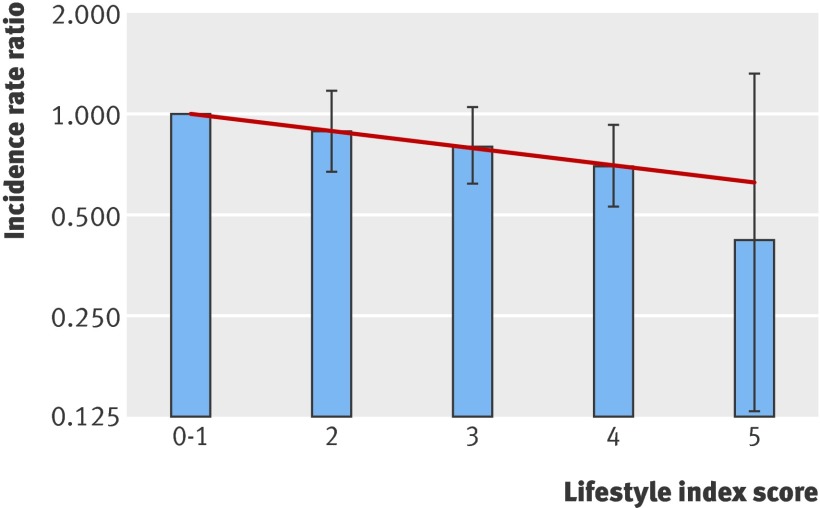
Incidence rate ratios for colorectal cancer and lifestyle index points for men and women combined in the study cohort. The bars show the incidence rate ratios and 95% confidence intervals for the categorical analyses, with the group scoring 0-1 as the reference group. The line shows the linear association between the incidence rate ratio and the lifestyle index score
Table 3.
Association between 55 487 participants’ combined lifestyle index score and risk of colorectal, colon, and rectal cancer. Incidence rate ratios and population attributable fractions are for cancer per one point higher lifestyle index score at baseline (linear trend estimate)
| No of cases | Incidence rate ratios (95% CI) | Population attributable fraction (95% CI) (%) | |||
|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Per +1 point† | For 5 points‡ | ||
| Colorectal cancer: | |||||
| Men and women§ | 678 | 0.89 (0.82 to 0.96) | 0.89 (0.82 to 0.96) | 13 (4 to 22) | 23 (9 to 37) |
| Men | 379 | 0.85 (0.77 to 0.95) | 0.85 (0.76 to 0.94) | 13 (4 to 22) | 19 (7 to 31) |
| Women | 299 | 0.94 (0.83 to 1.07) | 0.95 (0.83 to 1.07) | 4 (−5 to 14) | 6 (−7 to 20) |
| Colon cancer: | |||||
| Men and women | 420 | 0.88 (0.80 to 0.98) | 0.88 (0.80 to 0.98) | 10 (1 to 18) | 14 (3 to 25) |
| Men | 225 | 0.84 (0.73 to 0.97) | 0.84 (0.73 to 0.97) | 14 (2 to 26) | 20 (5 to 36) |
| Women | 195 | 0.94 (0.80 to 1.10) | 0.94 (0.81 to 1.10) | 5 (−7 to 16) | 7 (−10 to 24) |
| Rectal cancer: | |||||
| Men and women | 258 | 0.89 (0.78 to 1.02) | 0.89 (0.78 to 1.02) | 9 (−2 to 19) | 13 (−2 to 27) |
| Men | 154 | 0.86 (0.73 to 1.02) | 0.86 (0.72 to 1.02) | 12 (−2 to 26) | 18 (−1 to 37) |
| Women | 104 | 0.95 (0.77 to 1.18) | 0.95 (0.77 to 1.18) | 4 (−12 to 20) | 6 (−18 to 29) |
Assuming a causal and unbiased relationship between the lifestyle index and risk of colorectal cancer, we calculated the full population attributable fractions (PAF) of cases that might be associated with lack of adherence to all the recommendations as43:
- PAF = ({∑_Pi_[IRRi − 1]}/{1 + ∑_Pi_[IRRi − 1]}) × 100
and the proportion of cases that might be associated with lack of adherence to merely one additional recommendation for the entire cohort, except the healthiest group was:
- PAF+1 = ({∑_Pi_[IRRi − IRRi+1]}/{1 + ∑_Pi_[IRRi − IRRi+1]}) × 100 =
- PAF+1 = ({∑[Pi − Pi+1][IRRi − 1]}/{1 + ∑[Pi − Pi+1][IRRi − 1]}) × 100
where Pi is the prevalence of the participants for each group i at baseline and IRRi is the age adjusted incidence rate ratio of group i, based on the linear estimated incidence rate ratio for one additional point in the lifestyle index. We calculated approximate 95% confidence intervals for the estimated proportions of cases that might be preventable using the delta-method.
We examined the possibility of bias due to undiagnosed colorectal cancer cases at study entry by means of sensitivity analyses with and without a delayed entry of two and four years. Further sensitivity analyses were performed by combining the groups with four and five points because of the few participants achieving five points. In each sensitivity analysis the results were similar (data not shown).
We considered two-tailed P values lower than 0.05 to be statistically significant. All analyses were performed with SAS statistical software on a TextPad platform (release 9.1; SAS Institute, Cary NC, USA).
Results
Among the 55 487 study participants, 35 512 (64%) were non-smokers (among these, 56% had never smoked and 44% were former smokers), 32 737 (59%) had alcohol intake within the recommended limits, 45 499 (82%) followed the recommendation for physical activity, 42 170 (76%) had a waist circumference within the recommended range, and 1110 (2%) followed all four dietary recommendations (table 1). The median follow-up time (5th–95th centile range) in the cohort was 9.9 years (4.5–11.4). A total of 678 (1.22%) participants had colorectal cancer diagnosed during the follow-up time, 420 (0.76%) with colon cancer and 258 (0.46%) with rectal cancer.
Table 1.
Adherence to five lifestyle recommendations among 55 487 study participants
| Lifestyle factor and index score | No (%) of participants |
|---|---|
| Smoking: | |
| 0 (current smoker) | |
| 1 (non-smoker*) | 35 512 (64) |
| Alcohol: | |
| 0 (>7 and >14 drinks/week for women and men) | |
| 1 (≤7 and ≤14 drinks/week for women and men) | 32 737 (59) |
| Physical activity: | |
| 0 (<30 minutes moderate activity/day) | |
| 1 (≥30 minutes of moderate activity/day or light or heavy occupational physical activity) | 45 499 (82) |
| Waist circumference: | |
| 0 (>88 and >102 cm for women and men) | |
| 1 (≤88 and ≤102 cm for women and men) | 42 170 (76) |
| Diet†: | |
| 0 (0-3 points in dietary index) | |
| 1 (4 points in dietary index) | 1110 (2) |
Table 2 shows the baseline characteristics of the groups categorised by their lifestyle index score: 4570 (8%) participants scored zero or one, 14 173 (26%) scored two, 22 428 (40%) scored three, 13 806 (25%) scored four, and only 510 (1%) scored the maximum five. The total cohort’s median age was 56 (5th–95th centile range 50–64), and 26 634 (48%) participants were men.
Table 2.
Baseline characteristics of participants by their lifestyle index score. Values are numbers (percentages) of participants unless stated otherwise
| Cohort | Lifestyle index points | |||||
|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | 4 | 5 | ||
| All participants: | 55 487 (100) | 4 570 (8) | 14 173 (26) | 22 428 (40) | 13 806 (25) | 510 (1) |
| Men | 26 634 (48) | 2 514 (55) | 7 228 (51) | 10 317 (46) | 6 351 (46) | 61 (12) |
| Cases of colorectal cancer | 678 (1.2) | 68 (1.5) | 187 (1.3) | 272 (1.2) | 148 (1.1) | 3 (0.6) |
| Potential confounders | ||||||
| Median (90 centile range*) age (years) | 56 (50–64) | 56 (50–64) | 56 (50–64) | 56 (50–64) | 56 (50–64) | 56 (51–64) |
| Length of school education (years): | ||||||
| ≤7 | 18 311 (33) | 1 691 (37) | 4 961 (35) | 7 177 (32) | 4 280 (31) | 122 (24) |
| 8–10 | 25 524 (46) | 2 011 (44) | 6 378 (45) | 10 317 (46) | 6 627 (48) | 235 (46) |
| >10 | 11 653 (21) | 868 (19) | 2 835 (20) | 4 934 (22) | 2 899 (21) | 153 (30) |
| First degree family history of cancer | 26 634 (48) | 2 194 (48) | 6 803 (48) | 10 767 (48) | 6 627 (48) | 245 (48) |
| NSAID use monthly | 18 311 (33) | 1 554 (34) | 4 677 (33) | 7 401 (33) | 4 280 (31) | 138 (27) |
| HRT use in women: | ||||||
| Never | 15 581 (54) | 1 110 (54) | 3 681 (53) | 6 540 (54) | 4 175 (56) | 242 (54) |
| Past | 4 616 (16) | 370 (18) | 1 181 (17) | 1 817 (15) | 1 044 (14) | 72 (16) |
| Current | 8 656 (30) | 576 (28) | 2 084 (30) | 3 754 (31) | 2 237 (30) | 135 (30) |
| Factors included in lifestyle index | ||||||
| Non-smoker | 35 512 (64) | 640 (14) | 5 102 (36) | 15 700 (70) | 13 668 (99) | 510 (100) |
| Median (90 centile range*) physical activity (minutes/day) | 52 (8–163) | 21 (0–112) | 43 (8–163) | 56 (13–172) | 60 (26–172) | 77 (30–206) |
| Median (90 centile range*) alcohol intake (No of drinks/week)†: | ||||||
| Men | 11 (1–47) | 25 (4–62) | 21 (1–54) | 10 (1–38) | 7 (1–13) | 4 (0–11) |
| Women | 5 (0–24) | 12 (1–39) | 9 (0–32) | 6 (0–23) | 3 (0–7) | 3 (0–6) |
| Median (90 centile range*) waist circumference (cm)†: | ||||||
| Men | 95 (81–114) | 105 (85–122) | 98 (82–117) | 94 (81–111) | 92 (81–101) | 90 (81–101) |
| Women | 80 (67–103) | 92 (71–110) | 85 (68–108) | 80 (67–103) | 78 (67–88) | 77 (66–87) |
| Median (90 centile range*) dietary intake: | ||||||
| Dietary fibre (g/MJ dietary energy) | 2.1 (1.3–3.2) | 1.8 (1.1–2.7) | 1.9 (1.2–2.9) | 2.2 (1.4–3.1) | 2.3 (1.6–3.4) | 3.6 (3.1–4.8) |
| Fat energy (% of total dietary energy) | 33 (24–41) | 32 (23–40) | 33 (24–41) | 33 (24–41) | 33 (24–40) | 25 (18–29) |
| Red and processed meat (g/week) | 743 (298–1563) | 823 (371–1656) | 795 (348–1649) | 737 (312–1549) | 692 (253–1463) | 317 (15–486) |
| Fruit and vegetables (g/day) | 347 (111–786) | 263 (80–643) | 308 (95–705) | 354 (117–771) | 395 (147–840) | 801 (617–1272) |
A higher proportion of participants with higher scores had a longer education, a smaller proportion used non-steroidal anti-inflammatory drugs regularly, and a higher proportion were women. The median values for the participants who achieved all five points on the lifestyle index were 77 minutes of physical activity a day, four alcoholic drinks a week for men and three drinks a week for women, and a waist circumference of 90 cm for men and 77 cm for women, and all were non-smokers. Their median dietary values were 3.6 g of dietary fibre per MJ of energy, 801 g of fruit and vegetables a day, 317 g of red and processed meat a week, and 25% of total energy from fat.
In contrast, the participants who received zero or one point on the lifestyle index had median values of 21 minutes of physical activity a day, 25 alcoholic drinks a week for men and 20 a week for women, and a waist circumference of 105 cm for men and 92 cm for women, and 86% were smokers. Their median dietary intake was 1.8 g of dietary fibre per MJ of energy, 263 g of fruit and vegetables a day, 823 g of red and processed meat a week, and their diet contained 33% of total energy from fat (table 2).
Table 3 shows the association between participants’ combined lifestyle index score and risk of colorectal cancer. The lifestyle index was significantly linearly related to the risk of colorectal cancer and of colon cancer among men and women combined. For a one point higher lifestyle index score at baseline, the incidence rate ratio was 0.89 (95% confidence interval 0.82 to 0.96) for colorectal cancer, 0.88 (0.80 to 0.98) for colon cancer, and 0.89 (0.78 to 1.02) for rectal cancer. The estimated population attributable fraction for colorectal cancer was 13% (4% to 22%) if all participants had complied with one additional recommendation.
The figure shows the linear and the categorical analyses. Compared with the group who had the lowest lifestyle index scores in the categorical analysis, the groups who achieved two or three points had non-significantly lower risks of colorectal cancer, but achieving four points was associated with a significant 30% reduction in risk (incidence rate ratio 0.70 (0.53 to 0.93)) for colorectal cancer. If all five recommendations were followed, a 58% lower risk was seen, but the wide confidence interval meant this was not significant (incidence rate ratio 0.42 (0.13 to1.32)).
Based on the linear estimates, 23% (95% confidence interval 9% to 37%) of the colorectal cancer cases, 14% (3% to 25%) of the colon cancer cases, and 13% (−2% to 27%) of the rectal cancer cases might have been preventable if all participants had followed the five recommendations and had a lifestyle distribution for the five lifestyle factors at the levels found in the group who scored five points.
Among women, the results were less pronounced, but we found a weak, non-significant reduction in risk of colorectal, colon, and rectal cancer with a higher lifestyle index score. Among men, the association between the lifestyle index score and colorectal, colon, and rectal cancer were stronger and highly significant for colorectal and colon cancer. However, there was no significant sex difference in the associations between the lifestyle index points and risk of colorectal, colon, and rectal cancer (P for heterogeneity: 0.21 for colorectal cancer, 0.30 for colon cancer, and 0.47 for rectal cancer).
In addition to the simple equally weighted lifestyle index, we examined a lifestyle index weighted by the β coefficients of each lifestyle factor estimated in the multivariate model in relation to the risk of colorectal cancer, colon cancer, or rectal cancer. This was because some factors might be more or less related to the risk of colorectal cancer, although a recent meta-analysis found almost consistent associations among modifiable risk factors in relation to colorectal cancer.3 This method revealed similar results to those with the equally weighted lifestyle index (data not shown), which supports the use of the equally weighted method in public health practice.
Discussion
In this prospective study of Danish middle aged men and women it was clear that following the public health recommendations on smoking, alcohol intake, physical activity, waist circumference, and diet was associated with a substantially lower risk of colorectal cancer. More points achieved in the lifestyle index, corresponding to meeting additional recommendations, was associated with a markedly lower risk of colorectal cancer. If all participants had followed merely one additional recommendation we estimate that 13% of the cases of colorectal cancer might have been prevented. Furthermore, we estimate that 23% of the colorectal cancer cases in this cohort were associated with lack of adherence to the recommendations for the five lifestyle factors included in our study.
Strengths of study
Strengths of our study include the prospective design, which minimises potential selection bias and information bias. The linkage by the unique personal identification number to the Danish Cancer Registry and the Danish Pathology Databank ensures valid and complete ascertainment of incident cases, and the detailed baseline information enabled us to control for many possible confounding variables. Studies on the combined effect of lifestyle factors on risk of colorectal cancer are sparse despite numerous studies on individual lifestyle factors. However, the complex nature and multiple dimensions of health behaviours may be better captured in analyses of lifestyle factors in combination, like lifestyle patterns, than in analyses based on single lifestyle factors.
Limitations of study
Some misclassification of exposure to risk factors is inevitable in self reported questionnaires of lifestyle. But the dichotomised exposure used in this study did not require detailed information on exposure, and since few of the recommendations were well known at baseline, misclassification from over-reporting of healthy lifestyle and under-reporting of unhealthy lifestyle is not likely. However, by dichotomising the lifestyle factors, we lost information about any dose-response relation. Some extra analyses using cut points by the quartiles showed a slightly stronger association, but not significantly different from the original lifestyle index. The simple strategy and the possibility of considering the effect of adherence to the recommendations are lost by using quartiles as cut points.
The participation of only 35% of those invited to do so could introduce selection bias to our study, but only if non-participation is related to both the exposure and the outcome.
Healthy living people perhaps more often seek opportunistic screening, which will affect the diagnostic intensity. This could lead to greater likelihood of diagnosis of colorectal cancer (diagnostic bias) and potential attenuation of a beneficial association between the lifestyle index and incidence of colorectal cancer.
Adjustment for potential confounding factors did not change the estimates, but residual confounding from unknown confounding still remains possible.
Lifestyle changes before and after baseline assessments were not taken into account; since the relevant exposure period for colorectal cancer is unknown and might be decades before the diagnosis of colorectal cancer, the relevance of a single measurement at a given point in time depends on the degree to which the exposure and confounders track over time. Many participants retired during the follow-up, and they might then become more physically active and health conscious. The observed incidence rate ratios might be diminished according to these changes. However, a study on lifestyle and risk of mortality from all causes that accounted for within-person variation in the three lifestyle factors—smoking, physical activity, and body mass index—during the 20 years of follow-up period (with five follow-up assessments) found that correction for within-person variation had only a small effect on estimated risk differences and population attributable risk fractions when people were grouped into risk categories based on their lifestyle.44 Future cohort studies with repeated measurements of lifestyle factors will be required to investigate the impact of changing lifestyle.
Waist circumference might be an intermediate factor in the relation of diet and physical activity with colorectal cancer in the present study. However, previous studies have found both a total and a direct and independent relation between obesity and colorectal cancer.8 10 We examined a lifestyle index excluding waist circumference, and the risk estimates were slightly weaker, thus the other lifestyle aspects were not only mediated through waist circumference.
Choice of factors in lifestyle index
In other studies body mass index is often used as an estimate for overweight; but it seems that waist circumference is a better predictor for overweight45 and studies have shown that fat distribution and abdominal fat may be a more important risk factor for colorectal cancer than body mass index.9 10 Using body mass index in our lifestyle index instead of waist circumference slightly attenuated the associations between the lifestyle index and the risk of colorectal cancer, which indicates that waist circumference might be the better predictor of colorectal cancer risk.
We examined a modified lifestyle index that allowed participants to follow only the recommendation for fruit and vegetables to achieve one point for diet in the lifestyle index. This was for three reasons. Firstly, the contribution of adherence to the full dietary recommendations in the combined risk estimate was limited, as only 2% of the participants followed all four dietary recommendations. Secondly, intake of fruit and vegetables may be a surrogate marker for particular dietary patterns, because we observed a correlation among the dietary factors. Thirdly, estimating total energy from fat and dietary fibre intake required detailed dietary information, which limits the ease of use of the lifestyle index in populations. The results for the modified lifestyle index were similar to those for the original lifestyle index except for a slightly smaller risk reduction for the healthiest group compared with the least healthy group. This, indicates the potential to use the lifestyle index without having comprehensive dietary information. The slightly greater risk reduction for the healthiest group in the original lifestyle index could be explained by a stronger protective effect of adherence to the four dietary recommendations or a lack of sufficient statistical power because so few participants were in the healthiest group.
For the recommendation for non-smoking, we pooled former smokers and never smokers. However, other studies have found that former smokers have a significantly higher risk of colorectal cancer than never smokers.4 5 So we also examined a lifestyle index with former smokers pooled with current smokers, but the results were similar.
Generalisability and population attributable fractions
In our study cohort the recommendation that was most likely to be followed was for physical activity, and the second most likely was for waist circumference. The recommendation that was least likely to be followed was for diet (see table 1 and appendix on bmj.com).
Our observed association between the lifestyle index score and the risk of colorectal cancer may be generalised to other populations, but the strength of the association and the public health implications must be considered population-specific as the baseline incidence rate for colorectal cancer, the prevalence of the individual lifestyle risk factors, and the lifestyle factor patterns vary between populations. Consequently, our results primarily concern middle aged men and women of higher socioeconomic status living in cities (from the characteristics of our cohort).28 However, this also implies that the population attributable fractions estimated in our study probably underestimate the preventable proportion of colorectal cancer cases in the general population, as this proportion depends on the exposure prevalence and higher socioeconomic status might indicate a healthier lifestyle.46
Interpretation of the population attributable fractions should take into account that they rely on the distribution of lifestyle habits in the present cohort. The healthiest group consisted of a range of participants, from those who only just met the recommendations to those who were much healthier. Furthermore, population attributable fractions assume the exposures are causal, but only interventional studies can prove this.
Lifestyle index results in relation to other studies
To our knowledge only three prospective studies have examined a combination of lifestyle factors in relation to colorectal cancer or colon cancer.27 47 48 The comparability of the studies is limited, however, because they used different lifestyle factors, different cut-off points, and a different weighting of the lifestyle factors.
One study, on men, based its combination of lifestyle factors on generally good health behaviours—body mass index ≤25, physical activity ≥15 metabolic equivalent task (MET) hours/week, folic acid intake ≥100 μg/day, alcohol consumption <15 g/day and not being a former alcohol consumer or 15–30 g/day if supplemental folic acid intake ≥100 μg/day, early cumulative cigarette smoking ≤3 pack-years, and red meat consumption ≤2 servings/week. They found a population attributable fraction of 71% in relation to colon cancer.27
In the report Policy and Action for Cancer Prevention the World Cancer Research Fund and American Institute of Cancer Research looked at several lifestyle factors in relation to colorectal cancer risk, using risk estimates and prevalence from previous studies they calculated population attributable fractions for each lifestyle exposure. They estimated that 43% of colorectal cancer cases in the United Kingdom are preventable with a dietary fibre intake ≥30 g/day, intake of red and processed meat <10 g/day, drinking no alcohol, being physically active for ≥150 minutes/day, and having a body mass index <25.49
In comparison, we found a population attributable fraction for colorectal cancer of 23% if all participants had followed all the recommendations, and for colon cancer the population attributable fraction was 20% if all men had followed at least four recommendations.
The two other prospective studies, one of men and one of women, investigated the combination of lifestyle factors in a more complex manner. They both found a significant, fourfold to sixfold higher risk among those with an unhealthy lifestyle compared with the groups with a healthy lifestyle.47 48
In the present study we observed a stronger association between the lifestyle index and colorectal cancer among men than women. This was also found in a study with a lifestyle assessment and lifestyle index similar to ours but in relation to pancreatic cancer.50 The stronger association among men in our study is in agreement with meta-analyses investigating single lifestyle factors and risk of colorectal cancer.4 6 8 12 The non-significant sex difference we found could be due to some biological differences or to a difference in the level or quality of reporting lifestyle. A validation of the food frequency questionnaire used in this study showed that women tended to overestimate the consumption of “desirable” items more than men.29 Additionally, it is possible that our study may have lacked sufficient statistical power in the analyses of women only, as fewer colorectal cancer cases were diagnosed than among men.
Conclusions
The number of points achieved in a combined lifestyle index based on recommendations for smoking, alcohol intake, physical activity, waist circumference, and diet was associated with risk of colorectal cancer among middle aged Danish men and women. If all participants managed to improve their lifestyle by following merely one additional recommendation, 13% of the cases of colorectal cancer could potentially have been prevented.
Our study reveals the useful public health message that even modest differences in lifestyle might have a substantial impact on colorectal cancer risk and emphasises the importance of continuing vigorous efforts to convince people to follow the lifestyle recommendations.
However, our study findings should be confirmed in other studies with a diverse lifestyle among participants to give a comprehensive evaluation of lifestyle patterns and risk of colorectal cancer.
What is already known on this topic
- A healthier lifestyle is usually linked to prevention of cardiovascular disease
- Many studies have found an association between individual lifestyle factors and risk of colorectal cancer
What this study adds
- The combined effect of adherence to recommendations for five lifestyle factors has a protective effect on risk of colorectal cancer
- In the study cohort 13% of the colorectal cancer cases were associated with lack of adherence to one additional lifestyle recommendation among all participants except the healthiest
We thank Katja Boll, data manager, and Jytte Fogh Larsen, project coordinator, for their contribution in collection and handling of data.
Contributors: KO and AT collected the data. AT and NFJ had the idea for the study. HK did the data analysis, and JC and KF provided statistical expertise. HK wrote the first draft of the paper. All authors contributed to the interpretation of the results and critical revision of the manuscript and approved the final version of the manuscript. HK is the guarantor for the study.
Funding: This study was supported by the Danish Cancer Society.
Competing interests: None declared.
Ethical approval: The Diet, Cancer and Health Cohort Study and the present study were approved by the Regional Ethical Committees on human studies and by the Danish Data Protection Agency.
Data sharing: No additional data available
Cite this as: BMJ 2010;341:c5504
Web Extra. Extra material supplied by the author: commonest lifestyle patterns in each lifestyle index group
References
- 1.Ahmed FE. Effect of diet, life style, and other environmental/chemopreventive factors on colorectal cancer development, and assessment of the risks. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2004;22:91-147. [DOI] [PubMed] [Google Scholar]
- 2.Sundhedsstyrelsen (Denmark). [Cancer Registry 2005 and 2006—new figures from the National Board of Health.]Sundhedsstyrelsen, Sundhedsdokumentation, 2008.
- 3.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer 2009;125:171-80. [DOI] [PubMed] [Google Scholar]
- 4.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765-78. [DOI] [PubMed] [Google Scholar]
- 5.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406-15. [DOI] [PubMed] [Google Scholar]
- 6.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis 2005;7:204-13. [DOI] [PubMed] [Google Scholar]
- 7.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 2009;100:611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533-47. [DOI] [PubMed] [Google Scholar]
- 9.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 2007;13:4199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556-65. [DOI] [PubMed] [Google Scholar]
- 11.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med 2004;140:603-13. [DOI] [PubMed] [Google Scholar]
- 12.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer 2007;120:664-71. [DOI] [PubMed] [Google Scholar]
- 13.Reedy J, Mitrou PN, Krebs-Smith SM, Wirfalt E, Flood A, Kipnis V, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 2008;168:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA 2005;294:2849-57. [DOI] [PubMed] [Google Scholar]
- 15.Koushik A, Hunter DJ, Spiegelman D, Beeson WL, van den Brandt PA, Buring JE, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst 2007;99:1471-83. [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer 2006;119:2657-64. [DOI] [PubMed] [Google Scholar]
- 17.Sandhu MS, White IR, McPherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomarkers Prev 2001;10:439-46. [PubMed] [Google Scholar]
- 18.Boyle P, Levin B (eds). World cancer report 2008. International Agency for Research on Cancer, 2008.
- 19.Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation into Cancer and Nutrition—Potsdam study. Arch Intern Med 2009;169:1355-62. [DOI] [PubMed] [Google Scholar]
- 20.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med 2008;5:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen I, Katzmarzyk PT, Church TS, Blair SN. The Cooper Clinic Mortality Risk Index: clinical score sheet for men. Am J Prev Med 2005;29:194-203. [DOI] [PubMed] [Google Scholar]
- 22.Meng L, Maskarinec G, Lee J, Kolonel LN. Lifestyle factors and chronic diseases: application of a composite risk index. Prev Med 1999;29:296-304. [DOI] [PubMed] [Google Scholar]
- 23.Van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 2008;337:a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estaquio C, Castetbon K, Kesse-Guyot E, Bertrais S, Deschamps V, Dauchet L, et al. The French National Nutrition and Health Program score is associated with nutritional status and risk of major chronic diseases. J Nutr 2008;138:946-53. [DOI] [PubMed] [Google Scholar]
- 25.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA 2004;292:1433-9. [DOI] [PubMed] [Google Scholar]
- 26.Haveman-Nies A, de Groot LP, Burema J, Cruz JA, Osler M, van Staveren WA, et al. Dietary quality and lifestyle factors in relation to 10-year mortality in older Europeans: the SENECA study. Am J Epidemiol 2002;156:962-8. [DOI] [PubMed] [Google Scholar]
- 27.Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 2000;11:579-88. [DOI] [PubMed] [Google Scholar]
- 28.Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, et al. Study design, exposure variables, and socioeconomic determinants of participation in diet, cancer and health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health 2007;35:432-41. [DOI] [PubMed] [Google Scholar]
- 29.Tjonneland A, Overvad K, Haraldsdottir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol 1991;20:906-12. [DOI] [PubMed] [Google Scholar]
- 30.Tjonneland A, Haraldsdottir J, Overvad K, Stripp C, Ewertz M, Jensen OM. Influence of individually estimated portion size data on the validity of a semiquantitative food frequency questionnaire. Int J Epidemiol 1992;21:770-7. [DOI] [PubMed] [Google Scholar]
- 31.Overvad K, Tjonneland A, Haraldsdottir J, Ewertz M, Jensen OM. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol 1991;20:900-5. [DOI] [PubMed] [Google Scholar]
- 32.Cust AE, Smith BJ, Chau J, van der Ploeg HP, Friedenreich CM, Armstrong BK, et al. Validity and repeatability of the EPIC physical activity questionnaire: a validation study using accelerometers as an objective measure. Int J Behav Nutr Phys Act 2008;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pols MA, Peeters PH, Ocke MC, Slimani N, Bueno-de-Mesquita HB, Collette HJ. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int J Epidemiol 1997;26(suppl 1):181-9S. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. A 5 year action plan—smoke free Europe. WHO,1987.
- 35.Perdersen BK, Saltin B. [Physical activity—handbook of prevention and treatment.]Sundhedsstyrelsen, Center for Forebyggelse, 2004.
- 36.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. American Institute for Cancer Research, 2007.
- 37.Alexander J, Andersen SA, Antti A (eds). Nordic nutrition recommendations 2004—integrating nutrition and physical activity. 4th ed. Nordic Council of Ministers, 2004.
- 38.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO, 2000. [PubMed]
- 39.Hallund J, Dragsted LO, Halkjær J, Madsen C, Ovesen L, Rasmussen HH, et al. Fruits, vegetables and health—an update of the scientific basis of the Danish recommendation (2002-2006). DTU Food—National Food Institute, 2007.
- 40.Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry—history, content, quality and use. Dan Med Bull 1997;44:535-9. [PubMed] [Google Scholar]
- 41.Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd ed. Blackwell Science, 2003.
- 42.Andersen PK, Borgan O, Gill RD, Keiding N. Regression models. In: Andersen PK, Borgan O, Gill RD, Keiding N (eds). Statistical models based on counting processes. Springer-Verlag, 1993. p. 476-656.
- 43.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control 2007;18:571-9. [DOI] [PubMed] [Google Scholar]
- 44.Emberson JR, Whincup PH, Morris RW, Wannamethee SG, Shaper AG. Lifestyle and cardiovascular disease in middle-aged British men: the effect of adjusting for within-person variation. Eur Heart J 2005;26:1774-82. [DOI] [PubMed] [Google Scholar]
- 45.Seidell JC, Kahn HS, Williamson DF, Lissner L, Valdez R. Report from a Centers for Disease Control and Prevention Workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr 2001;73:123-6.11124761 [Google Scholar]
- 46.Lahelma E, Lallukka T, Laaksonen M, Martikainen P, Rahkonen O, Chandola T, et al. Social class differences in health behaviours among employees from Britain, Finland and Japan: the influence of psychosocial factors. Health Place 2010;16:61-70. [DOI] [PubMed] [Google Scholar]
- 47.Driver JA, Gaziano JM, Gelber RP, Lee IM, Buring JE, Kurth T. Development of a risk score for colorectal cancer in men. Am J Med 2007;120:257-63. [DOI] [PubMed] [Google Scholar]
- 48.Wei EK, Colditz GA, Giovannucci EL, Fuchs CS, Rosner BA. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses’ Health Study. Am J Epidemiol 2009;170:863-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Cancer Research Fund/American Institute for Cancer Research. Policy and action for cancer prevention. Food, nutrition, and physical activity: a global perspective. American Institute for Cancer Research, 2009.
- 50.Jiao L, Mitrou PN, Reedy J, Graubard BI, Hollenbeck AR, Schatzkin A, et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med 2009;169:764-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.