Use of Continuous Glucose Monitoring in Subjects With Type 1 Diabetes on Multiple Daily Injections Versus Continuous Subcutaneous Insulin Infusion Therapy: A prospective 6-month study (original) (raw)
Abstract
OBJECTIVE
To compare use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injection (MDI) therapy versus continuous subcutaneous insulin infusion (CSII) therapy for 6 months.
RESEARCH DESIGN AND METHODS
Sixty type 1 diabetic adults with similar baseline characteristics, using either MDI (n = 30) or CSII (n = 30) therapy, were enrolled in this 6-month prospective study. Subjects were instructed to wear the DexCom SevenPLUS continuous glucose monitor at all times throughout the study. All subjects were initially blinded from the continuous glucose monitoring (CGM) glucose data. After 4 weeks of blinded CGM use, the CGM was unblinded, making glucose data available to the patient. The CGM remained in the unblinded state for the remainder of the study (20 weeks). Clinic visits occurred every 4 weeks, at which time A1C values were collected and CGM data were downloaded.
RESULTS
Mean baseline (± SD) A1C was 7.61 (± 0.76) and 7.63 (± 0.68) for CSII and MDI, respectively (P > 0.05). Without any significant therapy change, A1C decrease at 12 weeks was similar in both groups (P = 0.03). When compared with the blinded phase, unblinded use of CGM was associated with similar but significant reductions in glycemic control and variability parameters. In addition, both therapy groups had similar changes in mean glucose and glucose variability indexes at 3 and 6 months (ITT analysis, P > 0.05). Predefined per protocol analysis (sensor use at least 6 days/week) showed greater improvement in time spent in target range glycemia, 3.9–10.0 mmol/L (70–180 mg/dL), in the CSII group.
CONCLUSIONS
We conclude that CGM provides similar benefits in glucose control for patients using MDI or CSII therapy.
For patients with type 1 diabetes using intensive insulin therapy (IIT), there are two approaches for insulin delivery: multiple daily injection (MDI) therapy and continuous subcutaneous insulin infusion (CSII) therapy (1,2). With both therapies, insulin is dosed using basal/bolus regimens. With MDI, a long-acting (basal) insulin analog is injected subcutaneously once or twice daily, providing a relatively constant insulin level. In contrast, CSII administers basal insulin by a continuous infusion of rapid-acting insulin that can be adjusted throughout the day based on an individual’s insulin requirements. In both approaches, basal insulin is adjusted to avoid hypo- and hyperglycemia during interprandial periods. Additionally, mealtime or “bolus” insulin is dosed based on several factors including anticipated meal carbohydrate content, current blood glucose, and postprandial glucose trends.
Severe hypoglycemia is a concern for all people with type 1 diabetes. Despite performing frequent self-monitoring of blood glucose (SMBG) four or more times daily, severe hypoglycemic episodes increase by threefold in IIT patients regardless of the method of insulin delivery (2). The recently available continuous glucose monitoring (CGM) is a device that provides patients with the ability to view real-time glucose values, review recent glucose trends, and receive hypo- or hyperglycemic alarms. The CGM systems provide a complete real-time glucose profile by measuring glucose levels at 1- to 5-min intervals. This allows for an accurate, large-scale representation of overall glucose trends, as compared with the isolated values offered by fingerstick SMBG (3–5). Furthermore, CGM use has been reported in both controlled (6–8) and nonrandomized trials (9–12) to improve glucose control, reduce hypo- and hyperglycemic excursions (10,11), and improve glucose variability (13,14). Despite limited data, it is commonly believed that optimal diabetes management can best be achieved when a CGM is used by IIT patients, especially when combined with insulin pump (CSII) therapy.
This prospective study was conducted comparing the usefulness of CGM in adult patients with type 1 diabetes using MDI or CSII.
RESEARCH DESIGN AND METHODS
Study population
Sixty subjects with type 1 diabetes were enrolled at the Barbara Davis Center for Diabetes at the University of Colorado Denver. The first 60 adult patients (30 patients using MDI therapy and 30 on CSII therapy) who qualified based on the inclusion and exclusion criteria were included in this study. The baseline A1C values were within 6.5–10.0%, and patients were willing to monitor their glucose (SMBG) at least four times a day. In addition, all subjects were willing to wear the CGM continuously throughout the 6-month study period. Subjects who had used CGM for ≥6 weeks during the past 3 months were excluded from the study. This was a nonrandomized prospective real-life clinical trial. Baseline demographics were similar in both groups (Table 1).
Table 1.
Baseline demographic* and A1C results for study population
| Study population mean (SD)* | ITT | Population | ||
|---|---|---|---|---|
| MDI | CSII | MDI | CSII | |
| n | 30 | 30 | 17 | 17 |
| Age | 39.0 (11.35) | 36.8 (8.84) | 40.1 (7.49) | 37.9 (12.05) |
| Sex* | 15 (52%) | 18 (60%) | 13 (77%) | 12 (71%) |
| Ethnicity* | 30 (100%) | 29 (96.7%) | 17 (100%) | 17 (100%) |
| Height (cm) | 173.6 (10.50) | 171.5 (10.77) | 172.5 (12.09) | 174.3 (8.65) |
| Weight (kg) | 81.3 (16.35) | 81.5 (17.09) | 83.7 (18.24) | 82.3 (13.8) |
| BMI (kg/m2) | 26.9 (4.53) | 27.6 (4.65) | 28.1 (5.27) | 27.0 (3.77) |
| Type 1 diabetes duration | 22.2 (10.14) | 21.9 (11.02) | 26.1 (10.02) | 23.0 (13.73) |
| Screening A1C (%)† | 7.62 (0.68) | 7.61 (0.76) | 7.56 (0.56) | 7.41 (0.49) |
| A1C at week 4, end of blinded CGM (%) baseline | 7.74 (0.74) | 7.97 (0.96) | 7.77 (0.66) | 7.72 (0.53) |
| A1C at 3 months‡ | 7.40 (0.71) | 7.35 (0.68) | 7.36 (0.68) | 7.18 (0.45) |
| A1C at 6 months, end of study (%) | 7.78 (1.03) | 7.59 (0.91) | 7.56 (0.72) | 7.39 (0.64) |
Study procedures
The protocol was approved by the institutional review board, and all subjects provided written informed consent before enrollment. Subjects were enrolled prospectively and scheduled for a total of seven clinic visits (at 0, 4, 8, 12, 16, 20, and 24 weeks). Throughout this study, subjects used the DexCom SevenPLUS CGM system comprised of a 7-day transcutaneous sensor, a transmitter, and a receiver for 7-day wearing periods. Initially, subjects were blinded to glucose data for the first 4 weeks and thereafter unblinded for the remainder of the study (20 weeks). At each clinic visit, A1C values were collected, patients were screened for adverse events, and sensor insertion sites were assessed. Digital data from all CGM receivers and SMBG meters were downloaded for analysis. The data collected during the blinded phase served as the control period.
All subjects were provided with an SMBG meter (OneTouch Ultra, LifeScan, Milpitas, CA) and test strips, which were used for receiver calibration and diabetes self-management purposes. Subjects were instructed to use only the meter(s) provided to them for all SMBG measurements taken throughout the study duration.
Subjects were instructed to wear the study device during all normal daily activities and to capture events such as bolus insulin dose, carbohydrate intake, and exercise using the CGM event input function. At the beginning of the study, the providers reviewed general principles of continuous glucose monitoring with the subjects. In addition, providers evaluated trends and alarms on the CGM and counseled patients on diabetes management including insulin dosing, treatment of hypoglycemia, and SMBG. All subjects were provided guidance (according to subjects’ needs) for adjusting insulin dose and/or food intake based on glucose trends, rate of change of glucose, and absolute glucose value in the case of hypoglycemia. Subjects were also instructed in timing of insulin bolus 15–30 min before the meals in an attempt to achieve euglycemia in the postprandial phase. General guidelines for increasing or decreasing the insulin dose were also provided, as have been previously described (15,16). Subjects were also asked to wear the device and the sensor continuously for the study period.
During the period of unblinded CGM use, receivers provided real-time continuous glucose data to the patients. When making treatment changes, subjects were instructed to use CGM data as an adjunct to, and not as a replacement for, SMBG values as per U.S. Food and Drug Administration label.
Outcome measures
The primary outcome for both IIT therapy groups was defined as change in A1C, comparing screening values to 3- and 6-month follow-up P values. The secondary outcome was defined by measuring glycemic variability, including the amount of time spent within the target range (WTR 3.9–10.0 mmol/L [70–180 mg/dL]), hypoglycemic excursions (below target range [BTR] <3.9 mmol/L [70 mg/dL]), hyperglycemia excursions (above target range [ATR] ≥10.0 mmol/L [180 mg/dL]), mean and SD of CGM glucose, and other glycemic variability indexes (17). Compliance comparisons of CGM use between both groups were made by analyzing the number of sensors used, and adverse effects of the device such as insertion site reactions and adhesive-related inflammation or bleeding were recorded.
Statistical analysis
Descriptive statistics for continuous variables were summarized using mean, SD, median, minimum, and maximum. Categorical variables such as patient diabetes history and baseline characteristics are summarized using counts and percentages. The repeated-measures ANCOVA model was used to evaluate the A1C change over time and therapy groups, adjusted for random subjects. Change in A1C between the 6-month follow-up and baseline within an individual was calculated and compared between therapy groups using an independent t test. The two-sided 95% CI was then constructed based on the model, and the noninferiority of therapy group was then concluded if the upper limit was <0.4% A1C, a prespecified margin of indifference. The analysis was performed on the full intent-to-treat population (subjects enrolled for whom at least one A1C was collected during the study). Similar analyses were conducted using data from only subjects who were categorized as compliant, and a similar approach was used for other continuous end points (i.e., time spent WTR, hypo- and hyperglycemia excursions, glycemic variability measurements). The blinded CGM period was considered to be the baseline for these measurements. A P value ≤0.05 was considered as statistically significant. All analyses were performed using SAS software (version 9.1.3; SAS Institute, Cary, NC).
RESULTS
Of the 60 subjects enrolled, 58 completed all study visits. One subject withdrew from the study after baseline and one subject did not complete the last two visits because of scheduling conflicts. At the 6-month mark, 34 subjects met the per-protocol requirements of using the CGM at least 6 days a week. An equal number of per-protocol subjects were on MDI and CSII therapy (n = 17, Table 1). All patients were provided with unlimited sensors; however, because of schedule conflicts, losing the sensor or being bothered by the alarms were the main reasons for noncompliance in 26 subjects.
A1C-ITT and per-protocol analysis
Over the 6-month study period, there was a tendency of similar A1C values between MDI and CSII groups (P = 0.584). Significant changes in A1C during follow-up were observed (P < 0.001), with lowest A1C values of 7.4% for MDI and 7.3% for CSII at week 12. The mean (± SD) change in A1C within subjects from screening to 6 months was 0.16 ± 0.84% for MDI and 0.02 ± 0.59% for CSII. The 95% CI for difference in change of A1C between MDI and CSII groups was 0.26% (less than the 0.4% predetermined noninferiority margin). The difference in A1C from screening to 6 months was not statistically significant in both MDI and CSII therapy groups (P = 0.3273).
Similar results were obtained for per-protocol population analysis (Table 1). For the MDI group, A1C reduction from screening to 6 months among protocol-compliant subjects was −0.16 ± 0.59% (mean ± SD) compared with an increasing A1C of 0.36 ± 0.85% for noncompliant subjects. For the CSII group, the protocol compliant subjects showed changes in A1C similar to noncompliant subjects (−0.02 vs. 0.07%, respectively).
Glycemic excursions
At the 6-month follow-up visit, the overall study population’s (ITT) time spent WTR (3.9–10.0 mmol/L [70–180 mg/dL]) increased by an average of 0.7 h/day for the CSII group and 1.4 h/day for the MDI group when compared with the CGM-blinded baseline period (Table 2, P = 0.009). Similarly, the time spent in the hypoglycemic range (<3.9 mmol/L [70 mg/dL]) was reduced by 21% in the CSII group and 30% in the MDI group (Table 2, _P_ > 0.05).
Table 2.
Glucose control and variability indexes in the two groups
| Blinded first month | Unblinded sixth month | *P value for CSII vs. MDI | ‡P value for month 6 unblinded vs. blinded | |||
|---|---|---|---|---|---|---|
| MDI | CSII | MDI | CSII | |||
| ITT population* | ||||||
| Glucose average | 9.8 mmol/L (176.4 mg/dL) | 9.9 mmol/L (178.3 mg/dL) | 9.69 mmol/L (174.0 mg/dL) | 9.3 mmol/L (166.7 mg/dL) | 0.304 | 0.001 |
| Glucose SD | 4.3 mmol/L (77.0 mg/dL) | 4.2 mmol/L (75.1 mg/dL) | 3.7 mmol/L (67.4 mg/dL | 3.8 mmol/L (68.0 mg/dL) | 0.089 | <0.001 |
| Hours/day in hypoglycemia (<3.9 mmol/L [70 mg/dL]) | 1.3 | 1.3 | 1.0 | 1.0 | 0.728 | <0.001 |
| Hours/day in hyperglycemia (>10.0 mmol/L [180 mg/dL]) | 8.2 | 8.9 | 8.0 | 6.9 | 0.421 | <0.001 |
| Hours/day within target range (70–180 mg/dL) | 9.7 | 9.8 | 10.4 | 11.2 | 0.297 | 0.009 |
| MAGE | 10.6 mmol/L (190.2 mg/dL) | 10.1 mmol/L (182.8 mg/dL) | 9.3 mmol/L (166.8 mg/dL) | 9.4 mmol/L (168.5 mg/dL) | 0.133 | <0.001 |
| GRADE | 11.5 | 11.7 | 10.8 | 9.9 | 0.890 | <0.001 |
| Per-protocol population† | ||||||
| Glucose average | 9.8 mmol/L (177.3 mg/dL) | 9.5 mmol/L (171.2 mg/dL) | 9.5 mmol/L (170.7 mg/dL) | 8.8 mmol/L (158.2 mg/dL) | 0.001 | <0.001 |
| Glucose SD | 4.3 mmol/L (78.0 mg/dL) | 4.1 mmol/L (73.5 mg/dL) | 3.8 mmol/L (67.7 mg/dL) | 3.6 mmol/L (64.2 mg/dL) | 0.055 | <0.001 |
| Hours/day in hypoglycemia (<70 mg/dL) | 1.4 | 1.5 | 1.1 | 1.1 | 0.599 | 0.008 |
| Hours/day in hyperglycemia (>180 mg/dL) | 8.8 | 8.6 | 8.4 | 6.8 | 0.038 | 0.004 |
| Hours/day within target range (70–180 mg/dL) | 9.8 | 10.7 | 11.3 | 13.5 | 0.017 | <0.001 |
| MAGE | 10.8 mmol/L (194.4 mg/dL) | 9.9 mmol/L (178.1 mg/dL) | 9.4 mmol/L (169.8 mg/dL) | 8.7 mmol/L (155.9 mg/dL) | 0.007 | <0.001 |
| GRADE | 11.7 | 10.9 | 10.5 | 8.9 | 0.013 | <0.001 |
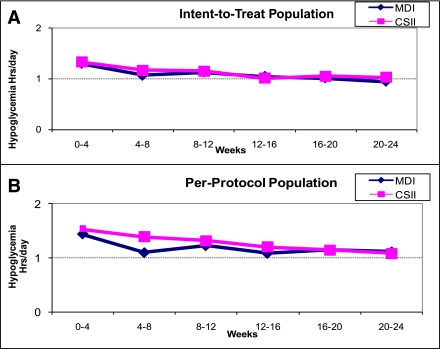
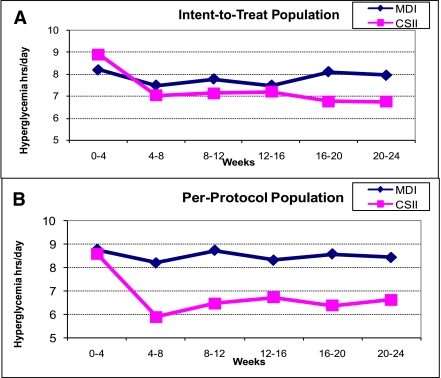
For both therapy groups, the number of glycemic excursions (hyper- and hypoglycemic) were decreased (P < 0.001; Figs. 1 and 2) similarly. When compared with the CGM-blinded phase, subjects spent less time in hypo- and hyperglycemia during the unblinded CGM phase.
Figure 1.
Changes in hypoglycemic excursions during the study period were similar in ITT (n = 60) (A) and per-protocol analysis (n = 34) (B).
Figure 2.
Changes in hyperglycemic excursions during the study period were also similar in the ITT analysis (n = 60) (A). However, hyperglycemic excursions were significantly lower in the CSII group in the subgroup analysis (per-protocol analysis, n = 34, B).
This analysis was repeated for unblinded CGM data on the per-protocol population (17 in each group; Figs. 1_B_ and 2_B_). Reduction in time spent in both hyper- and hypoglycemic ranges was observed (23 and 12% for MDI and CSII groups, respectively). The average time spent in hypoglycemic (<3.9 mmol/L [70 mg/dL]) and hyperglycemic (>10.0 mmol/L [180 mg/dL]) ranges was further evaluated at each clinic visit (∼1 month). The reductions of these glycemic excursions were apparent at 1 month after unblinded CGM use. The reduction in hypoglycemia between MDI and CSII therapy groups were similar for all study populations (Fig. 1). A greater reduction in time spent in hyperglycemic ranges was observed for subjects who used CSII, particularly for the per-protocol population (Fig. 2_B_, P < 0.05).
Mean glucose and SD
From baseline measurements to the 6-month follow-up visit, the mean glucose concentration decreased in both therapy groups (P < 0.001; Table 2). Subjects showed a tendency of mean glucose values from 9.8 mmol/L (176 mg/dL) to 9.7 mmol/L (174 mg/dL) for MDI and from 9.9 mmol/L (178 mg/dL) to 9.2 mmol/L (166 mg/dL) for CSII (P = 0.468). The SD of glucose within subjects also reduced from 4.3 mmol/L (77 mg/dL) to 3.8 mmol/L (67 mg/dL) for MDI and from 4.1 mmol/L (75 mg/dL) to 3.6 mmol/L (68 mg/dL) for CSII (P = 0.364).
Similar trends were observed in the per-protocol population, where the reduction in glucose and glycemic variability was greater. In the MDI group, the mean CGM glucose changed from 9.8 mmol/L (177 mg/dL) to 9.5 mmol/L (171 mg/dL) and 9.5 mmol/L (171 mg/dL) to 8.8 mmol/L (158 mg/dL) from baseline to the 6-month follow-up (Table 2). The CSII group showed greater reduction in CGM glucose and glucose variation compared with the MDI group (P = 0.03 and 0.04, respectively; Table 2).
Usability
CGM compliance was similar between the therapy groups. In both therapy groups, similar numbers of sensors were provided to study subjects and similar numbers of CGM devices were used (29 and 28 for MDI and CSII, respectively). In the noncompliant subjects (as defined per-protocol), fewer sensors were used by subjects who used CSII (24 devices) than subjects who used MDI (28 devices). Retrospectively, 93% of CGM data were available for the protocol-compliant population, whereas the availability of CGM data dropped to 68% for the noncompliance population.
Glycemic variability indexes
Independent of baseline A1C, the glycemic variability indexes including mean amplitude of glycemic excursions (MAGE) (12) and glycemic risk assessment diabetes equation score (GRADE) (13) were reduced in the 6-month unblinded period when compared with the blinded period. No statistical difference in these measures was observed for ITT analysis. The reductions of these indexes were more prominent in the CSII group for the per-protocol population (P = 0.02).
Safety
Over the 6-month duration of this study, 158 insertion areas were inspected. Of these inspections, the incidence of adverse effects associated with sensor insertion sites were as follows: 37 (23%) mild erythema, 5 (3%) moderate erythema, 7 (4%) mild edema, 2 (1%) moderate edema, 10 (6%) mild bruising, and 1 (0.6%) severe bruising. Adverse effects associated with sensor adhesive included the following: 40 (25%) mild erythema, 7 (4%) moderate erythema, 2 (1%) severe erythema, 2 (1.3%) mild edema, 3 (1.9%) moderate edema, 1 (0.6%) severe edema, 5 (3%) mild bruising, and 3 (1.9%) moderate bruising. All adverse events were resolved or stable upon completion of the study. No sensor insertion site infections or hypoglycemic events requiring assistance were reported. Similar adverse events were observed in both therapy groups.
CONCLUSIONS
To the best of our knowledge, this is the first (pilot) prospective real-life study showing similar glycemic benefits of using CGM for IIT patients on MDI or CSII. Recent studies demonstrated that the use of real-time CGM systems with or without pump therapy resulted in reduction in A1C, improved glycemic excursions, and reduced incidences of hypoglycemia (6,7,18). The Juvenile Diabetes Research Foundation studies included both patients on MDI and CSII therapy. The Juvenile Diabetes Research Foundation study, however, did not report the subgroup analyses comparing patients on MDI therapy to those on CSII therapy. The STAR3 study provided only comparison between integrated CGM with the pump system and injection therapy (MDI) where CGM was not used (18). Thus, it is possible that STAR3 results may entirely be due to use of CGM in the integrated pump and CGM group.
Although a statistically significant but similar decrease in A1C was observed at 3 months after the initial screening, the A1C measurements at 6 months were similar to the screening values in both MDI and CSII groups (both IIT and protocol-compliant populations). The study population was relatively well controlled with a mean baseline A1C of 7.6% in both groups. All subjects who were included in this study did qualify based on the inclusion and exclusion criteria as described above. Despite patients being informed of continuous use of the sensor throughout the study period, only 34 patients followed the protocol. This has been noted in many other clinical trials (6).
Patients were initially educated on trends and pattern management while using the CGM. The protocol allowed for real-life use of CGM without specific instruction by a physician or diabetes educator for insulin adjustments during the duration of the protocol. As discussed above in the study procedures, all subjects were provided guidance for adjusting insulin dose and/or food intake based on glucose trends rate of change of glucose. No follow-up education on CGM use was planned during this study. Continued diabetes education may be a key component of successful long-term CGM outcomes after 3 months.
Despite the insignificant A1C reduction at the conclusion of this study, there was an improvement of glycemic variability (MAGE, GRADE) with real-time CGM use. There was reduction in time spent in both hypoglycemic and hyperglycemic ranges and improvement in time WTR for both groups during the unblinded phase. There was a greater reduction in time spent in hyperglycemia in subjects using CSII, which is not surprising, since administrating correction boluses is easier with CSII. The difference in reduction of time spent in hypoglycemia between both therapy groups was not statistically significant. Different glucose variability indexes have also been previously shown to improve similarly with MDI and CSII treatment groups in many retrospective analyses (17,19–22).
We conclude that 6-month use of real-time CGM showed similar benefits in usability, persistence, and glycemic control in CSII- and MDI-treated adult patients with type 1 diabetes, especially when patients are unblinded.
Acknowledgments
The authors thank DexCom, Inc. (San Diego, CA), for sponsoring this study through a grant provided to the University of Colorado, Denver. S.K.G. received honoraria for giving lectures for DexCom Inc. M.K.V. is on the advisory board for DexCom and has received honoraria. H.A.M. received honoraria from DexCom for giving presentations. No other potential conflicts of interest relevant to this article were reported.
This study was investigator-initiated by S.K.G. and conducted at the Barbara Davis Center for Diabetes. S.K.G. was the principal investigator of the study, helped in data analysis, wrote the manuscript, and reviewed all versions of the manuscript. M.K.V. and C.R.B. were the coordinators of the study and helped with writing the manuscript. L.B.C. helped run the study. H.A.M., B.J.F., and R.M.H. helped with reviewing and editing the manuscript.
Data from this trial were presented in part at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010, and at the 46th European Association for the Study of Diabetes (EASD) Annual Meeting, Stockholm, Sweden, 20–24 September 2010.
Footnotes
Clinical trial reg. no. NCT01104142, clinicaltrials.gov.
References
- 1.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.Chase HP, Roberts MD, Wightman C, et al. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics 2003;111:790–794 [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933–938 [DOI] [PubMed] [Google Scholar]
- 5.Tanenberg R, Bode B, Lane W, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc 2004;79:1521–1526 [DOI] [PubMed] [Google Scholar]
- 6.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 7.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 9.Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract 1999;46:183–190 [DOI] [PubMed] [Google Scholar]
- 10.Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care 2004;27:734–738 [DOI] [PubMed] [Google Scholar]
- 11.Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care 2006;29:44–50 [DOI] [PubMed] [Google Scholar]
- 12.Garg SK, Kelly WC, Voelmle MK, et al. Continuous home monitoring of glucose: improved glycemic control with real-life use of continuous glucose sensors in adult subjects with type 1 diabetes. Diabetes Care 2007;30:3023–3025 [DOI] [PubMed] [Google Scholar]
- 13.Rodbard D, Jovanovic L, Garg S. Responses to continuous glucose monitoring in patients with type 1 diabetes using multiple daily injections and insulin pumps. Diabetes Technol Ther 2009;11:757–765 [DOI] [PubMed] [Google Scholar]
- 14.Moser EG, Crew LB, Garg SK. Role of continuous glucose monitoring in diabetes management. Avances en Diabetologia 2010;26:73–79 [Google Scholar]
- 15.Jenkins AJ, Krishnamurthy B, Best JD, et al. Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial. Diabetes Care 2010;33:1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch IB. Algorithms for care in adults using continuous glucose monitoring. J Diabetes Sci Tech 2007;1:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodbard D, Bailey T, Jovanovic L, Zisser H, Kaplan R, Garg SK. Improved quality of glycemic control and reduced glycemic variability with use of continuous glucose monitoring. Diabetes Technol Ther 2009;11:717–723 [DOI] [PubMed] [Google Scholar]
- 18.Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 19.Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med 2007;24:753–758 [DOI] [PubMed] [Google Scholar]
- 20.Garg SK. The future of continuous glucose monitoring. Diabetes Technol Ther 2009;11(Suppl. 1):S1–S3 [DOI] [PubMed] [Google Scholar]
- 21.Garg SK. Role of continuous glucose monitoring in patients with diabetes using multiple daily insulin injections. Infusystems USA 2009;6:9–14 [Google Scholar]
- 22.Blevins TC, Bode BW, Garg SK, et al. AACE Continuous Monitoring Task Force. Statement by the American Association of Clinical Endocrinologists Consensus Panel on continuous glucose monitoring. Endocr Pract 2010;16:730–745 [DOI] [PubMed] [Google Scholar]