HIV-1 Viral Escape in Cerebrospinal Fluid of Subjects on Suppressive Antiretroviral Treatment (original) (raw)
Abstract
Background. Occasional cases of viral escape in cerebrospinal fluid (CSF) despite suppression of plasma human immunodeficiency virus type 1 (HIV-1) RNA have been reported. We investigated CSF viral escape in subjects treated with commonly used antiretroviral therapy regimens in relation to intrathecal immune activation and central nervous system penetration effectiveness (CPE) rank.
Methods. Sixty-nine neurologically asymptomatic subjects treated with antiretroviral therapy >6 months and plasma HIV-1 RNA <50 copies/mL were cross-sectionally included in the analysis. Antiretroviral therapy regimens included efavirenz, lopinavir/ritonavir or atazanavir/ritonavir combined with tenofovir, abacavir, or zidovudine and emtricitabine or lamivudine. HIV-1 RNA was analyzed with real-time polymerase chain reaction assays. Neopterin was analyzed by enzyme-linked immunosorbent assay.
Results. Seven (10%) of the 69 subjects had detectable CSF HIV-1 RNA, in median 121 copies/mL (interquartile range, 54–213 copies/mL). Subjects with detectable CSF virus had significantly higher CSF neopterin and longer duration of treatment. Previous treatment interruptions were more common in subjects with CSF escape. Central nervous system penetration effectiveness rank was not a significant predictor of detectable CSF virus or CSF neopterin levels.
Conclusions. Viral escape in CSF is more common than previously reported, suggesting that low-grade central nervous system infection may continue in treated patients. Although these findings need extension in longitudinal studies, they suggest the utility of monitoring CSF responses, as new treatment combinations and strategies modify clinical practice.
Human immunodeficiency virus type 1 (HIV-1) invades the central nervous system (CNS) early during primary infection and remains detectable in the cerebrospinal fluid (CSF) in a majority of untreated subjects during the entire infectious course [1–3]. As immune function deteriorates, some individuals develop a more invasive encephalitis, which may lead to CNS dysfunction known as AIDS dementia complex, or HIV-associated dementia [4, 5]. Combination antiretroviral therapy (ART) has had a major impact on morbidity and mortality in HIV-1-infected patients, restoring immune function and thereby preventing opportunistic infections and other disease complications [6, 7].
Current guidelines recommend the use of 2 nucleoside/ nucleotide reverse transcriptase inhibitors (NRTI) in combination with either a ritonavir-boosted protease inhibitor (PI), a nonnucleoside reverse transcriptase inhibitor (NNRTI), or the integrase inhibitor raltegravir for treatment of chronic HIV-1 infection [8, 9]. In recent years, the most commonly used antiretroviral therapy (ART) combinations have been based on the NNRTI efavirenz or the PIs atazanavir/ritonavir or lopinavir/ritonavir, in combination with the NRTIs tenofovir or abacavir with either emtricitabine or lamivudine. Previously, zidovudine was preferred as part of the NRTI backbone, and although currently considered an alternative drug, many patients continue zidovudine as part of their ART regimens.
In the CNS compartment, entry of antiretroviral drugs is restricted by the blood-brain barrier and the blood-CSF barrier, and concern has been raised that virus in the CNS may escape therapy and act as a reservoir for viral persistence and evolution of drug resistance. Despite concerns about treatment difficulties in the CNS, HIV-1 in CSF is usually suppressed in subjects on effective therapy [2, 10]. ART has proved to be effective in preventing neurological complications of chronic HIV-1 infection, and the incidence of HIV-associated dementia has markedly declined since the introduction of combination antiretroviral therapy [6]. Although ART is often effective in suppressing CSF virus, the infectious process is associated with a local chronic inflammatory process that remains measurable even in individuals undergoing effective therapy [11, 12], albeit on a lower level than in untreated subjects [1]. It is unclear whether this residual intrathecal immune activation results from ongoing viral replication within the brain itself, transitory exposure of the CSF compartment to virus originating from migrating blood cells, or other mechanisms [11–13].
Few published studies have reported CSF “viral escape,” with HIV-1 RNA above levels of detection of standard assays in CSF despite having undetectable levels in blood [14–16]. A recent report describes a group of subjects with neurologic symptoms attributed to HIV-1 CNS infection and detectable CSF viral load despite suppression of plasma viremia [17]. The frequency of viral escape in neurologically asymptomatic individuals successfully treated with ART is largely unknown. To investigate CSF viral escape in this setting, here defined as having CSF HIV-1 RNA above the level of detection of standard assays while having HIV-1 RNA <50 copies/mL in blood, we have conducted a cross-sectional study of neurologically asymptomatic or stable individuals successfully treated with standard ART regimens. In addition, we examine our findings in relation to intrathecal immune activation drug regimens and CNS-penetration effectiveness rank (CPE rank) [18].
Methods
Study design. In a cross-sectional analysis using archived CSF and plasma specimens from 2 clinical centers in Gothenburg, Sweden, and San Francisco, California, we identified neurologically asymptomatic or stable (referred to subsequently as neuroasymptomatic) subjects who had received continuous antiretroviral therapy ⩾6 months, with no change in treatment regimen ⩾3 months before sampling. All subjects were evaluated by clinical neurological examination; however, formal neuropsychiatric testing was not performed in all subjects. A neuroasymptomatic condition was defined as either wholly without neurological symptoms or signs, or with neurological findings that could be attributed to static CNS conditions; past or present CNS opportunistic infections were excluded. Subjects with plasma HIV-1 RNA <50 copies/mL using treatment combinations that included either efavirenz, ritonavir-boosted lopinavir, or ritonavir-boosted atazanavir in combination with 2 NRTIs consisting of either tenofovir, abacavir, or zidovudine in combination with either emtricitabine or lamivudine, were included in the analysis. For subjects with >1 valid sampling point according to inclusion criteria, the most recent visit was included in the study. CSF samples were obtained for research purposes in separate local protocols evaluating CSF responses to antiretroviral treatment. The protocols followed in the present study were approved by the Research Ethics Committee of the University of Gothenburg and by the University of California San Francisco Committee on Human Research, and all subjects provided informed consent to participate.
CSF and blood measurements. After venous and lumbar puncture, paired samples of blood and CSF were centrifuged, and cell-free plasma and CSF were aliquoted and subsequently stored at −70°C for later analysis. Neopterin, a marker of macrophage activation and inflammation, was analyzed in CSF and plasma by a commercially available radioimmunoassay (Henningtest Neopterin; BRAHMS) or enzyme-linked immunosorbent assay (BRAHMS) with a normal reference value of <8.8 nmol/L in serum and <5.8 nmol/L in CSF [19]. HIV-1 RNA in CSF and plasma was measured using Cobas TaqMan HIV- 1 version 1 or 2 (Hoffmann-La Roche) or Abbott RealTime Viral Load Test on the m2000 system (Abbott RT/m2000 assay; Abbot), assays with a dynamic range of 20 (TaqMan software, version 2) or 40 to 1×107 copies/mL. CSF white blood cell count and peripheral blood CD4+ T lymphocyte determination was also performed using routine methods.
CNS penetration scores for antiretroviral medications. To investigate the impact of antiretroviral drug combinations on residual CSF HIV-1 RNA and immune activation, we calculated the CPE of drug regimens as devised and reported by Letendre et al [18]. In addition, we also calculated the revised CPE rank according to a recently proposed revision of ranking score by the same investigators [20].
Statistical analysis. Descriptive statistics were performed using Prism software (version 5; GraphPad) or SPSS PC software (version 17.0; SPSS). Continuous variables were log10 transformed where appropriate for the tests used. For 2-group comparisons, a t test or Mann-Whitney U test was used. Correlations were calculated using Spearman's rank correlation test. Comparisons of multiple categorical variables were analyzed using χ2 test or Fisher's exact test when appropriate.
Results
Sixty-nine subjects who fulfilled the inclusion criteria were identified and included in the analysis, 56 from Gothenburg and 13 from San Francisco. Samples included in the analysis were collected between 2002 and 2010. Table 1 shows the subject characteristics stratified by detectable CSF viral RNA. Subject groups were similar with respect to age, sex, CD4 cell count, and CD4 nadir (Table 1).
Table 1.
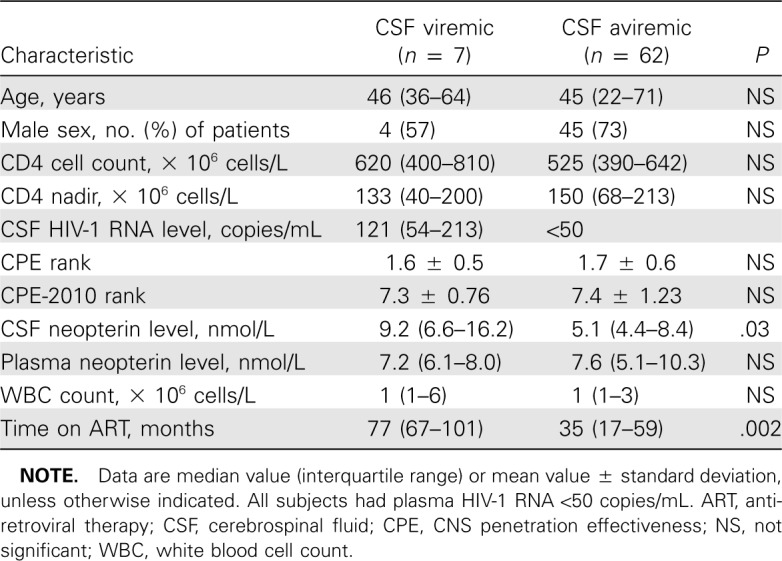
Subject Characteristics
Seven (10%) of the 69 subjects had detectable HIV-1 RNA in CSF. The median (range) CSF HIV-1 RNA was 121 (52–860) copies/mL in these 7 subjects. As determined by the inclusion criteria, all subjects had plasma HIV-1 RNA <50 copies/ mL. The frequency of detectable CSF HIV-1 RNA was similar in the 2 study sites.
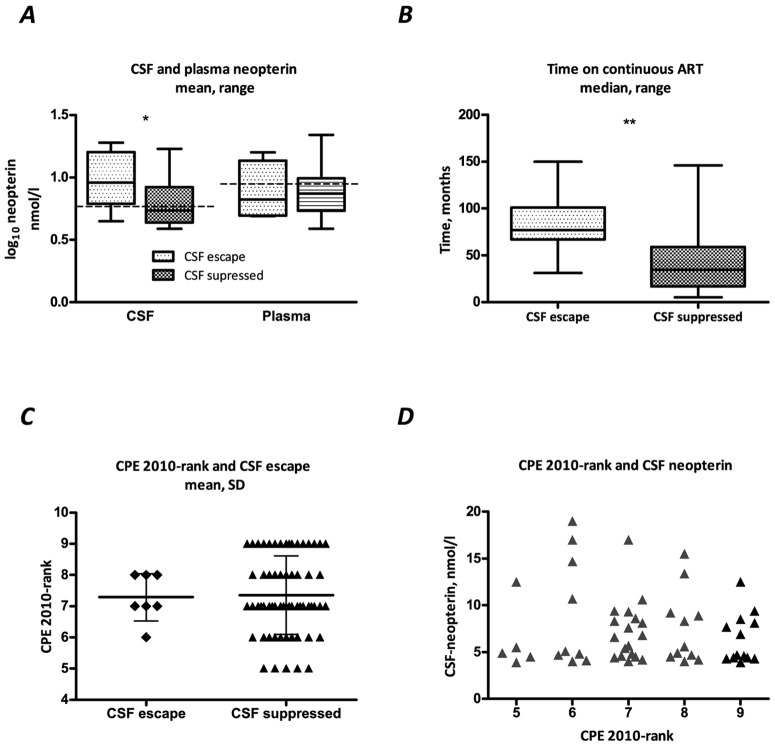
CSF and plasma neopterin were available for 5 of 7 of subjects with detectable CSF virus and 50 of 62 subjects with undetectable CSF HIV-1 RNA. CSF neopterin was significantly higher in subjects with detectable CSF HIV-1 RNA compared with subjects with undetectable CSF viral load (Figure 1_A_). Median (interquartile range [IQR]) CSF neopterin in subjects with detectable and undetectable CSF HIV-1 RNA was 9.2 (6.6–16.2) and 5.1 (4.4–8.4) nmol/L, respectively (_P_=.03, t test, neopterin log10 transformed for analysis). Four of the 5 subjects with available CSF neopterin and detectable CSF HIV-1 RNA had CSF neopterin levels above the normal reference value for healthy subjects (5.8 nmol/L) compared with 20 (40%) of the 50 subjects with available CSF neopterin and undetectable CSF HIV-1 RNA. In plasma, no significant difference was seen between subjects with detectable and subjects with undetectable CSF HIV-1 RNA, in median (IQR) 7.2 (6.1–8.0) and 7.6 (5.1–10.3) nmol/L, respectively (_P_=.9) (Figure 1_A_).
Figure 1.
A, Cerebrospinal fluid (CSF) and plasma neopterin in relation to CSF human immunodeficiency virus type 1 (HIV-1) RNA. Neopterin values are log10 transformed. Dotted line represents normal reference value for neopterin. CSF neopterin was significantly higher in CSF viremic subjects (_P_=.03, t test). B, Total treatment time was significantly longer in CSF viremic subjects (_P_=.002 ; Mann-Whitney U test). C and D, the impact of central nervous system penetration effectiveness 2010 rank (CPE 2010-rank) on CSF viral load (_C; P_=.88) and CSF neopterin (D; _r_=0.009, _P_=.9).
CSF pleocytosis was not significantly different in subjects with or without detectable CSF virus. Median (range) CSF white blood cell count was 1 (0–20) × 106 and 1 (0–9) × 106 cells/L in subjects with detectable and undetectable CSF HIV- 1 RNA, respectively (_P_=.4) (Table 1).
Figure 1_B_ shows the continuous treatment time on combination ART for the study subjects. The 7 subjects with detectable CSF HIV-1 RNA had a significantly longer treatment time on combination ART, in median (IQR) 77 (67–101) months, compared with 35 (17–59) months for subjects with undetectable CSF HIV-1 RNA (_P_=.002). Similarly, the total time on therapy was significantly longer in subjects with detectable CSF virus compared with subjects with suppressed CSF virus, in median (IQR) 100 (80–183) and 38 (18–63) months, respectively (P < .001).
The number of previous viral blips, defined as a single plasma HIV-1 RNA >50 copies/mL during ongoing therapy, was higher in the group of subjects with detectable CSF viral RNA, in median (IQR) 2.5 (1–4) compared with 0 (0–1) for subjects with undetectable CSF virus (_P_=.001). In addition, 5 (71%) of the 7 subjects with detectable CSF HIV-1 RNA had a history of 1 or more treatment interruptions, compared with 8 (15%) of the 54 subjects with available treatment history and viral suppression in CSF (P < .01). Treatment interruptions were defined as episodes of total ART abstinence >2 weeks followed by a subsequent increase in plasma viremia.
The distribution of antiretroviral drugs used in subjects with detectable CSF HIV-1 RNA is shown in Table 2. There was no significant difference in frequency of detectable CSF virus between study drugs (_P_=.5, χ2 test), although a trend was seen for comparison of tenofovir, abacavir, and zidovudine (_P_= .08). Among NRTIs, 4 (22%) of 14 subjects treated with abacavir and 3 (9%) of 34 subjects treated with tenofovir had detectable CSF RNA. None of the 17 zidovudine-treated subjects had signs of CSF escape (Table 2). Four (15%) of 27 subjects treated with efavirenz had detectable HIV-1 RNA in CSF. The corresponding frequencies for ritonavir-boosted atazanavir and ritonavir-boosted lopinavir were 2 (10%) of 21 subjects and 1 (5%) of 21 subjects, respectively.
Table 2.
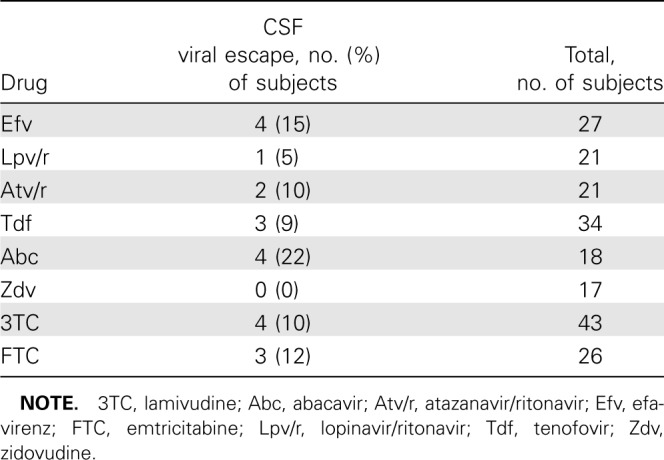
Distribution of Cerebrospinal Fluid (CSF) Viral Escape for Antiretroviral Drugs
Figure 1_C_–1_D_ shows the impact of CPE rank on CSF viral load and immune activation. The CPE rank was not a predictor of CSF escape in this analysis. Mean (± standard deviation) CPE rank was 1.6±0.5 for subjects with detectable CSF HIV- 1 RNA and 1.7±0.6 for subjects with undetectable CSF HIV- 1 RNA (_P_=.95, t test). Using the CPE-2010 score, mean (± standard deviation) rank was 7.3±0.76 and 7.4±1.27 for subjects with or without detectable CSF HIV-1 RNA, respectively (_P_=.88). Furthermore, CPE rank was not correlated with neopterin levels in CSF (CPE rank: _r_=−0.09, _P_=.5; CPE-2010 rank: _r_=−0.04, _P_=.8) or plasma (CPE rank: _r_=−0.18, _P_=.2; CPE-2010 rank: _r_=0.01, _P_=.9) in this study.
Discussion
In this cross-sectional evaluation of neurologically asymptomatic patients successfully treated with commonly used first-line ART combinations, we demonstrate that 10% of subjects had CSF HIV-1 RNA >50 copies/mL. Subjects with detectable CSF virus had significantly longer exposure to ART and higher levels of intrathecal immune activation; treatment interruptions were also more common in these subjects. Previous studies have shown that CSF HIV-1 RNA generally responds well to antiretroviral therapy [2, 10], although persistent HIV-1 RNA in CSF has occasionally been reported in subjects with suppressed plasma viral load [14–16]. Our findings, however, suggest that viral escape in CSF, even in subjects with successful systemic treatment with contemporary regimens, is a more common occurrence than previously reported. Several factors may influence the frequency of detectable CSF viral load found in our study population. First, the composition of ART regimens has been continuously modified as newer drugs have become available. Previously, ART regimens frequently included the PIs indinavir or lopinavir as well as the NRTI zidovudine, drugs that have a well-documented effect in the CNS [21–23], and detectable CSF HIV-1 RNA was uncommon in subjects treated with lopinavir or zidovudine in our analysis (Table 2). More recent regimens are commonly based on the NNRTI efavirenz or the PI atazanavir in combination with the NRTI tenofovir, drugs that likely penetrate less well into the CNS. Second, CSF samples in our study were consistently analyzed using newer reverse-transcription polymerase chain reaction assays that have improved quantification properties for low-level viral load. The level of HIV-1 RNA in subjects with detectable CSF viral load in our study population was low, in median (range) 121 (52–860) copies/mL, and in this interval the change from older amplification assays is likely to have an impact on the frequency of detectable CSF viral load.
No significant difference in frequency of detectable CSF HIV- 1 RNA was detected between study drugs, although we did see a trend toward significance when comparing NRTIs zidovudine, tenofovir, and abacavir. The efficacy of individual or combinations of antiretroviral drugs in the CNS is still not sufficiently elucidated, which may raise difficulties in estimating the antiretroviral potency of ART combinations. The CPE ranking system has been proposed as a simple method for estimating the combined CNS effectiveness of ART regimens and has been shown to correlate with CSF viral load in a cohort study [18]. In our subjects, however, CPE ranking (published version and CPE-2010 ranking) was not correlated with either detectable CSF HIV-1 RNA or level of intrathecal immune activation, suggesting that use of a simple categorical scale may not be sufficient in judging CNS efficacy and that a degree of caution in implementing CPE rank in routine clinical practice is indicated. However, the limited size of the study population may have influenced the lack of association between CSF viral escape and study drugs, as well as CPE rank.
Subjects with detectable CSF virus had significantly longer exposure to ART than subjects with no detectable virus in CSF. Given the comparatively lower drug concentrations in CNS than in plasma, adherence is likely of even greater importance in the treatment of HIV-1 in the CNS than systemically. The increased frequency of plasma viral blips found in the subjects with detectable CSF virus may represent slightly less adherent drug intake in these subjects. It is possible that longer treatment duration may influence adherence negatively, although other intrapatient related factors are likely as important. All subjects in our analysis were effectively suppressed in blood, suggesting that adherence in this population was good, although we cannot rule out that adherence may influence results.
While it is still unclear if ongoing full-cycle replication of HIV-1 occurs in the CNS during effective ART, it has been shown that HIV-1 may persist in the CNS during antiretroviral therapy due to insufficient CNS penetration of some antiretroviral drugs [24]. HIV-1 infection in the CNS becomes increasingly compartmentalized during disease progression [25], and it has been proposed that autonomous infection may be sustained by longer-lived cells within the CNS, that does not require replenishment from the blood [2]. Increased levels of intrathecal immune activation are found in a majority of subjects successfully treated with ART, although it is unclear if immune activation results from viral replication within theCNS or as a response to other factors [11]. The SMART study [7] established that subjects undergoing structured treatment interruptions were more likely to experience adverse disease events than subjects on continuous suppressive therapy. Treatment interruptions lead to rapid resurgence of active HIV-1 replication systemically, and also in the CNS compartment, resulting in neuronal injury, measurable in CSF as increased levels of neurofilament light protein, a marker of axonal injury, as well as increased levels of intrathecal immune activation [26]. Treatment interruptions were significantly more common in subjects with measurable CSF virus, and intermittent reseeding of the CNS during interruption of therapy leading to increased intrathecal immune activation may be of importance for establishing an autonomous CNS infection, and may contribute to the CSF viral escape found in these subjects. Autonomous viral replication in the brain even during ART remains a possibility, and may over time give rise to measurable CSF HIV- 1 RNA, as suggested by the longer time on treatment in subjects with CSF escape.
Ongoing replication in the CNS during ART may constitute a risk for evolution of viral resistance, and reports have demonstrated different resistance profiles in viral populations from blood and CSF, as well as in brain autopsy [27, 28]. Although selective resistance causing isolated viral escape in CSF seems to be rare, autonomous replication in the CNS may potentially cause independent evolution of drug resistance in the CSF [17]. In addition, a recent report demonstrated selection of enfuvirtide- resistant virus in CSF, causing subsequent loss of viral suppression in plasma, thus illustrating the importance of adequate penetration of antiretroviral drugs into the CNS [16]. CSF viral load in our subjects with detectable HIV-1 RNA was too low to allow resistance analysis, and although background resistance in our study population was rare, drug-resistant virus remains an important possible cause for the CSF viral escape found in 10% of our subjects.
Subjects included in the current analysis were neurologically asymptomatic or without evidence of active CNS disease, suggesting that viral escape in CSF may, at least in the short term, be clinically benign or silent in otherwise effectively treated individuals. Antiretroviral therapy has been successful in preventing HIV-associated dementia [6], although neurocognitive impairment remains prevalent in some chronically HIV-1-infected individuals [29]. However, a group of subjects with neurologic symptoms and viral escape in CSF despite systemically effective therapy was recently described, which illustrates that autonomous CNS HIV-1 replication and ongoing intrathecal immune activation may indeed signify a risk for neurologic complications in certain subjects [17]. For this reason, longitudinal follow-up in subjects with and without CSF viral control
In conclusion, viral escape in the CNS may be a more common occurrence than previously recognized. Ten percent of neurologically asymptomatic, systemically suppressed subjects still had detectable CSF virus and increased intrathecal immune activation. The increased frequency of previous treatment interruptions in subjects with CSF viral escape suggests that continuous viral suppression is of importance in controlling HIV- 1 CNS infection. The findings illustrate the importance of including analysis of CSF responses to antiretroviral therapy, especially when implementing new treatment strategies, for example, NRTI-sparing regimens, as well as when introducing new drugs. Our findings need to be extended in longitudinal studies both with respect to the frequency and consistency of viral escape and the neurological consequences. If indeed CSF viral escape is a persistent and consistent finding in a subset of patients, it may warrant integration into long-term strategies of virus and disease control.
Footnotes
Potential conflicts of interest: A.E. has received travel grants from Bristol-Myers Squibb. L.H. has received consultancy fees and/or lecture honoraria from Abbott, Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Tibotec/Janssen-Cilag. M.G. has received research grants, and/or consultancy fees, and/or honoraria from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, F Hoffmann-La Roche, Gilead, GlaxoSmithKline, Merck, Sharp and Dohme, and Tibotec/Janssen-Cilag.
Presented in part: 17th Conference on Retroviruses and Opportunistic Infections (CROI), 16–19 February 2010, San Francisco, California (abstract 432).
Financial support: This work was supported by The Sahlgrenska Academy at University of Gothenburg (project ALFGBG-11067); Swedish Research Council (project 2007-7092); National Institutes of Health (grants R01 MH62701, K23 MH074466, and NCRR UCSF-CTSI UL1 RR024131).
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of these funding agencies. The funding sources had no role in study design, data collection and analysis. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
References
- 1.Gisslen M, Fuchs D, Svennerholm B, Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21:271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome: HIV neurobehavioral research center group. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 4.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158:1079–1083. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- 6.d'Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 7.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed February 2010]. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Published 1 December 2009.
- 9.Josephson F, Albert J, Flamholc L, et al. Treatment of HIV infection: Swedish recommendations 2009. Scand J Infect Dis. 2009;41:788–807. doi: 10.3109/00365540903214322. [DOI] [PubMed] [Google Scholar]
- 10.Mellgren A, Antinori A, Cinque P, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10:701–707. [PubMed] [Google Scholar]
- 11.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after 14 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair E, Ronquillo R, Lollo N, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvey LJ, Everitt A, Winston A, Mackie NE, Benzie A. Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment. AIDS. 2009;23:1443–1444. doi: 10.1097/QAD.0b013e32832d077c. [DOI] [PubMed] [Google Scholar]
- 15.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 16.van Lelyveld SF, Nijhuis M, Baatz F, et al. Therapy failure following selection of enfuvirtide-resistant HIV-1 in cerebrospinal fluid. Clin Infect Dis. 2010;50:387–390. doi: 10.1086/649874. [DOI] [PubMed] [Google Scholar]
- 17.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 18.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagberg L, Andersson LM, Abdulle S, Gisslen M. Clinical application of cerebrospinal fluid neopterin concentrations in HIV infection. Pteridines. 2004;15:102–106. [Google Scholar]
- 20.Letendre S, Ellis R, Deutsch R, et al. Correlates of time-to-loss-of-viralresponse in CSF and plasma in the CHARTER cohort. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 16–19 February 2010; San Francisco, California. [Google Scholar]
- 21.Haas DW, Stone J, Clough LA, et al. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type 1 infection. Clin Pharmacol Ther. 2000;68:367–374. doi: 10.1067/mcp.2000.109391. [DOI] [PubMed] [Google Scholar]
- 22.Letendre SL, van den Brande G, Hermes A, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;45:1511–1517. [Google Scholar]
- 23.Yilmaz A, Stahle L, Hagberg L, Svennerholm B, Fuchs D, Gisslen M. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36:823–828. doi: 10.1080/00365540410025320. [DOI] [PubMed] [Google Scholar]
- 24.Gisolf EH, Enting RH, Jurriaans S, et al. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14:1583–1589. doi: 10.1097/00002030-200007280-00014. [DOI] [PubMed] [Google Scholar]
- 25.Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5:e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gisslen M, Rosengren L, Hagberg L, Deeks SG, Price RW. Cerebrospinal fluid signs of neuronal damage after antiretroviral treatment interruption in HIV-1 infection. AIDS Res Ther. 2005;2:6. doi: 10.1186/1742-6405-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bestetti A, Presi S, Pierotti C, et al. Long-term virological effect of highly active antiretroviral therapy on cerebrospinal fluid and relationship with genotypic resistance. J Neurovirol. 2004;10(Suppl 1):52–57. doi: 10.1080/753312753. [DOI] [PubMed] [Google Scholar]
- 28.Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol. 2004;78:10133–10148. doi: 10.1128/JVI.78.18.10133-10148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]