Interactions Between Obesity and Obstructive Sleep Apnea: Implications for Treatment (original) (raw)
Abstract
Obstructive sleep apnea (OSA) adversely affects multiple organs and systems, with particular relevance to cardiovascular disease. Several conditions associated with OSA, such as high BP, insulin resistance, systemic inflammation, visceral fat deposition, and dyslipidemia, are also present in other conditions closely related to OSA, such as obesity and reduced sleep duration. Weight loss has been accompanied by improvement in characteristics related not only to obesity but to OSA as well, suggesting that weight loss might be a cornerstone of the treatment of both conditions. This review seeks to explore recent developments in understanding the interactions between body weight and OSA. Weight loss helps reduce OSA severity and attenuates the cardiometabolic abnormalities common to both diseases. Nevertheless, weight loss has been hard to achieve and maintain using conservative strategies. Since bariatric surgery has emerged as an alternative treatment of severe or complicated obesity, impressive results have often been seen with respect to sleep apnea severity and cardiometabolic disturbances. However, OSA is a complex condition, and treatment cannot be limited to any single symptom or feature of the disease. Rather, a multidisciplinary and integrated strategy is required to achieve effective and long-lasting therapeutic success.
Our understanding of the implications of obstructive sleep apnea (OSA) on disease pathophysiology has been evolving rapidly. OSA is thought to adversely affect multiple organs and systems and may be especially relevant to cardiovascular disease.1,2 It has been implicated in the etiology of hypertension3,4 and in the progression of several established medical conditions such as congestive heart failure, atrial fibrillation, diabetes, and pulmonary hypertension.1 However, whether OSA is causally linked to the development of these latter disease states remains to be proven.1,2
In the adult population, the prevalence of OSA is estimated to be ~25%, and as high as 45% in obese subjects.5-10 Obesity predisposes to and potentiates OSA. The prevalence of OSA and its consequences are likely to increase in light of the current obesity epidemic. Recent estimates suggest that 60% of the adult population in industrialized countries is overweight (BMI ≥ 25 kg/m2) and at least 30% is obese (BMI ≥ 30 kg/m2).11 Sleep fragmentation is an important consequence of OSA; whether such interrupted sleep results in pathophysiologically similar mechanisms such as sleep deprivation is not known. Experimental sleep deprivation, as well as self-reported short sleep (< 6 h/night),12-15 have been linked to metabolic dysregulation independent of obesity and OSA, suggesting important interactions between these conditions and increasing the complexity of their treatment.
At present, continuous positive airway pressure (CPAP) is considered the mainstay of treatment of OSA. However, despite the benefits of CPAP therapy seen in numerous clinical trials,16 noncompliance is evident in a significant proportion of patients,17,18 suggesting that other therapies are needed.
With few exceptions, weight loss may be an effective therapy, especially in overweight and obese patients, who may comprise > 70% of subjects with OSA.19 Weight loss, especially through bariatric surgery, has been shown to reduce the severity and symptoms of OSA and sometimes, though not always, result in its complete resolution.20 In addition, weight loss may elicit beneficial changes in cholesterol, insulin resistance, leptin, inflammatory markers, and endothelial function,21,22 abnormalities that are not only associated with OSA but also with obesity, experimental sleep deprivation, and self-reported short sleep.
Epidemiology of OSA
The best prevalence estimates of OSA in the general population are derived from six large studies conducted worldwide. These studies suggest that approximately 25% of adults with a BMI between 25 kg/m2 and 28 kg/m2 have at least mild OSA (apnea-hypopnea index [AHI] ≥ 5).5-10 However, the prevalence of OSA varies according to gender (~30% in men and ~15% in women), age, and body weight. Men’s risk for OSA is twofold-higher than that of women.6 Postmenopausal women are at higher risk for OSA than premenopausal women,7 suggesting a potential role for hormonal differences in OSA pathophysiology. OSA is also affected by age, as prevalence increases until age 65 years, when for unclear reasons the prevalence reaches a plateau.23 Data also suggest that the interaction between body weight (measured using BMI) and OSA in elderly subjects might be different from that in younger adults. Janssen24 followed 4,968 elderly subjects (≥ 65 years) for 9 years and showed that the risk of OSA was not different between overweight (BMI 25-29.9 kg/m2) and normal-weight (BMI 20-24.9 kg/m2) elderly subjects. However, these results might be explained by the poor diagnostic performance of BMI in detecting an excess of body fat in the elderly.25 These findings may also be relevant to the “obesity paradox,” which relates to a better survival in overweight subjects when compared with normal-weight subjects,26 findings that are beyond the scope of this review. However, because of the important interaction between OSA and body weight, this subject is discussed below in greater detail.
Obesity and OSA
Obesity is considered a major risk factor for the development and progression of OSA.8,19,20,27,28 The prevalence of OSA in obese or severely obese patients is nearly twice that of normal-weight adults. Furthermore, patients with mild OSA who gain 10% of their baseline weight are at a sixfold-increased risk of progression of OSA, and an equivalent weight loss can result in a more than 20% improvement in OSA severity.28 Moreover, the higher prevalence of OSA in obese subjects is not limited to adults; recent data show that obese children have a 46% prevalence of OSA when compared with children seen in a general pediatric clinic (33%).29 This finding is further aggravated by the obesity epidemic among children and adolescents.30 In fact, there are data suggesting that children and adolescents with OSA have more than a sixfold-increased risk of having metabolic syndrome,31 when compared with children and adolescents without OSA. These findings highlight the need to develop screening and prevention for these conditions, even as early as in childhood.
It is possible that obesity may worsen OSA because of fat deposition at specific sites. Fat deposition in the tissues surrounding the upper airway appears to result in a smaller lumen and increased collapsibility of the upper airway, predisposing to apnea.32,33 Moreover, fat deposits around the thorax (truncal obesity) reduce chest compliance and functional residual capacity, and may increase oxygen demand.34 Visceral obesity is common in subjects with OSA.35 However, the relationship between OSA and obesity is complex. Although there is compelling evidence showing that obesity, as well as visceral obesity, may predispose to OSA, and that losing weight results in OSA improvement, recent studies suggest that OSA may itself cause weight gain.36,37 Factors such as reduced activity levels and increased appetite, particularly for refined carbohydrates, may conceivably contribute to weight gain in OSA patients.13 Whether OSA predisposes to preferential accumulation of visceral fat remains to be determined.38 CPAP treatment of OSA reduces the amount of visceral fat (as measured by abdominal CT scanning), even in patients without significant weight loss.39 Finally, there are data showing substantial overlap in genetic substrates between OSA and obesity. Patel et al40 reported a significant correlation between AHI and anthropomorphic adiposity measures (ranging from 0.57 to 0.61), suggesting that obesity could explain nearly 40% of the genetic variance in sleep apnea. In another study, Popko et al41 showed that polymorphisms (Arg/Arg and Gln/Arg when compared with Gln/Gln) of the leptin receptor, which is involved in energy homeostasis and body weight regulation, are significantly correlated with both OSA and obesity when compared with healthy controls. These studies suggest that genetic polymorphisms may influence both sleep apnea and obesity, and may be importantly interrelated in the development of these conditions.
OSA, Sleep Deprivation, and Metabolic Dysregulation
Several cardiometabolic alterations have been associated with OSA, independent of obesity and other potential confounders. Among the most important are glucose intolerance and insulin resistance, which are risk factors for the development of diabetes and cardiovascular disease.42,43 Moreover, OSA has been associated with a heightened systemic inflammatory state, as shown by increases in cytokines,38,44 serum amyloid A,45 and, in some but not all studies, C-reactive protein.46,47 Subjects with OSA who received effective treatment with CPAP have shown improvement in some of these metabolic and inflammatory abnormalities.39,48
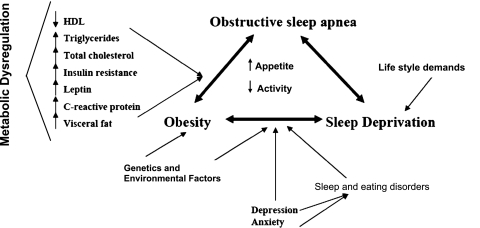
Systemic inflammation has also been described in subjects with short sleep duration.49 Population studies show a dose-response relationship between self-reported short sleep duration and increased body weight, suggesting that sleep duration may be an important regulator of body weight and metabolic and endocrine function. These close interactions between obesity, sleep deprivation, and OSA (Fig 1) share the common pathophysiologic feature of metabolic dysregulation. Weight loss may improve all of these conditions and might constitute an important potential intervention for these patients. In fact, studies assessing changes in sleep architecture after weight loss achieved through bariatric surgery (gastric banding) suggest that, 1 year later, there are significant increases in rapid eye movement and slow-wave sleep, with reduced daytime sleepiness.50
Figure 1.
Interaction between obstructive sleep apnea, obesity, sleep deprivation, and metabolic abnormalities. HDL = high-density lipoprotein.
Other metabolic disturbances present in OSA that could potentially improve with weight loss include lipid abnormalities. OSA patients have been shown to have increased triglycerides, total cholesterol/high-density lipoprotein (HDL) ratio and low-density lipoprotein and lower HDL values.51 Intermittent hypoxia, a key feature of OSA, causes an increase in the liver content of triglycerides in mice.52,53 OSA patients may also have reduced HDL-mediated inhibition of low-density lipoprotein oxidation ex vivo.54 The independent roles of OSA and obesity in these abnormalities remain unclear, but these measures may improve with weight loss achieved through diet and exercise, again speaking to a close interaction between these conditions (Fig 1).
Finally, hormones related to obesity, weight control, satiety, and energy expenditure may be altered in OSA. Leptin is a hormone produced by adipose tissue and binds to the ventral medial nucleus of the hypothalamus, known as the satiety center. Binding of leptin to this nucleus signals to the brain that the body has had enough to eat, a sensation of satiety.55 Sleep deprivation in the short term inhibits leptin production, suggesting a potential mechanism for the early development of obesity.56 Paradoxically, subjects with obesity have higher levels of leptin, likely due to increased fat mass. This hyperleptinemia is believed to be accompanied by desensitized cellular responses to leptin so that the effect of leptin is not achieved.57 Leptin also modulates ventilatory control, and may therefore be implicated in abnormal breathing patterns in obesity.58-60 Other adipokines, such as tumor necrosis factor α and interleukin-6, are also elevated in obesity and may be linked to depression of CNS activity and airway neuromuscular control, thus perhaps increasing OSA severity, which consequently triggers proinflammatory substances, creating a vicious circle.60 Indeed, leptin levels in OSA patients are higher than would be expected because of the obesity alone,37,38 and leptin levels are reduced after as little as 4 days of CPAP use.39 Another adipokine closely related to obesity and OSA is adiponectin.61,62 Increased levels of serum adiponectin have been shown to improve glucose and lipid metabolism and prevent inflammation and atherosclerosis. Adiponectin is low in obesity and also in OSA. Furthermore, adiponectin levels have been shown to increase with CPAP treatment, suggesting that treating OSA could potentially reduce the metabolic disturbances and potentially reduce cardiovascular risk.61,62 Ghrelin, a hormone produced by cells lining the stomach, stimulates appetite and is considered a counter-regulator to leptin.63 Interestingly, ghrelin is increased during the night in obese subjects,64 and reduced sleep65 has been shown to increase the production of ghrelin, which ultimately stimulates appetite, and might lead to obesity and worsening of OSA.13
Weight Loss as a Treatment of OSA
Most studies assessing the effects of weight loss on OSA have had methodologic limitations, including lack of either randomization and/or a control group for comparisons, inadequate adjustment for potential confounders, and limited follow-up. Many of the more recent studies assessing weight loss on OSA severity and outcomes were in the context of bariatric surgery.
In the last few years, three randomized trials have addressed whether weight loss can improve OSA through nonsurgical treatments.66,67 Kajaste and colleagues,66 using a cognitive-behavioral program and an initial low-calorie diet with (n = 14) or without (n = 17) additional CPAP therapy, followed 31 obese men for 2 years to assess changes in the severity of OSA. Patients had lost an average of 13.5% of their initial body weight at 6 and 12 months when compared with baseline, but at 24 months the weight loss had diminished to 9% when compared with baseline. At baseline, the oxygen desaturation index (desaturation events per hour of sleep exceeding 4% from baseline) was 51 ± 31 and was reduced to 23 ± 18 and 25 ± 23 at 6 and 12 months, respectively. After 24 months, patients began to regain the lost weight and the oxygen desaturation index began to increase (32 ± 26) but was still significantly lower when compared with baseline.66 Interestingly, the group of patients in this study who also underwent CPAP therapy did not differ in weight loss or oxygen desaturation index when compared with patients without additional CPAP therapy, although adherence was not measured objectively. This randomized clinical trial suggests that weight loss in obese patients with OSA might be an important therapeutic intervention. However, as in most weight-loss programs, patients started to regain the lost weight after 2 years followed by a worsening of their OSA severity. In addition, patients never reached a “normal” oxygen desaturation index.
In the second trial, Lam et al67 randomized 101 patients (79% men) with mild-to-moderate OSA to one of three treatment groups: (1) conservative measures (sleep hygiene) alone or (2) with the addition of CPAP or (3) with the addition of oral appliances. All overweight subjects (83% of the sample) were referred to a weight-reduction class. After 10 weeks, all three groups of patients lost an average of 1.5 kg. Only CPAP therapy was associated with improvements in OSA severity, daytime sleepiness, and quality-of-life measures. Firm conclusions cannot be drawn from these two studies, but they do highlight the problems of recidivism and limited efficacy that may diminish the value of some weight-loss programs.
Very recently, Tuomilehto and colleagues68 randomized a small group of subjects (70% men, mean BMI 32 kg/m2) with mild, mostly supine-position predominant OSA (mean AHI 10) to either a 600 to 800 kcal/day diet plus supervised lifestyle counseling or routine lifestyle counseling over 1 year of study. The treatment group was rigorously followed, with 14 visits over 1 year, each lasting 60 to 90 min. At 1 year, the treatment group lost just more than 10 kg of weight, associated with a reduction in AHI of 4, whereas the controls lost an average of 2.4 kg, without measurable changes in AHI.68 Longer-term follow-up is needed to determine the durability of such lifestyle modifications, but the rigor of this protocol raises the issue of whether such costly and time-intensive programs are of value in those with milder OSA.
A longitudinal cohort study followed 2,968 men and women for 5 years to assess the effects of weight loss or weight gain on OSA severity.69 Men were more likely to develop worsening OSA severity with a given increase in weight than were women. In adjusted models, men who gained at least 10 kg were five times more likely to have moderate-to-severe OSA when compared with women, in whom the risk increased 2.5 times. Conversely, a similar amount of weight loss was associated with a greater improvement in OSA severity in men, but to a lesser degree in women, suggesting that weight gain and weight loss could have different consequences for OSA severity in men vs women.
In earlier studies, from the 1980s and 1990s, improvements in OSA severity were reported consistently in subjects in whom weight loss had been achieved without surgery.70-79 In these studies, patients who had lost 9 to 18% of their initial body weight showed marked improvement in their AHI (47%-100%), suggesting that if patients were able to maintain the weight loss, the benefits in OSA severity and other metabolic and endocrine parameters would be sustained. However, this remains to be proven.
Probably because of the limited weight loss achieved with conservative therapy, more recent trials assessing weight loss and OSA severity have been in the context of bariatric surgery, which may result in dramatic weight reduction, often maintained for up to 10 years.80 Obese patients undergoing bariatric surgery have shown a prevalence of OSA of > 70%.20 These patients may or may not have had symptoms of OSA and often have other important comorbidities, such as diabetes, hypertension, and dyslipidemia.19 After bariatric surgery, significant improvement and occasionally even resolution of these comorbidities, as well as of OSA, have been noted. Table 1 displays some of the recent studies assessing weight loss through bariatric surgery, and associated changes in OSA severity.50,81-99 Overall, patients undergoing bariatric surgery (regardless of the type of intervention) showed an average reduction of 15 kg/m2 in BMI and 36 events/hour in the AHI, suggesting that every 1 unit reduction in BMI translated to a reduction of 2.3 units in the AHI. These results are very similar to a recently published metaanalysis showing that an average reduction of 17.9 kg/m2 (95% CI, 55.3-37.7 kg/m2) translates into a reduction of 38.2 in AHI (95% CI, 31.9-44.4 AHI).100 However, as this metaanalysis also points out, despite showing a significant drop in the average AHI in most of the patients, OSA resolved completely (AHI < 5) in only 4% of subjects, and most continued to have at least moderate OSA.98 Thus, persistence of some degree of OSA in many patients who undergo bariatric surgery can be expected because most of these subjects, despite significant reduction in weight, still remain overweight, if not obese.
Table 1.
—Association Between Weight Loss Achieved By Bariatric Surgery and Obstructive Sleep Apnea Severity
| BMI | AHI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | No. of Patients | Follow-up, mo | Year of Study | Type ofSurgery | Baseline | Follow-up | Reduction(% Change) | Baseline | Follow-up | Reduction(% Change) |
| Rasheid et al81 | 11 | 3-21 | 1998-2001 | GBP | 62 | 40 | −22 (−35.5) | 56 | 23 | −33 (−68.9) |
| Haines et al82 | 101 | 6-42 | 1998-2005 | GBP | 56 | 38 | −18 (−22.2) | 51 | 15 | −35 (−70.6) |
| Valencia-Flores et al83 | 29 | 13.7 | 1999-2002 | GBP | 56.5 | 39.2 | −17.3 (−30.7) | 53.7 | 8.6 | −45.1 (−83.9) |
| Lankford et al84 | 15 | 12 | 2002-NA | GBP | 48 | 32 | −16 (−33.4) | 32 | 11 | −21 (−65.7) |
| Dixon et al50 | 25 | 17 | 1999-2002 | GBP | 52.7 | 37.2 | −15.5 (29.5) | 61.6 | 13.4 | −48.2 (−78.3) |
| Guardiano et al85 | 8 | 28 | NA | GBP | 49 | 34 | −15 (−30.7) | 55 | 14 | −41 (−74.6) |
| Busetto et al86 | 17 | 17 | NA | IGB | 55.8 | 48.6 | −7.2 (−12.9) | 52.1 | 14 | −38.1 (−73.2) |
| Charuzi et al91 | 46 | 46 | 1978-1986 | GBP and VBG | 47.5 | 32.1 | −15.4 (−32.5) | 58.8 | 7.8 | −51 (−86.8) |
| Scheuller et al88 | 15 | 12-144 | NA | GBP and VBG | 160a | 105a | −55b (−65.6) | 97 | 11.3 | −85.7 (−88.4) |
| Sugerman et al89 | 40 | 69.6 | 1980-1990 | GBP,VBG, and HG | 56 | 40 | −16 (−28.6) | 64 | 26 | −38 (−59.4) |
| Pillar et al90 | 14 | 90 | NA | GBP | 45 | 35 | −10 (−22.3) | 40 | 24 | −16 (−40) |
| Summers et al99 | 1 | 6 | NA | VBG | 54 | 37 | −17 (−31.5) | 40 | <5 | −36 (−90) |
| Lettieri et al98 | 24 | 12 | 2003-2005 | GBP | 51.0 | 32.1 | −18.9 (−37.1) | 47.9 | 24.5 | −23.4 (−48.8) |
| Buchwald et al92 | 1195 | ∼24 | 1990-2003 | GBP,VBG, HG, and BPD | NA | NA | −14 | NA | NA | −33 |
| Angrisani et al93 | 1 | 60 | 2000 | GBP and VBG | ∼43.4 | ∼35.5 | −7.9 (−28.3) | NA | <5 | 100% resolved |
| Skroubis et al94 | 4 | 29.3 | 1994-2005 | GBP and BPD | ∼45 | ∼26 | −19 (−42.3) | NA | <5 | 100% resolved |
| Nelson et al95 | 9 | 21 | 1999-2005 | GBP | 51 | 37 | −14 (−27.5) | NA | NA | 77% resolved |
| Nelson et al96 | 85 | 12-148 | 1985-2004 | GBP | 61 | 37 | −24 (−39.4) | NA | NA | 48% resolved |
| Cleator et al97 | 20 | 12 | 1997-2002 | Ileogastrostomy | 42.3 | 36 | −6.3 (−14.9) | NA | NA | 85% improved |
Although bariatric surgery may be considered an “extreme measure” for shedding weight, it provides support for the concept that reduced body fatness translates into important improvements and even resolution of several harmful conditions, including OSA. Some studies even relate bariatric surgery to better survival and fewer cardiovascular events at mid- and long-term follow-up.101-103 However, bariatric surgery is not exempt from complications and the 30-day mortality has been reported to be as high as 2% and 4.6% at 1 year,104 highlighting that for every patient, a careful balance between possible benefits and complications must be made. In addition, some questions regarding weight loss remain to be resolved. For example, does the presence of sleep apnea blunt the success of weight loss and/or promote weight gain late after surgery? Also not known is whether residual OSA after surgery depends on the persistence of visceral fat, or some other variable, after surgery. The timing of a repeat polysomnography following weight loss in patients with OSA is not certain, but could be conducted when weight has been stable for several months.
CPAP Treatment of OSA
CPAP is considered the mainstay of treatment of OSA and has shown benefits in dozens of randomized controlled trials. These benefits include reducing daytime sleepiness, improving quality of life, and lowering blood pressure.105 In addition, short-term data suggest that CPAP may possibly attenuate some of the cardiometabolic alterations that are present not only in OSA but common also to obesity and sleep deprivation. CPAP therapy has also been associated with reductions in visceral fat and total cholesterol and increased HDL.106 As for OSA patients with concomitant type 2 diabetes, CPAP therapy has been linked to better glycemic control and improved insulin sensitivity, although these results have not been consistent.107-109 Finally, CPAP has been associated with attenuation in inflammatory biomarkers and perhaps even improved endothelial function. Indeed, observational studies have reported better survival and fewer cardiovascular events in patients treated with CPAP when compared with patients with poor CPAP adherence or those who remained untreated.18,110
However, important questions regarding CPAP treatment remain unanswered. Most trials have involved male subjects, and therefore we have limited insight into how women may benefit from CPAP therapy. The benefits of CPAP therapy on metabolic parameters in subjects with mild and moderate OSA also remain unclear. CPAP therapeutic effects rely on patient compliance, which can be problematic.17,111,112 Most important, definitive evidence that CPAP prevents cardiovascular events and reduces mortality remains to be obtained. Only carefully designed large prospective studies will answer these important questions.
Conclusions
Weight loss appears to confer benefits not only on OSA severity but also in terms of mitigating cardiometabolic consequences related to both OSA and obesity. Unfortunately, weight loss through diet, exercise, and/or medications has been hard to achieve and maintain. Bariatric surgery may be an alternative treatment of severe or complicated obesity, and important and sometimes impressive changes have been noted in cardiovascular risk factors, metabolic markers, and OSA severity. In addition, CPAP therapy may contribute to the weight loss process and may also, in and of itself, improve some of the metabolic abnormalities characteristic of OSA and obesity. However, it is clear that treatment of OSA cannot be limited to any single strategy, but rather requires a multidisciplinary approach, effective provider-patient communications, and systematic long-term follow-up to achieve an effective and long-lasting therapeutic success.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Romero-Corral is an advisor for Select Research. Dr Caples has received research funding from the ResMed Foundation, Restore Medical, and Ventus. Dr Somers has served as a consultant for ResMed, Respironics, GlaxoSmithKline, Sepracor, and Cardiac Concepts; he has received research grants from the ResMed Foundation, the Respironics Sleep and Respiratory Research Foundation, Sorin, Inc., and Select Research, Inc., and works with Mayo Health Solutions and iLife on intellectual property related to sleep and to obesity. Dr Lopez-Jimenez serves as a coinvestigator of an investigator-initiated grant for Select Research.
Abbreviations
AHI
apnea-hypopnea index
CPAP
continuous positive airway pressure
HDL
high-density lipoprotein
OSA
obstructive sleep apnea
Footnotes
Funding/Support: At the time of the writing of this manuscript, Dr Romero-Corral was supported by a Postdoctoral Fellowship from the American Heart Association. Dr Caples is supported by NIH grant HL99534. Dr Lopez-Jimenez is a recipient of a Clinical Scientist Development Award from the American Heart Association. Dr Somers is supported by NIH grants HL-65176, HL-73211, and 1 UL1 RR024150, and by the Mayo Clinic College of Medicine.
References
- 1.Somers VK, White DP, Amin R, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology. American Heart Association Stroke Council. American Heart Association Council on Cardiovascular Nursing. American College of Cardiology Foundation Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79(8):1036–1046. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 8.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170(10):1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130(1):149–156. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 12.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7(4):297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 15.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airway pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006:CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 17.McNicholas WT. Compliance with nasal CPAP therapy for obstructive sleep apnoea: how much is enough? Eur Respir J. 1997;10(5):969–970. doi: 10.1183/09031936.97.10050969. [DOI] [PubMed] [Google Scholar]
- 18.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 19.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25(5):669–675. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 20.Fritscher LG, Mottin CC, Canani S, Chatkin JM. Obesity and obstructive sleep apnea-hypopnea syndrome: the impact of bariatric surgery. Obes Surg. 2007;17(1):95–99. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415. [PubMed] [Google Scholar]
- 22.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation on obesity. Geneva, Switzerland: World Health Organization; 1998. [PubMed] [Google Scholar]
- 23.Young T, Shahar E, Nieto FJ, et al. Sleep Heart Health Study Research Group Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 24.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (Silver Spring) 2007;15(7):1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32(6):959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 27.Wolk R, Somers VK. Obesity-related cardiovascular disease: implications of obstructive sleep apnea. Diabetes Obes Metab. 2006;8(3):250–260. doi: 10.1111/j.1463-1326.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 28.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 29.Rudnick EF, Walsh JS, Hampton MC, Mitchell RB. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg. 2007;137(6):878–882. doi: 10.1016/j.otohns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120(2):340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 31.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176(4):401–408. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148(2):462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 33.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 34.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 36.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17(9):1297–1300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 37.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 38.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 39.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100(7):706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 40.Patel SR, Larkin EK, Redline S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int J Obes (Lond) 2008;32(5):795–800. doi: 10.1038/sj.ijo.0803803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popko K, Gorska E, Wasik M, et al. Frequency of distribution of leptin gene polymorphism in obstructive sleep apnea patients. J Physiol Pharmacol. 2007;58(Pt. 2) Suppl 5:551–561. [PubMed] [Google Scholar]
- 42.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 43.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep Heart Health Study Investigators Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 44.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94(1):179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 45.Minoguchi K, Yokoe T, Tazaki T, et al. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175(6):612–617. doi: 10.1164/rccm.200608-1141OC. [DOI] [PubMed] [Google Scholar]
- 46.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 47.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27(8):1507–1511. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 48.Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009;136(1):125–129. doi: 10.1378/chest.08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 50.Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29(9):1048–1054. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 51.Börgel J, Sanner BM, Bittlinsky A, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27(1):121–127. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Grigoryev DN, Ye SQ, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99(5):1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97(7):698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 54.Tan KC, Chow WS, Lam JC, et al. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184(2):377–382. doi: 10.1016/j.atherosclerosis.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Tomaszuk A, Simpson C, Williams G. Neuropeptide Y, the hypothalamus and the regulation of energy homeostasis. Horm Res. 1996;46(2):53–58. doi: 10.1159/000184996. [DOI] [PubMed] [Google Scholar]
- 56.Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 57.Wolk R, Somers VK. Leptin and vascular function: Friend or foe? Eur Heart J. 2006;27(19):2263–2265. doi: 10.1093/eurheartj/ehl246. [DOI] [PubMed] [Google Scholar]
- 58.Polotsky VY, Smaldone MC, Scharf MT, et al. Impact of interrupted leptin pathways on ventilatory control. J Appl Physiol. 2004;96(3):991–998. doi: 10.1152/japplphysiol.00926.2003. [DOI] [PubMed] [Google Scholar]
- 59.Tankersley CG, O’Donnell C, Daood MJ, et al. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol. 1998;85(6):2261–2269. doi: 10.1152/jappl.1998.85.6.2261. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang XL, Yin KS, Li C, Jia EZ, Li YQ, Gao ZF. Effect of continuous positive airway pressure treatment on serum adiponectin level and mean arterial pressure in male patients with obstructive sleep apnea syndrome. Chin Med J (Engl) 2007;120(17):1477–1481. [PubMed] [Google Scholar]
- 62.Nakagawa Y, Kishida K, Kihara S, et al. Nocturnal reduction in circulating adiponectin concentrations related to hypoxic stress in severe obstructive sleep apnea-hypopnea syndrome. Am J Physiol Endocrinol Metab. 2008;294(4):E778–E784. doi: 10.1152/ajpendo.00709.2007. [DOI] [PubMed] [Google Scholar]
- 63.Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18(3):439–456. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 64.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101(28):10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5(2):125–131. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62(4):354–359. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuomilehto HP, Seppä JM, Partinen MM, et al. Kuopio Sleep Apnea Group Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(4):320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 69.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 70.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103(6(Pt 1)):850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 71.Rubinstein I, Colapinto N, Rotstein LE, Brown IG, Hoffstein V. Improvement in upper airway function after weight loss in patients with obstructive sleep apnea. Am Rev Respir Dis. 1988;138(5):1192–1195. doi: 10.1164/ajrccm/138.5.1192. [DOI] [PubMed] [Google Scholar]
- 72.Aubert-Tulkens G, Culée C, Rodenstein DO. Cure of sleep apnea syndrome after long-term nasal continuous positive airway pressure therapy and weight loss. Sleep. 1989;12(3):216–222. doi: 10.1093/sleep/12.3.216. [DOI] [PubMed] [Google Scholar]
- 73.Pasquali R, Colella P, Cirignotta F, et al. Treatment of obese patients with obstructive sleep apnea syndrome (OSAS): effect of weight loss and interference of otorhinolaryngoiatric pathology. Int J Obes. 1990;14(3):207–217. [PubMed] [Google Scholar]
- 74.Rajala R, Partinen M, Sane T, Pelkonen R, Huikuri K, Seppäläinen AM. Obstructive sleep apnoea syndrome in morbidly obese patients. J Intern Med. 1991;230(2):125–129. doi: 10.1111/j.1365-2796.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144(3 Pt 1):494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 76.Suratt PM, McTier RF, Findley LJ, Pohl SL, Wilhoit SC. Effect of very-low-calorie diets with weight loss on obstructive sleep apnea. Am J Clin Nutr. 1992;56(1 suppl):182S–184S. doi: 10.1093/ajcn/56.1.182S. [DOI] [PubMed] [Google Scholar]
- 77.Kiselak J, Clark M, Pera V, Rosenberg C, Redline S. The association between hypertension and sleep apnea in obese patients. Chest. 1993;104(3):775–780. doi: 10.1378/chest.104.3.775. [DOI] [PubMed] [Google Scholar]
- 78.Nahmias J, Kirschner M, Karetzky MS. Weight loss and OSA and pulmonary function in obesity. N J Med. 1993;90(1):48–53. [PubMed] [Google Scholar]
- 79.Noseda A, Kempenaers C, Kerkhofs M, Houben JJ, Linkowski P. Sleep apnea after 1 year domiciliary nasal-continuous positive airway pressure and attempted weight reduction. Potential for weaning from continuous positive airway pressure. Chest. 1996;109(1):138–143. doi: 10.1378/chest.109.1.138. [DOI] [PubMed] [Google Scholar]
- 80.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 81.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13(1):58–61. doi: 10.1381/096089203321136593. [DOI] [PubMed] [Google Scholar]
- 82.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141(3):354–358. doi: 10.1016/j.surg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Valencia-Flores M, Orea A, Herrera M, et al. Effect of bariatric surgery on obstructive sleep apnea and hypopnea syndrome, electrocardiogram, and pulmonary arterial pressure. Obes Surg. 2004;14(6):755–762. doi: 10.1381/0960892041590773. [DOI] [PubMed] [Google Scholar]
- 84.Lankford DA, Proctor CD, Richard R. Continuous positive airway pressure (CPAP) changes in bariatric surgery patients undergoing rapid weight loss. Obes Surg. 2005;15(3):336–341. doi: 10.1381/0960892053576749. [DOI] [PubMed] [Google Scholar]
- 85.Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest. 2003;124(4):1615–1619. doi: 10.1378/chest.124.4.1615. [DOI] [PubMed] [Google Scholar]
- 86.Busetto L, Enzi G, Inelmen EM, et al. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128(2):618–623. doi: 10.1378/chest.128.2.618. [DOI] [PubMed] [Google Scholar]
- 87.Charuzi I, Fraser D, Peiser J, Ovnat A, Lavie P. Sleep apnea syndrome in the morbidly obese undergoing bariatric surgery. Gastroenterol Clin North Am. 1987;16(3):517–519. [PubMed] [Google Scholar]
- 88.Scheuller M, Weider D. Bariatric surgery for treatment of sleep apnea syndrome in 15 morbidly obese patients: long-term results. Otolaryngol Head Neck Surg. 2001;125(4):299–302. doi: 10.1067/mhn.2001.119139. [DOI] [PubMed] [Google Scholar]
- 89.Sugerman HJ, Fairman RP, Sood RK, Engle K, Wolfe L, Kellum JM. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr. 1992;55(2 suppl):597S–601S. doi: 10.1093/ajcn/55.2.597s. [DOI] [PubMed] [Google Scholar]
- 90.Pillar G, Peled R, Lavie P. Recurrence of sleep apnea without concomitant weight increase 7.5 years after weight reduction surgery. Chest. 1994;106(6):1702–1704. doi: 10.1378/chest.106.6.1702. [DOI] [PubMed] [Google Scholar]
- 91.Charuzi I, Ovnat A, Peiser J, Saltz H, Weitzman S, Lavie P. The effect of surgical weight reduction on sleep quality in obesity-related sleep apnea syndrome. Surgery. 1985;97(5):535–538. [PubMed] [Google Scholar]
- 92.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 93.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3(2):127–132. doi: 10.1016/j.soard.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Skroubis G, Anesidis S, Kehagias I, Mead N, Vagenas K, Kalfarentzos F. Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion in a non-superobese population: prospective comparison of the efficacy and the incidence of metabolic deficiencies. Obes Surg. 2006;16(4):488–495. doi: 10.1381/096089206776327251. [DOI] [PubMed] [Google Scholar]
- 95.Nelson LG, Lopez PP, Haines K, et al. Outcomes of bariatric surgery in patients > or = 65 years. Surg Obes Relat Dis. 2006;2(3):384–388. doi: 10.1016/j.soard.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Nelson WK, Fatima J, Houghton SG, et al. The malabsorptive very, very long limb Roux-en-Y gastric bypass for super obesity: results in 257 patients. Surgery. 2006;140(4):517–522. doi: 10.1016/j.surg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 97.Cleator IG, Birmingham CL, Kovacevic S, Cleator MM, Gritzner S. Long-term effect of ileogastrostomy surgery for morbid obesity on diabetes mellitus and sleep apnea. Obes Surg. 2006;16(10):1337–1341. doi: 10.1381/096089206778663850. [DOI] [PubMed] [Google Scholar]
- 98.Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med. 2008;4(4):333–338. [PMC free article] [PubMed] [Google Scholar]
- 99.Summers CL, Stradling JR, Baddeley RM. Treatment of sleep apnoea by vertical gastroplasty. Br J Surg. 1990;77(11):1271–1272. doi: 10.1002/bjs.1800771123. [DOI] [PubMed] [Google Scholar]
- 100.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 101.Batsis JA, Romero-Corral A, Collazo-Clavell ML, et al. Effect of weight loss on predicted cardiovascular risk: change in cardiac risk after bariatric surgery. Obesity (Silver Spring) 2007;15(3):772–784. doi: 10.1038/oby.2007.589. [DOI] [PubMed] [Google Scholar]
- 102.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 103.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 104.Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294(15):1903–1908. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 105.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 106.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9(5):679–687. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 107.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 108.Harsch IA, Schahin SP, Brückner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71(3):252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 109.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 110.McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnoea syndrome. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1666–1670. doi: 10.1152/ajpregu.00401.2007. [DOI] [PubMed] [Google Scholar]
- 111.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127(6):2085–2093. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]