Potential beneficial effects of butyrate in intestinal and extraintestinal diseases (original) (raw)
Abstract
The multiple beneficial effects on human health of the short-chain fatty acid butyrate, synthesized from non-absorbed carbohydrate by colonic microbiota, are well documented. At the intestinal level, butyrate plays a regulatory role on the transepithelial fluid transport, ameliorates mucosal inflammation and oxidative status, reinforces the epithelial defense barrier, and modulates visceral sensitivity and intestinal motility. In addition, a growing number of studies have stressed the role of butyrate in the prevention and inhibition of colorectal cancer. At the extraintestinal level, butyrate exerts potentially useful effects on many conditions, including hemoglobinopathies, genetic metabolic diseases, hypercholesterolemia, insulin resistance, and ischemic stroke. The mechanisms of action of butyrate are different; many of these are related to its potent regulatory effects on gene expression. These data suggest a wide spectrum of positive effects exerted by butyrate, with a high potential for a therapeutic use in human medicine.
Keywords: Short-chain fatty acids, Dietary fiber, Colon, Ion transport, Inflammation, Carcinogenesis, Intestinal barrier, Oxidative stress, Visceral perception
INTRODUCTION
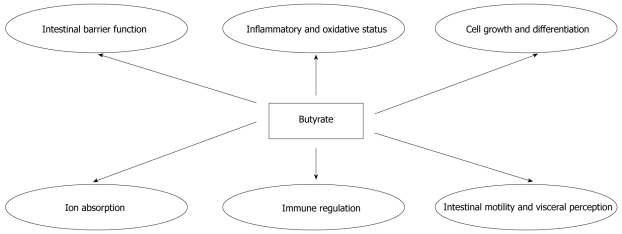
The development of the intestinal ecosystem is crucial for many gastrointestinal functions and body health. The intestinal ecosystem essentially comprises the epithelium, immune cells, enteric neurons, intestinal microflora, and nutrients. The coordinate interplay between all these components is the object of intensive research efforts to design new strategies for many intestinal and extraintestinal diseases. In this context, short-chain fatty acids (SCFAs), produced by intestinal microflora, represent a clear example of the importance of the intestinal ecosystem. SCFAs are organic acids produced by intestinal microbial fermentation of mainly undigested dietary carbohydrates, specifically resistant starches and dietary fiber, but also in a minor part by dietary and endogenous proteins. SCFAs are 2-carbon to 5-carbon weak acids, including acetate (C2), propionate (C3), butyrate (C4), and valerate (C5). SCFAs are essentially produced in the colon. The ratio of SCFA concentrations in the colonic lumen is about 60% acetate, 25% propionate, and 15% butyrate. As a result of increasing concentrations of acidic fermentation products, the luminal pH in the proximal colon is lower. This pH seems to boost the formation of butyrate, as mildly acidic pH values allow butyrate-producing bacteria to compete against Gram-negative carbohydrate-utilizing bacteria, such as Bacteroides spp.[1]. The ability to produce butyrate is widely distributed among the Gram-positive anaerobic bacteria that inhabit the human colon. Butyrate-producing bacteria represent a functional group, rather than a coherent phylogenetic group. Numerically, two of the most important groups of butyrate producers appear to be Faecalibacterium prausnitzii, which belongs to the Clostridium leptum (or clostridial cluster IV) cluster, and Eubacterium rectale/Roseburia spp., which belong to the Clostridium coccoides (or clostridial cluster XIVa) cluster of firmicute bacteria[2]. Butyrate is the major energy source for colonocytes and is involved in the maintenance of colonic mucosal health[3]. Recently several intestinal and extraintestinal effects of butyrate have been demonstrated (Figure 1 and Table 1). This review is focused on new evidence for possible applications of butyrate in human medicine.
Figure 1.
The multiple effects of butyrate at intestinal level.
Table 1.
Main butyrate effects potentially useful in human medicine
| Intestinal level | Extraintestinal level |
|---|---|
| Ion absorption | Insulin sensitivity |
| Cell proliferation | Cholesterol synthesis |
| Cell differentation | Energy expenditure |
| Intestinal barrier function | Ammonia scavenger |
| Immune-regulation | Stimulation of β-oxidation of very long chain fatty acids and peroxisome proliferation |
| Oxidative stress | CFTR function |
| Intestinal motility | Neurogenesis |
| Visceral perception and rectal compliance | HbF production |
EFFECTS OF BUTYRATE AT THE INTESTINAL LEVEL
Effects on transepithelial ion transport
Potentially, SCFAs are absorbed by each intestinal segment, as demonstrated in animal models and human volunteers. The colonocytes absorb butyrate and other SCFAs through different mechanisms of apical membrane SCFA uptake, including non-ionic diffusion, SCFA/HCO3- exchange, and active transport by SCFA transporters. The transport proteins involved are monocarboxylate transporter isoform 1 (MCT1), which is coupled to a transmembrane H+-gradient, and SLC5A8, which is Na+-coupled co-transporter[3,4]. The absorption of these fatty acids has a significant impact on the absorption of NaCl and on the electrolyte balance generally[5]. In particular, butyrate is able to exert a powerful pro-absorptive stimulus on intestinal NaCl transport and an anti-secretory effect towards Cl- secretion. The powerful regulatory pro-absorptive/anti-secretory effects induced by butyrate on the transepithelial ion transport occurs through several mechanisms: (1) Stimulation of NaCl absorption by the action of two coupled transport systems on the intestinal brush border: Cl-/HCO3- and Na+/H+ and Cl-/butyrate and Na+/H+; and (2) inhibition of Cl- secretion by blocking the activity of the cotransporter Na-K-2Cl (NKCC1) on the enterocyte basolateral membrane. In vitro studies have shown that butyrate has an inhibitory effect on Cl- secretion induced by prostaglandin E2, cholera toxin, and phosphocholine. This effect is due to reduced production of intracellular cAMP secondary to the expression and regulation of adenylate cyclase[4]. Comparison studies showed that the pro-absorptive and anti-secretory effects of butyrate are significantly higher than those of all other SCFAs[6]. Clinical studies in children with acute diarrhea caused by V. cholerae showed a reduction in stool volume and a more rapid recovery in patients who received oral rehydration therapy in addition to resistant starch, a precursor of butyrate, in the diet[7,8]. These results were confirmed in other forms of infectious diarrhea in children and in animal models studies[9,10]. Moreover, butyrate therapy is beneficial in patients affected by Congenital Chloride Diarrhea (CLD)[11,12]. This rare genetic disease is caused by mutations in the gene encoding the solute-linked carrier family 26-member A3 (SLC26A3) protein, which acts as a plasma membrane anion exchanger for Cl- and HCO3[13]. The mechanism underlying this therapeutic effect could be related, at least in part, to stimulation of the Cl-/butyrate exchanger activity[11]. It is also possible that butyrate could reduce mistrafficking or misfolding of the SLC26A3 protein, as demonstrated for other molecules involved in transepithelial ion transport[14]. Alternatively, butyrate may enhance gene expression: the SLC26A3 gene contains a 290-bp region between residues -398 and -688 that is crucial for high-level transcriptional activation induced by butyrate. This may explain the variable response of patients affected by CLD to butyrate[12]. In fact, depending on the patient’s genotype, mutations in the above-mentioned regulatory regions of the SLC26A3 gene could affect the gene transcription rate. It is also conceivable that other channels could be involved in the therapeutic effect of butyrate in CLD. SLC26A3, like other components of the SLC26 family, interacts with cystic fibrosis transmembrane conductance regulator (CFTR)[15,16]. The interaction between CFTR and these components is mediated by binding of the regulatory domain of CFTR to the sulfate transporter and anti sigma factor antagonist (STAS) domain of SLC26. The interaction is enhanced by phosphorylation of the regulatory domain by protein kinase A[17] and is modulated by PDZ-binding scaffold proteins. An important consequence of this interaction is that SLC26 anion exchange activity is enhanced when CFTR is activated by phosphorylation. Moreover, the two genes regulate each other: the overexpression of SLC26A3 or -A6 causes upregulation of CFTR and _vice versa_[18]. In patch-clamp experiments, protein kinase A-stimulated CFTR channel activity was six-fold higher in HEK293 cells co-expressing both SCL26 exchanger and CFTR than in HEK293 cells expressing CFTR alone[12,15,16,18]. Mutations may impair the interactions between channels and thus reduce the effect of butyrate therapy. Interestingly, it has been demonstrated that butyrate can act by different mechanisms in in vitro models of cystic fibrosis: it can increase the expression of the apical epithelial membrane of the CFTR, and it can act as a “chaperone-like” molecule, as shown in the ΔF508del CFTR cell line model[19]. Similar mechanisms could occur in CLD. Lastly, Clausen et al[20] demonstrated that antibiotic-associated diarrhea was related to reduced fecal concentrations and production rates of butyrate. Their results suggest that the antibiotic-associated diarrhea might be secondary to impaired colonic fermentation in otherwise disposed subjects, resulting in decreased butyrate and fluid absorption. In this case, the administration of butyrate could also alleviate the symptoms associated with antibiotic use.
Effects on cell growth and differentiation
Several epidemiological studies support the role of dietary fiber in the protection against colorectal cancer[21-26]. Different mechanisms have been proposed for fiber’s cancer preventive properties: reduction in transit time of the feces in the gut, which reduces exposure of the mucosa to luminal carcinogens; absorption of bile acids, biogenic amines, bacterial toxins, and production of butyrate. Most of the anticarcinogenic effects of butyrate are observed in in vitro carcinoma cell lines. In these models, addition of butyrate leads to inhibition of proliferation, induction of apoptosis, or differentiation of tumor cells[27-30]. Butyrate’s anticarcinogenic effects are in contrast with the effects of this compound in normal enterocytes. In fact, it has been shown that butyrate stimulates the physiological pattern of proliferation in the basal crypt in the colon, whereas it reduces the number and the size of aberrant crypt focus, which are the earliest detectable neoplastic lesions in the colon[31]. These contradictory patterns of butyrate represents the so called “butyrate paradox”[27]. An important mechanism by which butyrate causes biological effects in colon carcinoma cells is the hyperacetylation of histones by inhibiting histone deacetylase (HDAC). This compensates for an imbalance of histone acetylation, which can lead to transcriptional dysregulation and silencing of genes that are involved in the control of cell cycle progression, differentiation, apoptosis and cancer development[32,35]. In particular, in human colon cancer cell lines butyrate, acting as HDAC inhibitor, increases the p21 (WAF1) gene expression by selectively regulating the degree of acetylation of the gene-associated histones, and induces G1 cell cycle arrest[36]. A novel contributory mechanism to the chemopreventive effect of butyrate is the downregulation of the key apoptotic and angiogenesis regulator Neuropilin-1 (NRP-1), which has been shown to promote tumor cell migration and survival in colon cancer in response to vascular endothelial growth factor (VEGF) binding[37]. Several reports have shown that the apoptosis triggered by butyrate in vitro is associated with dysregulation of Bcl2 family proteins, especially upregulation of BAK and downregulation of BclxL[38,39], rather than cellular damage. A study by Thangaraju et al suggests a novel mode of action of butyrate in the colon involving GPR109A, a G-protein–coupled receptor for nicotinate[40,41], which recognizes butyrate with low affinity. This receptor is expressed in the normal colon on the lumen-facing apical membrane of colonic epithelial cells, but is silenced in colon cancer via DNA methylation. Thangaraju et al[42] showed that inhibition of DNA methylation in colon cancer cells induces GPR109A expression and that activation of the receptor causes tumor cell–specific apoptosis. Butyrate is an inhibitor of HDAC, but apoptosis induced by activation of GPR109A with its ligands in colon cancer cells does not involve inhibition of histone deacetylation. The primary changes in this apoptotic process include downregulation of Bcl-2, Bcl-xL, and cyclin D1 and upregulation of death receptor pathway. Moreover, a recent study suggested that the protective role of dietary fiber, and its breakdown product butyrate, against colorectal cancer could be determined by a modulation of canonical Wnt signaling, a pathway constitutively activated in the majority of colorectal cancers[43]. Butyrate is recognized for its potential to act on secondary chemoprevention, by slowing growth and activating apoptosis in colon cancer cells[44], but it can also act on primary chemoprevention. The mechanism proposed is the transcriptional upregulation of detoxifying enzymes, such as glutathione-S-transferases (GSTs). This modulation of genes may protect cells from genotoxic carcinogens, such as H2O2 and 4-hydroxynonenal (HNE)[45,46].
Effects on inflammatory and oxidative status
Butyrate has a role as an anti-inflammatory agent, primarily via inhibition of nuclear factor κB (NF-κB) activation in human colonic epithelial cells[47], which may result from the inhibition of HDAC. NF-κB regulates many cellular genes involved in early immune inflammatory responses, including IL-1b, TNF-α, IL-2, IL-6, IL-8, IL-12, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), intercellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-1 (VCAM-1), T cell receptor-α (TCR-α), and MHC class II molecules[48-50]. The activity of NF-κB is frequently dysregulated in colon cancer[51,52] and in inflammatory bowel diseases (IBDs), such as ulcerative colitis (UC) and Crohn’s disease (CD)[53-55]. In CD patients, butyrate decreases pro-inflammatory cytokine expression via inhibition of NF-κB activation and IκBα degradation[53]. The upregulation of peroxisome proliferator-activated receptor γ (PPARγ) a nuclear receptor highly expressed in colonic epithelial cells, and the inhibition of IFNγ signaling, are another two of butyrate’s anti-inflammatory effects[56,57]. Butyrate can act on immune cells through specific G-protein-coupled receptors (GPRs) for SCFAs, GPR41 (or FFA3) and GPR43 (or FFA2), which are both expressed on immune cells, including polymorphonuclear cells, suggesting that butyrate might be involved in the activation of leucocytes[58]. The possible immune-modulatory functions of SCFAs are highlighted by a recent study on GPR43 -/- mice. These mice exhibit aggravated inflammation, related to increased production of inflammatory mediators and increased immune cell recruitment[59].
Most clinical studies analyzing the effects of butyrate on inflammatory status focused on UC patients. Hallert et al[60] instructed 22 patients with quiescent UC to add 60 g oat bran (corresponding to 20 g dietary fiber) to their daily diet. Four weeks of this treatment resulted in a significant increase of fecal butyrate concentration and in a significant improvement of abdominal symptoms. In a double blind, placebo-controlled multicenter trial, Vernia et al[61] treated 51 patients with active distal UC with rectal enemas containing either 5-aminosalicylic acid (5-ASA) or 5-ASA plus sodium butyrate (80 mmol/L, twice a day). The combined treatment with topical 5-ASA plus sodium butyrate significantly improved the disease activity score more than 5-ASA alone. These and other intervention studies[62-64] suggested that the luminal administration of butyrate or stimulation of luminal butyrate production by the ingestion of dietary fiber results in an amelioration of the inflammation and symptoms in UC patients.
Numerous studies have reported that butyrate metabolism is impaired in intestinal inflamed mucosa of patients with IBD. Recent data show that butyrate deficiency results from the reduction of butyrate uptake by the inflamed mucosa through downregulation of MCT1. The concomitant induction of the glucose transporter GLUT1 suggests that inflammation could induce a metabolic switch from butyrate to glucose oxidation. Butyrate transport deficiency is expected to have clinical consequences. Particularly, the reduction of the intracellular availability of butyrate in colonocytes may decrease its protective effects toward cancer in IBD patients[65].
Limited evidence from pre-clinical studies shows that oxidative stress in the colonic mucosa can be modulated by butyrate. Oxidative stress is involved in both inflammation[66] and the process of initiation and progression of carcinogenesis[67]. During oxidative stress there is an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense mechanisms, leading to a cascade of reactions in which lipids, proteins, and/or DNA may get damaged. In healthy humans, it has been demonstrated that locally administered butyrate in physiological concentrations increased the antioxidant GSH and possibly decreased ROS production, as indicated by a decreased uric acid production[68]. As the human colon is continuously exposed to a variety of toxic stimuli, enhanced butyrate production in the colon could result in an enhanced resistance against toxic stimuli, thus improving the barrier function. This might be relevant for the treatment of gastrointestinal disorders, such as post-infectious irritable bowel syndrome (IBS), microscopic colitis, IBD, and diversion colitis.
Effects on non-specific intestinal defense mechanisms
The main components of nonspecific intestinal barrier defense mechanisms are the mucous layer covering the epithelium, the production of antimicrobial peptides, and tight junctions, which protect the gastrointestinal mucosa against pathogens. Evidence suggests a role for butyrate in reinforcing the colonic defense barrier. Butyrate stimulates MUC2 mucin production in a human colonocytes cell line (LS174T). The increased expression of MUC2 gene, and the induction of mucin synthesis, can affect the mucous layer leading to enhanced protection against luminal agents[69,70].
Combined with other components of the innate immune system, antimicrobial peptides (AMPs) form the first line of defense against infections. The two major classes of AMPs found in humans are defensins and cathelicidins. While the intestine expresses numerous defensins, LL-37 is the only cathelicidin-derived peptide expressed in humans. Several studies demonstrated an effect of butyrate on LL-37 gene expression and proposed that the molecular mechanism may be linked to an increase in histone acetylation and mitogen-activated protein (MAP) kinase signaling[71-75]. The use of HDAC inhibitors, such as butyrate, to enhance the expression of the LL-37 gene may become a novel approach for strengthening innate immunity to treat or prevent intestinal infections.
Butyrate also regulates the colonic defense barrier through its effects on intestinal permeability, which depends on its concentration. At low concentrations, butyrate induces a concentration-dependent reversible decrease in permeability in intestinal cell line models[76,77]. The effect of butyrate on the intestinal epithelial permeability involves the assembly of tight junctions via AMP-activated protein kinase (AMPK)[78].
Effects on visceral perception and intestinal motility
Little is known about the environmental and nutritional regulation of the enteric nervous system (ENS), which controls gastrointestinal motility. Butyrate regulates colonic mucosa homeostasis and can modulate neuronal excitability. Soret et al[79] investigated the effects of butyrate on the ENS and colonic motility, and showed, in vivo and in vitro, that butyrate significantly increased the proportion of choline acetyltransferase (ChAT), but not nitric oxide synthase (nNOS) immunoreactive myenteric neurons. Butyrate increases the cholinergic-mediated colonic circular muscle contractile response ex vivo. The authors suggest that butyrate might be used, along with nutritional approaches, to treat various gastrointestinal motility disorders associated with inhibition of colonic transit.
A recent study by Van Houten et al[80] shows that intraluminal administration of a physiologically relevant dose (50 to 100 mmol/L-) of butyrate into the distal colon increases compliance and decreases pain, urge, and discomfort measured with a rectal barostat procedure in healthy subjects. This study suggests a potential beneficial effect of butyrate in disorders that are associated with visceral hypersensitivity, such as IBS and infantile colics, and provides a basis for future trials with dietary modulation resulting in intracolonic butyrate production in both healthy and IBS subjects. The decrease in visceral perception induced by butyrate treatment could be due to an increased 5-HT release, as previously suggested by others[81]. Another possible mechanism by which butyrate could affect visceral perception is the previous reported inhibition of histone deacetylase. In fact, Chen et al[82] showed that these inhibitors induce microglyal apoptosis and attenuate inflammation-induced neurotoxicity in rats, which may affect visceral perception. Butyrate has been reported to induce enhancement of colonic motility via the release of 5-HT[83]. In functional studies, butyrate and propionate induced phasic and tonic contractions in rat colonic circular muscle. The dose-dependent contractile effect occurred only when SCFAs were applied on the mucosal side and disappeared in mucosal free preparations, suggesting the presence of sensory mechanisms near the epithelium[84,85].
EFFECTS AT THE EXTRAINTESTINAL LEVEL
Hemoglobinopathies
Clinical trials in patients with sickle cell disease and β-thalassemia confirmed the ability of butyrate to increase fetal hemoglobin (HbF) production[86-89]. Butyrate is an inducer of HbF through an epigenetic regulation of fetal globin gene expression via HDAC inhibition, resulting in global histone hyperacetylation, including nucleosomes at the γ-globin promoters[90]. Other experiments have shown that butyrate can cause a rapid increase in the association of γ-globin mRNA with ribosomes[91]. Other authors have demonstrated activation of p38 mitogen-activated protein kinases (MAPK) and cyclic nucleotide signaling pathways in association with butyrate induction of HbF[92]. Taken together, these studies suggest that global histone hyperacetylation induced by HDAC inhibition is not the unique mechanism underlying butyrate stimulation of HbF.
Genetic metabolic diseases
Sodium phenylbutyrate 4 (4-PBA) was approved by the Food and Drug Administration (FDA) for use in patients with urea cycle enzyme deficiency, in which it acts as a scavenger of ammonia. Indeed, 4-PBA is oxidized to phenylacetate, which binds to glutamine and determines the urinary excretion. In patients with ornithine transcarbamylase deficiency, the use of 4-PBA allows for better metabolic control and increased intake of natural protein in the diet[93].
The possible use of butyrate in the treatment of X-linked Adrenoleukodystrophy (X-ALD), a disorder of peroxisomes characterized by altered metabolism and accumulation of very long chain fatty acids, has also been studied. Sodium phenylbutyrate 4 induces, in vitro on fibroblasts from patients with X-ALD and in vivo in X-ALD knockout mice, an increase in β-oxidation of very long chain fatty acids and peroxisome proliferation[94].
Hypercholesterolemia
Under normal lipidemic conditions, the liver is the most important site of cholesterol biosynthesis, followed by the intestine. Biosynthesis in the liver and intestine account for about 15% and 10%, respectively, of the total amount of cholesterol biosynthesis each day[95,96]. In hypercholesterolemia, when cholesterol biosynthesis is suppressed in most organs by fasting, the intestine becomes the major site of cholesterol biosynthesis, and its contribution can increase up to 50%. Importantly, recent evidence shows that the global effect of butyrate is to downregulate the expression of nine key genes involved in intestinal cholesterol biosynthesis, potentially inhibiting this pathway[97].
Obesity and insulin resistance
Dietary supplementation with butyrate can prevent and treat diet-induced obesity and insulin resistance in mouse models. After a 5-wk treatment with butyrate, obese mice lost 10.2% of their original body weight. Consistent with the change in body weight, fat content was reduced by 10%. Furthermore, fasting glucose was reduced by 30%, insulin resistance was reduced by 50%, and intraperitoneal insulin tolerance was improved significantly by butyrate. The mechanism of butyrate action is related to promotion of energy expenditure and induction of mitochondrial function. Stimulation of peroxisome proliferator-activated receptor (PPAR) coactivator (PGC-1α) activity has been suggested as the molecular mechanism of butyrate. Activation of AMPK and inhibition of histone deacetylases may contribute to the PGC-1α regulation. These data suggest that butyrate may have potential application in the prevention and treatment of metabolic syndrome in humans[98].
Ischemic stroke
Cerebral ischemia enhances neurogenesis in neurogenic and non-neurogenic regions of the ischemic brain of adult animal models. A recent study demonstrated that post-insult treatment with sodium butyrate stimulated the incorporation of bromo-2’-deoxyuridine (BrdU) in the ischemic brain of rats subjected to permanent cerebral ischemia. Butyrate treatment also increased the number of cells expressing polysialic acid-neural cell adhesion molecule, nestin, glial fibrillary acidic protein, phospho-cAMP response element-binding protein (CREB), and brain-derived neurotrophic factor (BDNF) in various brain regions after cerebral ischemia[99]. Furthermore, extensive co-localization of BrdU and polysialic acid-neural cell adhesion molecule was observed in multiple regions after ischemia, and butyrate treatment upregulated protein levels of BDNF, phospho-CREB, and glial fibrillary acidic protein. Intraventricular injection of K252a, a tyrosine kinase B receptor antagonist, markedly reduced the long-lasting behavioral benefits of butyrate, inhibiting cell proliferation, nestin expression, and CREB activation[99]. Together, these results suggest that butyrate-induced cell proliferation, migration, and differentiation require BDNF-tyrosine kinase B signaling and may contribute to long-term beneficial effects of butyrate after ischemic injury.
ISSUES RELATED TO THE CLINICAL USE OF BUTYRATE
Data from literature and clinical experience of several research groups show a wide spectrum of possibilities for potential therapeutic use of butyrate by oral administration without having serious adverse events (Table 2). Some butyrate-based products are marketed, but their spread is still very limited and greatly understaffed in view of the wide spectrum of possible indications, especially in chronic diseases, where it is possible to predict a lasting use of the compound. The main problem is of the availability of formulations of butyrate that can be easily administered orally, in particular for pediatric patients, and to the extremely poor palatability of the products available on the market. The unpleasant taste and odor make oral administration of butyrate extremely difficult, especially in children. Thus, new formulations of butyrate with a better palatability, which can be easily administered orally, are needed. Another possible solution could be the modulation of intestinal microflora by probiotics. Probiotics are live and viable microorganisms, which, if given in adequate amounts, confer a beneficial effect to the host. Probiotic microorganisms generate small molecular metabolic byproducts, referred to as “postbiotics”, which exert beneficial regulatory influence on host biological functions, including butyrate[100].
Table 2.
Possible therapeutic indications of butyrate in gastroenterology
| Functions | Therapeutic indications | Potential applications |
|---|---|---|
| Regulation of fluid and electrolyte uptake | Acute gastroenteritis[9,10] | Irritable bowel syndrome[80] |
| Cholera[7,8] | Aspecific chronic diarrhea[4] | |
| Congenital chloride diarrhea[11,12] | Traveler’s diarrhea[4] | |
| Antibiotic associated diarrhea[4,20] | ||
| Chronic secretory diarrhea[4] | ||
| Cystic fibrosis[14,19] | ||
| Mucosal atrophy in malnutrition[101] | ||
| Effects on proliferation and differentiation of epithelial intestinal cells | Acute gastroenteritis[9,10] | Mucosal atrophy in total parenteral nutrition[101,102] |
| IBD[53-65] | Mucosal atrophy in radiotherapy or chemotherapy[101] | |
| Short bowel syndrome and intestinal failure[103] | ||
| Prevention of colorectal cancer[46] | ||
| Intestinal polyposis[104] | ||
| Anti-inflammatory effect | IBD[53-65] | Pouchitis[105] |
| Allergic colitis[106] |
CONCLUSION
The SCFA butyrate, a main end product of microbial fermentation of dietary fibers in the human intestine, plays an important role in the maintenance of intestinal homeostasis and overall health status. The effects exerted by butyrate are multiple and involve several distinct mechanisms of action. Its well-known epigenetic mechanism, through the inhibition of HDACs, results in the regulation of gene expression and in the control of cell fate. At the intestinal level, butyrate exerts multiple effects such as the prevention and inhibition of colonic carcinogenesis, the improvement of inflammation, oxidative status, epithelial defense barrier, and the modulation of visceral sensitivity and intestinal motility. At the extraintestinal level, potential fields of application for butyrate seem to be the treatment of sickle cell disease, β-thalassemia, cystic fibrosis, urea cycle enzyme deficiency, X-linked adrenoleukodystrophy, hypercholesterolemia, obesity, insulin resistance, and ischemic stroke.
In conclusion, a growing number of studies have revealed new mechanisms and effects of butyrate with a wide range of potential clinical applications from the intestinal tract to peripheral tissues. However, more clinical studies to elucidate the role of butyrate in health and diseases and new solutions for easier administration are needed.
Footnotes
Supported by A Grant from Agenzia Italiana del Farmaco (AIFA) grant code FARM6FJ728
Peer reviewers: Bruno Stieger, Professor, Department of Medicine, Division of Clinical Pharmacology and Toxicology, University Hospital, Zurich 8091, Switzerland; Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwels Strasse 30, 52074 Aachen, Germany
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 2.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 4.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 5.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 6.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989;96:989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- 7.Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Engl J Med. 2000;342:308–313. doi: 10.1056/NEJM200002033420502. [DOI] [PubMed] [Google Scholar]
- 8.Rabbani GH, Albert MJ, Rahman H, Chowdhury AK. Short-chain fatty acids inhibit fluid and electrolyte loss induced by cholera toxin in proximal colon of rabbit in vivo. Dig Dis Sci. 1999;44:1547–1553. doi: 10.1023/a:1026650624193. [DOI] [PubMed] [Google Scholar]
- 9.Rabbani GH, Teka T, Zaman B, Majid N, Khatun M, Fuchs GJ. Clinical studies in persistent diarrhea: dietary management with green banana or pectin in Bangladeshi children. Gastroenterology. 2001;121:554–560. doi: 10.1053/gast.2001.27178. [DOI] [PubMed] [Google Scholar]
- 10.Alam NH, Ashraf H. Treatment of infectious diarrhea in children. Paediatr Drugs. 2003;5:151–165. doi: 10.2165/00128072-200305030-00002. [DOI] [PubMed] [Google Scholar]
- 11.Berni Canani R, Terrin G, Cirillo P, Castaldo G, Salvatore F, Cardillo G, Coruzzo A, Troncone R. Butyrate as an effective treatment of congenital chloride diarrhea. Gastroenterology. 2004;127:630–634. doi: 10.1053/j.gastro.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 12.Wedenoja S, Holmberg C, Höglund P. Oral butyrate in treatment of congenital chloride diarrhea. Am J Gastroenterol. 2008;103:252–254. doi: 10.1111/j.1572-0241.2007.01562_14.x. [DOI] [PubMed] [Google Scholar]
- 13.Kere J, Höglund P. Inherited disorders of ion transport in the intestine. Curr Opin Genet Dev. 2000;10:306–309. doi: 10.1016/s0959-437x(00)00088-5. [DOI] [PubMed] [Google Scholar]
- 14.Roomans GM. Pharmacological approaches to correcting the ion transport defect in cystic fibrosis. Am J Respir Med. 2003;2:413–431. doi: 10.1007/BF03256668. [DOI] [PubMed] [Google Scholar]
- 15.Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol. 2003;549:3–19. doi: 10.1113/jphysiol.2003.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höglund P, Sormaala M, Haila S, Socha J, Rajaram U, Scheurlen W, Sinaasappel M, de Jonge H, Holmberg C, Yoshikawa H, et al. Identification of seven novel mutations including the first two genomic rearrangements in SLC26A3 mutated in congenital chloride diarrhea. Hum Mutat. 2001;18:233–242. doi: 10.1002/humu.1179. [DOI] [PubMed] [Google Scholar]
- 18.Gadsby DC, Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev. 1999;79:S77–S107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- 19.Rowe SM, Varga K, Rab A, Bebok Z, Byram K, Li Y, Sorscher EJ, Clancy JP. Restoration of W1282X CFTR activity by enhanced expression. Am J Respir Cell Mol Biol. 2007;37:347–56. doi: 10.1165/rcmb.2006-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clausen MR, Bonnén H, Tvede M, Mortensen PB. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology. 1991;101:1497–1504. doi: 10.1016/0016-5085(91)90384-w. [DOI] [PubMed] [Google Scholar]
- 21.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 22.Burkitt DP. Epidemiology of cancer of the colon and rectum. 1971. Dis Colon Rectum. 1993;36:1071–1082. doi: 10.1007/BF02047303. [DOI] [PubMed] [Google Scholar]
- 23.Cassidy A, Bingham SA, Cummings JH. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994;69:937–942. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe GR, Benito E, Castelleto R, Cornée J, Estève J, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 1992;84:1887–1896. doi: 10.1093/jnci/84.24.1887. [DOI] [PubMed] [Google Scholar]
- 25.Kim YI. AGA technical review: impact of dietary fiber on colon cancer occurrence. Gastroenterology. 2000;118:1235–1257. doi: 10.1016/s0016-5085(00)70377-5. [DOI] [PubMed] [Google Scholar]
- 26.Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294:2849–2857. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- 27.Comalada M, Bailón E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Gálvez J. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 2006;132:487–497. doi: 10.1007/s00432-006-0092-x. [DOI] [PubMed] [Google Scholar]
- 28.Hodin RA, Meng S, Archer S, Tang R. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ. 1996;7:647–653. [PubMed] [Google Scholar]
- 29.Chirakkal H, Leech SH, Brookes KE, Prais AL, Waby JS, Corfe BM. Upregulation of BAK by butyrate in the colon is associated with increased Sp3 binding. Oncogene. 2006;25:7192–7200. doi: 10.1038/sj.onc.1209702. [DOI] [PubMed] [Google Scholar]
- 30.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012–1017. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 31.Alrawi SJ, Schiff M, Carroll RE, Dayton M, Gibbs JF, Kulavlat M, Tan D, Berman K, Stoler DL, Anderson GR. Aberrant crypt foci. Anticancer Res. 2006;26:107–119. [PubMed] [Google Scholar]
- 32.Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Gibson PR, Rosella O, Wilson AJ, Mariadason JM, Rickard K, Byron K, Barkla DH. Colonic epithelial cell activation and the paradoxical effects of butyrate. Carcinogenesis. 1999;20:539–544. doi: 10.1093/carcin/20.4.539. [DOI] [PubMed] [Google Scholar]
- 34.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 35.Kouraklis G, Theocharis S. Histone deacetylase inhibitors: a novel target of anticancer therapy (review) Oncol Rep. 2006;15:489–494. [PubMed] [Google Scholar]
- 36.Chen YX, Fang JY, Lu J, Qiu DK. Regulation of histone acetylation on the expression of cell cycle-associated genes in human colon cancer cell lines. Zhonghua Yixue Zazhi. 2004;84:312–317. [PubMed] [Google Scholar]
- 37.Yu DC, Waby JS, Chirakkal H, Staton CA, Corfe BM. Butyrate suppresses expression of neuropilin I in colorectal cell lines through inhibition of Sp1 transactivation. Mol Cancer. 2010;9:276. doi: 10.1186/1476-4598-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruemmele FM, Dionne S, Qureshi I, Sarma DS, Levy E, Seidman EG. Butyrate mediates Caco-2 cell apoptosis via up-regulation of pro-apoptotic BAK and inducing caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase (PARP) Cell Death Differ. 1999;6:729–735. doi: 10.1038/sj.cdd.4400545. [DOI] [PubMed] [Google Scholar]
- 39.Ruemmele FM, Schwartz S, Seidman EG, Dionne S, Levy E, Lentze MJ. Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway. Gut. 2003;52:94–100. doi: 10.1136/gut.52.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303:364–369. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 41.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 42.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordonaro M, Lazarova DL, Sartorelli AC. Butyrate and Wnt signaling: a possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle. 2008;7:1178–1183. doi: 10.4161/cc.7.9.5818. [DOI] [PubMed] [Google Scholar]
- Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care 2004; 7: 563-567. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Pool-Zobel B, Veeriah S, Böhmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens-focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 48.Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 49.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 51.Shao J, Fujiwara T, Kadowaki Y, Fukazawa T, Waku T, Itoshima T, Yamatsuji T, Nishizaki M, Roth JA, Tanaka N. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–736. doi: 10.1038/sj.onc.1203383. [DOI] [PubMed] [Google Scholar]
- 52.Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, Moldawer LL, Copeland EM 3rd, Mackay S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 53.Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schröder O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF kappa B signalling. Mol Immunol. 2007;44:3625–3632. doi: 10.1016/j.molimm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res. 2003;1:855–862. [PubMed] [Google Scholar]
- 58.Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13:715–721. doi: 10.1097/MCO.0b013e32833eebe5. [DOI] [PubMed] [Google Scholar]
- 59.Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 60.Hallert C, Björck I, Nyman M, Pousette A, Grännö C, Svensson H. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm Bowel Dis. 2003;9:116–121. doi: 10.1097/00054725-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Vernia P, Annese V, Bresci G, d’Albasio G, D’Incà R, Giaccari S, Ingrosso M, Mansi C, Riegler G, Valpiani D, et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003;33:244–248. doi: 10.1046/j.1365-2362.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 62.Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–56. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- 63.Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37:458–466. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 64.Vernia P, Marcheggiano A, Caprilli R, Frieri G, Corrao G, Valpiani D, Di Paolo MC, Paoluzi P, Torsoli A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9:309–313. doi: 10.1111/j.1365-2036.1995.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 65.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16:684–695. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 66.Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 67.Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–406. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, Brummer RJ. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28:88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun. 2007;356:599–603. doi: 10.1016/j.bbrc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 70.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125:1613–1625. doi: 10.1053/j.gastro.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 72.Kida Y, Shimizu T, Kuwano K. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol Immunol. 2006;43:1972–1981. doi: 10.1016/j.molimm.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, Menzel T, Gostner A, Lührs H, Scheppach W. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinmann J, Halldórsson S, Agerberth B, Gudmundsson GH. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother. 2009;53:5127–5133. doi: 10.1128/AAC.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mariadason JM, Barkla DH, Gibson PR. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol. 1997;272:G705–G712. doi: 10.1152/ajpgi.1997.272.4.G705. [DOI] [PubMed] [Google Scholar]
- 77.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 78.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 80.Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009;21:952–976. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 81.Kilkens TO, Honig A, van Nieuwenhoven MA, Riedel WJ, Brummer RJ. Acute tryptophan depletion affects brain-gut responses in irritable bowel syndrome patients and controls. Gut. 2004;53:1794–1800. doi: 10.1136/gut.2004.041657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB, Gean PW, Chuang DM, Hong JS. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 84.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 85.Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59 Suppl 2:251–262. [PubMed] [Google Scholar]
- 86.Atweh GF, Sutton M, Nassif I, Boosalis V, Dover GJ, Wallenstein S, Wright E, McMahon L, Stamatoyannopoulos G, Faller DV, et al. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93:1790–1797. [PMC free article] [PubMed] [Google Scholar]
- 87.Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, Cai SP, Vichinsky EP, Olivieri NF. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993;328:81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- 88.Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–49. [PubMed] [Google Scholar]
- 89.Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;84:339–343. [PubMed] [Google Scholar]
- 90.Perrine SP, Rudolph A, Faller DV, Roman C, Cohen RA, Chen SJ, Kan YW. Butyrate infusions in the ovine fetus delay the biologic clock for globin gene switching. Proc Natl Acad Sci USA. 1988;85:8540–8542. doi: 10.1073/pnas.85.22.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinberg RS, Ji X, Sutton M, Perrine S, Galperin Y, Li Q, Liebhaber SA, Stamatoyannopoulos G, Atweh GF. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105:1807–1809. doi: 10.1182/blood-2004-02-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mabaera R, West RJ, Conine SJ, Macari ER, Boyd CD, Engman CA, Lowrey CH. A cell stress signaling model of fetal hemoglobin induction: what doesn’t kill red blood cells may make them stronger. Exp Hematol. 2008;36:1057–1072. doi: 10.1016/j.exphem.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Burlina AB, Ogier H, Korall H, Trefz FK. Long-term treatment with sodium phenylbutyrate in ornithine transcarbamylase-deficient patients. Mol Genet Metab. 2001;72:351–355. doi: 10.1006/mgme.2001.3156. [DOI] [PubMed] [Google Scholar]
- 94.Kemp S, Wei HM, Lu JF, Braiterman LT, McGuinness MC, Moser AB, Watkins PA, Smith KD. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat Med. 1998;4:1261–1268. doi: 10.1038/3242. [DOI] [PubMed] [Google Scholar]
- 95.Gylling H. Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. Int J Clin Pract. 2004;58:859–866. doi: 10.1111/j.1742-1241.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 96.Sviridov DD, Safonova IG, Talalaev AG, Repin VS, Smirnov VN. Regulation of cholesterol synthesis in isolated epithelial cells of human small intestine. Lipids. 1986;21:759–763. doi: 10.1007/BF02535408. [DOI] [PubMed] [Google Scholar]
- 97.Alvaro A, Solà R, Rosales R, Ribalta J, Anguera A, Masana L, Vallvé JC. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life. 2008;60:757–764. doi: 10.1002/iub.110. [DOI] [PubMed] [Google Scholar]
- 98.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110:1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–1231. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 101.Jacobasch G, Schmiedl D, Kruschewski M, Schmehl K. Dietary resistant starch and chronic inflammatory bowel diseases. Int J Colorectal Dis. 1999;14:201–211. doi: 10.1007/s003840050212. [DOI] [PubMed] [Google Scholar]
- 102.Stein TP, Yoshida S, Schluter MD, Drews D, Assimon SA, Leskiw MJ. Comparison of intravenous nutrients on gut mucosal proteins synthesis. JPEN J Parenter Enteral Nutr. 1994;18:447–452. doi: 10.1177/0148607194018005447. [DOI] [PubMed] [Google Scholar]
- 103.Tappenden KA. Emerging therapies for intestinal failure. Arch Surg. 2010;145:528–532. doi: 10.1001/archsurg.2010.102. [DOI] [PubMed] [Google Scholar]
- 104.Goldstein NS. Serrated pathway and APC (conventionUse)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol. 2006;125:146–153. [PubMed] [Google Scholar]
- 105.Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:90–95. doi: 10.1111/j.1365-2036.2006.03067.x. [DOI] [PubMed] [Google Scholar]
- 106.Diakos C, Prieschl EE, Säemann MD, Böhmig GA, Csonga R, Sobanov Y, Baumruker T, Zlabinger GJ. n-Butyrate inhibits Jun NH(2)-terminal kinase activation and cytokine transcription in mast cells. Biochem Biophys Res Commun. 2006;349:863–868. doi: 10.1016/j.bbrc.2006.08.117. [DOI] [PubMed] [Google Scholar]