Diagnosis, Treatment, and Prevention of Cerebral Palsy in Near-Term/Term Infants (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 9.
Published in final edited form as: Clin Obstet Gynecol. 2008 Dec;51(4):816–828. doi: 10.1097/GRF.0b013e3181870ba7
Abstract
Cerebral palsy is the most prevalent cause of persisting motor function impairment. In a majority of cases, the predominant motor abnormality is spasticity; other forms of cerebral palsy include dyskinetic (dystonia or choreoathetosis) and ataxic cerebral palsy. The care of individuals with cerebral palsy should include the provision of a primary care medical home for care coordination and suppor and diagnostic evaluations. Current strategies to decrease the risk of cerebral palsy include interventions to prolong pregnancy (e.g., 17α-progesterone), limiting the number of multiple gestations related to assisted reproductive technology, antenatal steroids for mothers expected to deliver prematurely, caffeine for extremely low birth weight neonates, and induced hypothermia for a subgroup of neonates diagnosed with hypoxic-ischemic encephalopathy.
Keywords: cerebral palsy, function impairment, neuroimaging classification, postnatal prevention
Introduction
Among the variety of disorders that severely impair motor function in young children, cerebral palsy is the most prevalent. In birth cohorts from developed countries, the prevalence is 1-2/1000 live births(1). The prevalence rises dramatically with decreasing gestational age at birth such that among extremely low gestational age newborns (i.e., gestational age < 28 weeks), the prevalence is about 100 per 1000 of surviving infant, a 100-fold higher risk than infants born at term. As a function of all live births, the prevalence has been remarkably stable for decades, but this has not been the case among very low birth weight and very preterm infants, among whom prevalence increased after the introduction of neonatal intensive care and has begun to decrease in the past decade(1).
In addition to motor manifestations, children with cerebral palsy frequently exhibit cognitive and sensory impairments, epilepsy, and nutritional deficiencies. Except in the mildest cases, cerebral palsy has a substantial impact on families’ well being and societal health care costs.
As a result of both laboratory-based and clinical research over the past several decades, a few perinatal interventions have been identified which probably are effective for lowering the risk of cerebral palsy. Successes from clinical research on cerebral palsy has been enhanced by efforts to increase the reliability (and thereby validity) of the diagnosis and classification of this disorder. These efforts have improved the efficiency of observational and experimental epidemiologic studies related to prevention, as well as intervention trials for individuals with cerebral palsy.
In this manuscript I will begin by reviewing research on the diagnosis and classification of cerebral palsy, including efforts to standardize diagnostic criteria, quantify severity in terms of motor impairment and impact on quality of life, and classify children with regard to location and type of neurological abnormality, associated non-motor impairments, and neuroimaging findings. I will then discuss the care of individuals particularly, children, with cerebral palsy, with a primary focus on evidence from randomized trials. In concluding I will discuss preventive interventions that appear likely to have an impact on the risk of cerebral palsy or that are currently under study in clinical trials. While some of these interventions are not applicable to obstetric care, it is likely that all will be of interest to obstetricians.
Diagnosis of Cerebral Palsy
The diagnosis of cerebral palsy is based on a clinical assessment, and not on laboratory testing or neuroimaging. A recent international working group offered the following definition for cerebral palsy: “Cerebral palsy is a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain.”(2) This definition allows for heterogeneity of clinical manifestations (“a group of ... disorders”) and emphasizes that impaired movement and posture due to a disturbance in the brain is the invariant clinical manifestation. While the underlying abnormality of the brain is presumed to be permanent and non-progressive, there is overwhelming evidence that clinical manifestations and severity of functional impairment often change over time. Thus from a patient’s perspective, the term “permanent” may connate an unduly pessimistic prognosis. In contrast to earlier definitions of cerebral palsy, that proposed by the aforementioned international working group included an explicit criterion for the lower limit of abnormality that must be exceeded in order to diagnose CP, i.e., “activity limitation”.
On the other hand, this working group did not offer a readily operational approach to the ascertainment of activity limitation. The approach that appears to be most widely used is based on a standardized measure of motor function, the Gross Motor Function Classification System (GMFCS)(3), which will be discussed below in greater detail. The GMFCS has been included in several recent randomized clinical trials as part of the definition of severity, and in these trials children with mild cerebral palsy without functional impairment were not considered to have the outcome of interest. The Western Australian Cerebral Palsy Register grades severity using the GMFCS, which allows for researchers to include or exclude cases without functional impairment. In an international study involving prematurely born children with cerebral palsy, “disabling”, as opposed to “non-disabling” cerebral palsy was ascertained with greater reliability, suggesting that the exclusion of cases without functional impairment may improve the validity of observational epidemiologic studies as well as randomized trials in which cerebral palsy is an outcome. Conversely, clinicians should regard with caution those studies of cerebral palsy in which information is lacking about the severity of cerebral palsy, as assessed with a reliable measure.
In clinical practice, the diagnosis of cerebral palsy is typically based on observations or parent reports of attained motor milestones, such as sitting, pulling to stand, and walking, and evaluation of posture, deep tendon reflexes, and muscle tone. Particularly among infants born prematurely, neurological abnormalities, observed in the early months of life, may not be associated with motor impairment and may resolve during the first one or two years of life. One such abnormality, transient dystonia, was described in early studies of premature infants and refers to abnormal neurological signs (e.g., hyperextension of the trunk) that are no longer present after one year of age. Because the diagnosis of cerebral palsy depends in part on neurological findings that are subject to inter-examiner variation, with regard to both the method used to elicit the neurological finding as well as the interpretation of the finding, and because neurological abnormalities may be transient, many clinicians avoid basing the diagnosis on a single aspect of the parent’s report or the clinician’s examination and typically will make a definitive diagnosis only after repeated examination(s).
Because cerebral palsy occurs in only 1-2 / 1000 live births, prospective ascertainment of cases has been limited primarily to cohorts of high risk infants, such as low birth weight babies, very preterm infants, and infants enrolled in clinical trials. For population-based studies, registries of children with cerebral palsy have been used, such as those established in Sweden, Western Australia, Metropolitan Atlanta, and Europe. Children listed in these registries are identified from records of human services providers and in some studies the diagnosis is confirmed by examination of medical records or of the child. These population studies have been particularly informative about trends in prevalence over time.
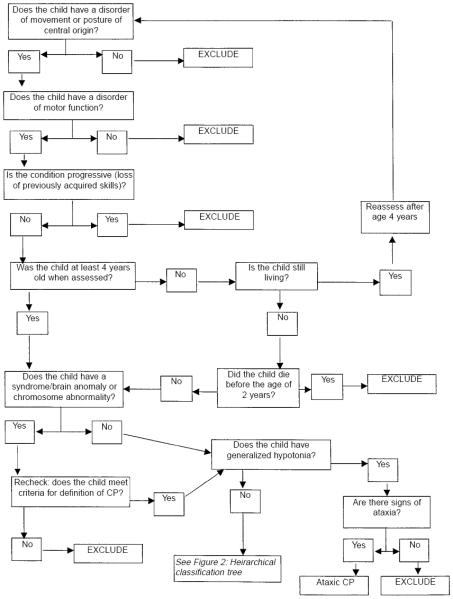
In many recent studies of high risk infants(4-6), painstaking efforts were directed at standardizing the definition of cerebral palsy used for prospective identification. With some minor differences between studies, each of these studies base the definition on a combination of delayed motor development (either observed or parent-reported), deep tendon reflexes, posture, and muscle tone (see Figure 1). For more details the reader is directed to reference(7).
Figure 1.
Decision tree for inclusion/exclusion of cases of cerebral palsy on SCPE register. Reproduced with permission from Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000; 42: 816-824
For both clinical as well as research purposes, cerebral palsy has often been classified according to the nature of the movement disorder (spasticity, ataxia, dystonia, and athetosis) and the anatomic, or topographic distribution of the motor abnormalities. The predominant abnormality among children born prematurely is spasticity, referring to a velocity dependent increase in muscle tone. Spasticity can be assessed clinically as the joint angle at which an increase muscle tone (resistance to stretch) is encountered. In a much smaller proportion of cases, referred to as having dyskinetic cerebral palsy, the predominant abnormality is either dystonia or choreo-athetosis. Dystonia refers to hypertonia and reduced activity; choreoathetosis, to irregular, spasmodic, involuntary movements of the limbs or facial muscles. With ataxic cerebral palsy there is a loss of orderly muscular coordination, so that movements are performed with abnormal force, rhythm, and accuracy.
A traditional classification of children with spastic cerebral palsy includes spastic diplegia (bilateral spasticity with leg involvement greater than arm), spastic hemiplegia (unilateral spasticity), or quadriplegia (bilateral spasticity with arm involvement equal to or greater than leg). In a large population-based study of very low birth weight children with cerebral palsy, 25% of children with spastic CP had hemiplegia, 37.5% had quadriplegia, and 37.5% had diplegia. Children with hemiplegia almost always develop independent ambulation, whereas a majority of those with quadriplegia do not. There is some evidence that the profile of risk factors differs for each of these topgraphic types of cerebral palsy. It should be noted, however, that the inter-rater reliability of examiners’ topographic classification is not high; thus the Surveillance for Cerebral Palsy in Europe (SCPE), a multi-center research collaboration, has proposed an alternative classification that includes unilateral spastic cerebral palsy, bilateral spastic cerebral palsy, dystonic cerebral palsy, choreo-athetoid cerebral palsy, and ataxia (see Figure 2). Currently no method of topographic classification has been broadly agreed upon.
Figure 2.
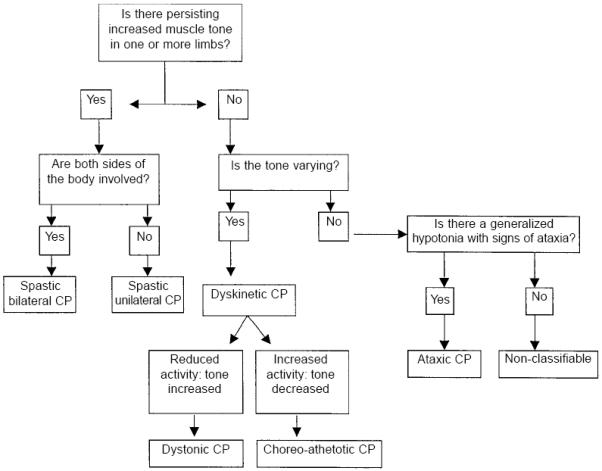
Hierarchical classification tree of cerebral palsy sub-types. Reproduced with permission from Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol 2000; 42: 816-824
More agreement has been achieved on classification of cerebral palsy in terms of the functional severity. More than a decade ago, Palisano and his colleagues developed the GMFCS, which defines five levels of gross motor function, which have been shown to correspond to five distinct “trajectories” of motor development. For example, among children younger than 2 years, those at level II can “maintain floor sitting but may need to use their hands for support to maintain balance,” whereas those at level IV have head control but trunk support is required for floor sitting”. The GMFCS correlates strongly with the World Health Organization International Classification of Impairments, Disabilities and Handicap code, but is considerably less time-consuming and can be derived from medical records. The inter-rater reliability of the GMFCS is high, as is the stability over time. The GMFCS level of children, as reported by parents, agrees well with that assigned by a physician; and the level self-reported by adults agrees well with that assigned by a physical therapist..
Other assessments have been developed for fine motor function, including the ABILHAND-Kids, the Bimanual Fine Motor Function (BFMF) Classification, and the Manual Ability Classification System. These assessments have not been studied as extensively as the GMFCS, but in one study the BFMF correlated strongly with the GMFCS, and in another, a high level of agreement about the MACS was found between pairs of therapists. Thus a comprehensive description of an individual with cerebral palsy would include both the GMFCS as well as a measure of upper extremity (fine motor) function.
In a large proportion of individuals with cerebral palsy, the motor impairment is accompanied by secondary musculoskeletal problems, epilepsy, and disturbances of sensation, perception, cognition, communication, and behavior(8). Among children registered in the SCPE Collaboration, 31% have severe intellectual disability, 11% have severe visual disability, and 21% have epilepsy. The likelihood of associated impairments varies according to topographic type. Less than one half of children with leg dominant bilateral spastic cerebral palsy have mental retardation, which is present in more than one half of children with other forms of spastic cerebral palsy. Severe visual impairment is present in more than one half of children with four leg dominant spastic CP but only less than 10% of children with two leg dominant spastic cerebral palsy.
Quality of life has been defined as “an individual’s perception of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns.” Using validated instruments which allow children to self-report on their quality of life, researchers have found that most children with cerebral palsy report similar quality of life to children not affected with this disorder and that quality of life is not worse with greater levels of functional impairment(9). In contrast, health related quality of life, which measures life satisfaction in areas such as self-care, mobility, and communication is influenced by the severity of impairment.
Neuroimaging classification
Until recently, neuroimaging studies of preterm infants with cerebral palsy were based largely on neonatal cranial ultrasound. The most widely used classification system, derived from computed tomography but applied readily to ultrasound findings, was described by Papile et al. In this approach to classification, grade 1 refers to hemorrhage limited to the subependymal germinal matrix region, grade 2 refers to hemorrhage in the cerebral ventricule(s), grade 3 refers to hemorrhage in the ventricle(s) with ventricular enlargement, and grade 4 refers to hemorrhage in the periventricular cerebrum. More recent approaches to classification, justified by extensive research, emphasize echolucency in the periventricular cerebrum and moderate-to-severe ventricular enlargement as most predictive of subsequent cerebral palsy(10). Very low birth weight infants with either of these abnormalities have a greater than 50% risk of developing cerebral palsy. For more details see reference (11)
Most children who are born at term and subsequently develop cerebral palsy do not undergo neuroimaging studies as neonates, but rather, after the diagnosis is suspected. Magnetic resonance imaging (MRI) is recommended for children with neurological findings suggestive of cerebral palsy in order to determine if a brain abnormality is present. In a study of 273 children with cerebral palsy who were born after 35 weeks gestation and underwent neuroimaging, one third of infants who underwent neuroimaging had normal studies(12). The most frequent observed abnormality was focal infarction, which was observed in 22% of the children and 45% of those with hemiplegia. The next most common finding was brain malformation, including schizencephaly, hydrocephalus, polymigrogyria, lissenencephaly, agenesis of the cropus callosum, holoprosencephaly, septooptic dysplasia, and cerebellar anomalies. In 12% of children neuroimaging revealed periventricular leukomalacia, a finding often is associated with prematurity. Of note is that only 5% of children had neuroimaging findings considered specific to hypoxia-ischemia(12).
Care of the child with cerebral palsy
Initial assessments (see Figure 3) for children diagnosed with cerebral palsy include neuroimaging if the etiology has not been established and metabolic and genetic studies if clinical history and findings on neuroimaging do not determine a specific etiology or if there are aspects of the history or physical examination that suggestive of a metabolic or genetic etiology (including a brain malformation). In addition, testing for prothrombotic abnormalities of coagulation should be considered in individuals with hemiplegia. All children with cerebral palsy should be screened for mental retardation, ophthalmologic and hearing impairment, and speech and language disorders, and nutrition and growth should be monitored(13).
Figure 3.
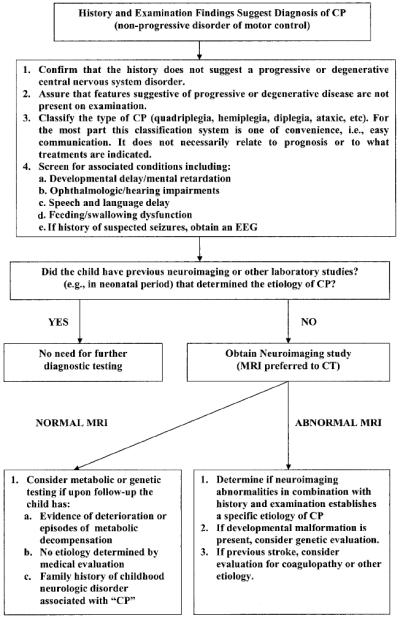
Alogorithm for evaluation of the child with cerebral palsy. Reprinted with permission from Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, Stevenson R; Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004 Mar 23;62(6):851-63
The American Academy of Pediatrics has emphasized the importance of a primary care “medical home” for children with chronic illnesses, including cerebral palsy(8). The roles of the medical home include care coordination to minimize redundant services and assure comprehensive services, monitoring of response to treatments and impact of the child’s illness on the family, interpreting findings to the family, orchestrating comanagment with specialists and specialty teams, and advocating for the patient with payers and providers such as the public schools(8). (Beginning in 1975, a series of United States public laws has specified that all handicapped children have available to them a free appropriate education designed to meet their unique needs. The most recent of these is Public Law 108-446, the Individuals with Disabilities Education Improvement Act of 2004.)
As discussed above, a large majority of children with cerebral palsy have spasticity. Active management of spasticity is needed to prevent painful contractures and deformities and promote optimal function. This management generally is provided by multidisciplinary teams that include physical therapists, orthopedic surgeons, and physiatrists. Physical therapy, which is uniformly utilized for cerebral palsy has not been subjected to randomized trials, but is widely accepted as a component of standard management. Table 1 describes the results of systematic reviews for five therapies used in the management of spasticity in patients with cerebral palsy. Those provided by the Cochrane Collaboration can be assessed at http://www.mrw.interscience.wiley.com/cochrane. For each of these therapies systematic reviews have provided evidence of benefit. Botulinum toxin type A for management of spasticity and casting for management of equinus deformity of the foot are probably the most widely available and used. Dorsal rhizotomy is a more invasive approach to the management of spastic diplegia and is reserved for the more difficult cases.
Table 1.
Therapies used to manage spasticity in cerebral palsy
| Therapy | # studies (# patients studied) | # randomized trials (# patients randomized) | Conclusions |
|---|---|---|---|
| Botulinum toxin type A in the treatment of lower limb spasticity | 3 (52) | 3 (52) | no strong controlled evidence found to support or refute the use of Botulinum toxin type A for the treatment of leg spasticity (1) |
| Botulinum toxin type A for treatment of spastic equinus foot | Not provided | 4 (183) | Botulinum toxin type A superior to placebo for improvement of gait (2) |
| Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy | 2 (44) | 2 (44) | one of the two randomized trials reported promising results in support of reduced muscle tone following Botulinum toxin A injections; evidence not sufficient to support or refute the use of this therapy as an adjunct to managing the upper limb in children with spastic cerebral palsy (3) |
| selective’ dorsal rhizotomy plus physiotherapy | 3 (90) | 3 (90) | selective’ dorsal rhizotomy (SDR) plus physiotherapy reduces spasticity in children with spastic diplegia slightly improves gross motor function (4) |
| casting for equinus | 21 (473) | 9 (238) | No randomized trials available comparing protocols of casting in current use with no treatment; no strong and consistent evidence that combining casting and Botulinum toxin A is superior to using either intervention along; no evidence that the order of these two treatments affects outcome (5) |
Other therapies to improve function in cerebral palsy are listed in Table 2. Therapies specific for hemiplegia, such as constraint-induced movement therapy (CIMT) and hand-arm bimanual intensive therapy (HABIT), have been studied in a relatively small number of individuals. With CIMT, the non-involved upper extremity is restrained and the involved extremity is engaged in targeted practice. With HABIT, structured tasks requiring bimanual function are practiced in the context of play and functional activities. While more study is warranted, these appear to hold promise of benefit. More research on the effects of hyperbaric oxygen therapy is required, as there is considerable uncertainty about its efficiency and adverse effects, and this treatment costs approximately $400 per 90-minute session.
Table 2. Therapies used to improve function in children with cerebral palsy.
| Therapy | # studies (# patients studied) | # randomized trials (# patients randomized) | Conclusions |
|---|---|---|---|
| Speech and language therapy to improve the communication skills | 11 (46) | 1 (20) | trend towards improved communication skills; evidence not sufficient to recommend change in practice (1) |
| Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy | 3 (94) | 2 (49) | significant benefit in a single trial; positive trend favoring constraint-induced movement therapy (CIMT) and Forced Use; “given the limited evidence, the use of CIMT, modified CIMT and Forced Use should be considered experimental in children with hemiplegic cerebral palsy.” (2) |
| Hyperbaric oxygen therapy | 6 (449) | 2 (137) | Hyperbaric oxygen therapy and pressurized room air improved function to a similar degree; a proportion of patients treated with this therapy experience seizures (3) |
Prognosis
As discussed above, prognosis for independent ambulation depends in large part on the type of motor impairment. Ambulation status, intelligence quotient, quality of speech, and hand function together are predictive of employment status. For example, in a study of adults with cerebral palsy, intelligence quotient ≥ 80, and understandable speech, who were ambulatory and independent of the need for “significant assistance”, 90% were employed in a “competitive job” (i.e., one which could be filled by a similarly qualified person without a disability).
Mortality also is strongly associated with both the level of functional impairment as well as associated non-motor impairments. In one study of over 2014 individuals with cerebral palsy, the strongest predictor of mortality was intellectual disability. For example, among those with profound intellectual disability (i.e., IQ < 20), only one half survived into adulthood; whereas among those with IQ > 35, 92% survived to adulthood. More generally, mortality risk increases incrementally with increasing number of impairments, including intellectual, limb function, hearing, and vision. In a recent population-based study, the shortest life expectancy was observed among those individuals who were unable to lift their head in prone, who had a life expectancy of 20 years.
Antenatal approaches to prevention
To an alarming extent, cerebral palsy is mistakenly attributed to acts of omission or commission by obstetricians. Among the most important lines of research during the past two decades is the investigation of the role of perinatal infection and inflammation in acquired brain damage in both term and preterm fetuses and newborns(7;14), and the role of prothrombotic factors and other causes of neonatal stroke in the pathogenesis of congenital hemiplegia(15). Although epidemiologic studies indicate that less than 10% of cerebral palsy results from intrapartum hypoxia, three quarters of United States obstetricians report being the subject of a litigation event, most frequently related to their allegedly causing cerebral palsy by not preventing or treating fetal hypoxia(16). In a study of 46 malpractice cases, investigators concluded that “the severity of the patient’s disability, not the occurrence of an adverse event or an adverse event due to negligence, was predictive of payment to the plaintiff”(17).
Many assumptions about the effectiveness of obstetric care for preventing cerebral palsy generally are based on weak or no evidence(16). Published checklists provide a more evidence-based approach to attribution(18;19). A intrapartum event as a cause of cerebral palsy is more likely if significant fetal acidosis (such as pH < 7.0) and neonatal encephalopathy are observed, and is more likely with spastic quadriplegia or dyskinetic cerebral palsy. While these events might be the result of intrapartum hypoxia, they might also be the result of fetal infection. Thus there is almost always considerable uncertainty as to the cause of cerebral palsy, particularly among infants born at or near term.
Nonetheless, because approximately one-half of all new cases of cerebral palsy arise from the group of neonates born prematurely, it is possible that interventions which either prolong gestation or decrease the risk of preterm delivery will also decrease the risk of cerebral palsy. Specific approaches to reduce the rate of preterm birth, that are supported by a high level of evidence, include limiting the number of embryos transferred with in vitro fertilization, smoking cessation during pregnancy, screening for and treatment of asymptomatic bacteriuria during pregnancy, antiplatelet drugs to prevent preeclampsia, 17α-progesterone caproate, and cervical cerclage for women with previous preterm birth and short cervix (i.e., < 2.5 centimeters)(20). In addition, interventions which have been shown to prolong pregnancy include calcium channel blockers and an oxytocin antagonist (atosiban) for women with preterm labor and erythromycin for women with premature rupture of the membranes(20). In addition to these measures, the results of a meta-analysis of four trials suggests that treatment of mothers expected to deliver before 36 weeks gestation with glucocorticoids (eg. β-methasone) reduces the risk of cerebral palsy. In the Australasian Collaborative Trial of Magnesium Sulphate, magnesium sulfate treatment of mothers at risk for preterm birth before 30 weeks gestation reduced the risk of substantial gross motor dysfunction, and in a multicenter randomized trial in France a trend towards a reduction in cerebral white matter damage was observed. More information about the possible beneficial of antenatal magnesium sulfate is expected soon from the National Institutes of Health Maternal Fetal Medicine Network trial (Benefits of Antenatal Magnesium Sulfate).
Postnatal approaches to prevention
Most term or near-term infants who develop cerebral palsy have uneventful neonatal courses. A notable exception are those who have neonatal encephalopathy. One of the presumed causes of neonatal encephalopathy is intrapartum cerebral hypoxia and ischemia, which in severe cases could result in permanent brain damage manifesting as cerebral palsy. In such infants, hypothermia, either selectively applied to the head or total body, appears to decrease the risk of neurodevelopmental impairments, including cerebral palsy(5). While this intervention appears to be effective, it is applicable to only a small proportion of children who subsequently develop cerebral palsy.
In preterm infants, caffeine is the only therapy that has been shown in a multicenter trial to decrease the risk of cerebral palsy. In this trial, extremely low birth weight infants were randomized to caffeine or placebo in the first days of life. While the results might apply only to a small proportion of preterm infants, it is this subgroup that is at highest risk of cerebral palsy. Postnatal steroids, given to premature infants to decrease lung inflammation and decrease the risk of bronchopulmonary dysplasia, increase the risk of cerebral palsy. Thus limiting the use of this treatment can be expected to reduce the risk of cerebral palsy. The goal of recently completed trial by the National Institutes of Health Neonatal Research Network was to study the effect of aggressive phototherapy for hyperbilirubinemia on neurodevelopmental outcome. The results of this trial may be relevant to the prevention of cerebral palsy in extremely low birth weight infants.
Summary
Cerebral palsy is relatively common cause of persistent motor impairment and is associated with a variety of developmental disabilities. In developing countries, approximately one half of cases occur in prematurely born infants, and in this group the prevalence of cerebral palsy in developed countries appears to be decreasing over the past decade. In full term infants, the prevalence has been remarkably stable despite the advent of continuous fetal heart rate monitoring. The care of children with cerebral palsy requires a multidisciplinary, comprehensive, and coordinated approach. In developed countries, the self-reported quality of life for adolescents with cerebral palsy, except for severe cases, is similar to that of adolescents without cerebral palsy. Interventions that hold promise for reducing the prevalence of cerebral palsy include interventions to decrease the risk of premature birth, antenatal steroids given to mothers expected to deliver prematurely, treatment of mothers who are expected to deliver prior to 30 weeks gestation with magnesium sulfate, hypothermia for neonates diagnosed with hypoxic-ischemic encephalopathy, and caffeine for extremely low birth weight infants.
Footnotes
Sources of support: none to report
Reference List
- (1).Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clinics in Perinatology. 2006;33(2):251. doi: 10.1016/j.clp.2006.03.011. + [DOI] [PubMed] [Google Scholar]
- (2).Rosenbaum P. A report: the definition and classification of cerebral palsy April 2006 (vol 49, pg 8, 2007) Developmental Medicine and Child Neurology. 2007;49(6):480. [PubMed] [Google Scholar]
- (3).Rosenbaum P, Walter SD, Hanna SE, Palisano R, Russell DJ, Raina P, et al. Prognosis for gross motor function in cerebral palsy. JAMA. 2002;288(11):1357–1363. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- (4).Surveillance of Cerebral Palsy in Europe (SCPE) Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- (5).Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. New England Journal of Medicine. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- (6).Kuban KCK, O’Shea M, Allred E, Leviton A, Gilmore H, DuPlessis A, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. Journal of Child Neurology. 2005;20(10):829–831. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- (7).O’Shea TM. Cerebral palsy in very preterm infants: New epidemiological insights. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8(3):135–145. doi: 10.1002/mrdd.10032. [DOI] [PubMed] [Google Scholar]
- (8).Cooley WC. Providing a primary care medical home for children and youth with cerebral. Pediatrics. 2004;114(4):1106–1113. doi: 10.1542/peds.2004-1409. [DOI] [PubMed] [Google Scholar]
- (9).Dickinson HO, Parkinson KN, Ravens-Sieberer U, Schirripa G, Thyen U, Arnaud C, et al. Self-reported quality of life of 8-12-year-old children with cerebral palsy: a cross-sectional European study. Lancet. 2007;369(9580):2171–2178. doi: 10.1016/S0140-6736(07)61013-7. [DOI] [PubMed] [Google Scholar]
- (10).Leviton A, Kuban K, Paneth N. Intraventricular haemorrhage grading scheme: time to abandon? Acta Paediatrica. 2007;96(9):1254–1256. doi: 10.1111/j.1651-2227.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- (11).Hintz SR, O’Shea M. Neuroimaging and neurodevelopmental outcomes in preterm infants. Semin Perinatol. 2008;32(1):11–19. doi: 10.1053/j.semperi.2007.12.010. [DOI] [PubMed] [Google Scholar]
- (12).Wu YW, Shah SJ, Newman TB, Najjar DV, Croen LA. Cerebral palsy in a term population: Risk factors and neuroimaging findings. Annals of Neurology. 2006;60:S115. doi: 10.1542/peds.2006-0278. [DOI] [PubMed] [Google Scholar]
- (13).Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, et al. Practice parameter: Diagnostic assessment of the child with cerebral palsy - Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004;62(6):851–863. doi: 10.1212/01.wnl.0000117981.35364.1b. [DOI] [PubMed] [Google Scholar]
- (14).Nelson KB. The epidemiology of cerebral palsy interm infants. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8(3):146–150. doi: 10.1002/mrdd.10037. [DOI] [PubMed] [Google Scholar]
- (15).Hunt RW, Inder TE. Perinatal and neonatal ischaemic stroke: A review. Thrombosis Research. 2006;118(1):39–48. doi: 10.1016/j.thromres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- (16).Hankins GDV, MacLennan AH, Speer ME, Strunk A, Nelson K. Obstetric litigation is asphyxiating our maternity services. Obstetrics and Gynecology. 2006;107(6):1382–1385. doi: 10.1097/01.AOG.0000220531.25707.27. [DOI] [PubMed] [Google Scholar]
- (17).Brennan TA, Sox CM, Burstin HR. Relation between negligent adverse events and the outcomes of medical-malpractice litigation. New England Journal of Medicine. 1996;335(26):1963–1967. doi: 10.1056/NEJM199612263352606. [DOI] [PubMed] [Google Scholar]
- (18).Di Tommaso M, Tranquilli AL. A checklist to identify the origin of cerebral palsy. Journal of Maternal-Fetal and Neonatal Medicine. 2006;15:281–286. doi: 10.1080/14767050410001712121. [DOI] [PubMed] [Google Scholar]
- (19).MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. British Medical Journal. 1999;319(7216):1054–1059. doi: 10.1136/bmj.319.7216.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Iams JD, Romero R, Culhane JF, Goldenberg RL. Preterm birth 2 - Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]