Constitutional telomerase mutations are genetic risk factors for cirrhosis (original) (raw)
. Author manuscript; available in PMC: 2012 May 1.
Published in final edited form as: Hepatology. 2011 May;53(5):1600–1607. doi: 10.1002/hep.24173
Abstract
Some patients with liver disease progress to cirrhosis, but the risk factors for cirrhosis development are unknown. Dyskeratosis congenita, an inherited bone marrow failure syndrome associated with mucocutaneous anomalies, pulmonary fibrosis, andcirrhosis, is caused by germ-line mutations of genesin the telomerase complex. We examined whether telomerase mutations also occurred in sporadic cirrhosis. One hundred thirty-four patients with cirrhosis of common etiologies treated at the Liver Research Institute, University of Arizona, between May 2008 and July 2009, and 528healthy subjects were screened for variation in the TERT and TERC genes by direct sequencing; an additional 1472 controls were examined for the most common genetic variation observed in patients. Telomere length of leukocytes was measured by quantitative polymerase chain reaction. Functional effects of genetic changes were assessed by transfection of mutation-containing vectors into telomerase-deficient cell lines, and telomerase activity was measured in cell lysates. Nine of the 134 patients with cirrhosis (7%) carried a missense variant in TERT, resulting in a cumulative carrier frequency significantly higher than in controls (_P_=0.0009). One patient was homozygous and eight were heterozygous. The allele frequency for the most common missense TERT variant was significantly higher in cirrhotic patients (2.6%) than in 2000 controls (0.7%; _P_=0.0011). One additional patient carried a TERC mutation. The mean telomere length of leukocytesin cirrhotic patients, including six mutant cases, was shorter than in age-matched controls(_P_=0.0004). Most TERT gene variants reduced telomerase enzymatic activity in vitro. Loss-of-function telomerase gene variants associated with short telomeres are risk factors for sporadic cirrhosis.
Keywords: telomere, regeneration, dyskeratosis congenital, aplastic anemia
Telomeres, the natural ends of linear chromosomes, cap and protect chromosomes against damage and from being mistaken for double-stranded DNA breaks.(1) When a cell divides, telomeres shorten due to DNA polymerase’s inability to fully replicate the 3′ ends of chromosomes, the “end-under-replication problem”.(2) If telomeres become critically short, cellular signaling cascades involving p53 and p21 are activated, resulting in cell senescence or apoptosis.(3, 4) To counter telomere attrition, cells with high proliferative capacity express telomerase, a reverse transcriptase enzyme that adds DNA repeats to telomeres.(5) Components of the telomerase complex include the reverse transcriptase enzyme, TERT, the RNA component that serves as template for telomere elongation, encoded by TERC, and associated proteins, including dyskerin(encoded by DKC1).(6, 7) Loss-of-function heterozygous or homozygous mutations in genes of the telomerase complex have been implicated in human diseases caused by a deficient tissue regeneration capacity,(3)including dyskeratosis congenita(8, 9), aplastic anemia,(10, 11)and familial idiopathic pulmonary fibrosis,(12, 13)and also with acute myeloid leukemia.(14, 15) Common genetic variants in the region of the TERT gene on chromosome 5p15.33 have been associated with susceptibility to braintumors, lung cancer, pancreatic cancer and additional cancers(16–20).
Dyskeratosis congenita is a rare genetic disease in which patients develop bone marrow failure and exhibit a mucocutaneous triad of abnormal reticular skin pigmentation, leukoplakia, and nail dystrophy.(21)Most cases are X-linked and caused by mutations in the DKC1 gene. Dyskeratosis congenita also may be autosomal dominant, in which heterozygous mutations in the telomere biology genes TERT, TERC, or TINF2 are etiologic, or autosomal recessive, due to mutations in NOLA2 or NOLA3, coding telomerase-associated proteins.(22, 23) The observation that lungdisease, mainly pulmonary fibrosis, is present in up to 20% of patients with dyskeratosis congenita led to the association of telomerase mutations with familial idiopathic pulmonary fibrosis.(12) Approximately, seven percent of dyskeratosis patients have a concurrent diagnosis of hepatic disease, including cirrhosis.(21) Fatal liver complications are a relatively common cause of death after hematopoietic stem-cell transplantation for bone marrow failure in dyskeratosis congenita, whereas fatal liver complications are infrequent following transplant for other disorders, suggestive of a related underlying mechanism.(24) In families of patients with telomerase mutation and aplastic anemia, severe hepatic disease in relatives tracks to mutation status.(25)
The relationship between telomere shortening and risk of cirrhosis has been examined in an experimental model of mice null for the telomerase reverse transcriptase gene. _Tert_-deficient mice had reduced regenerative activity following partial hepatectomy as well as more hepatic fibrosis and inflammation after exposure to CCl4 compared to normal mice.(26) In human cirrhosis, hepatocytes display excessive telomere shortening and senescence.(27, 28) These findings in experimental animals and observations in humans suggest that reduced telomerase activity may contribute to the development of cirrhosis. In the present study, we sought to determine whether germ-line missense sequence variants in the telomerase complex genes are more frequent in patients with non-familial cirrhosis due to a variety of causes.
Methods
Patients and Controls
Adults with cirrhosis who were patients at the liver clinic at the University of Arizona were recruited for the study. The diagnosis of cirrhosis was established by liver biopsy or clinical evidence of cirrhosis and portal hypertension(i.e. ascites, varices, or CT findings of cirrhosis). None of the patients had a family history of liver disease or the classical manifestation of dyskeratosis congenita (bone marrow failure, ungual dystrophy, abnormal skin pigmentation). Patients or their legal guardians provided informed consent for genetic testing, as specified by protocols approved by the institutional review board of the University of Arizona (IRB No. 08-0347-04). DNA was extracted from buccal mucosa swab using the Gentra Puregene buccal cell core kit (Qiagen, Gaithersburg, Maryland) or peripheral blood leukocytes using the automated Maxwell® 16 System (Promega, Madison, Wisconsin). Samples from 528healthy subjects were analyzed as controls for genetic screening, as previously described(control group 1).(11) An additional 1472 healthy subjects were screened for variations in TERT exon 15 (control group 2; 751 individuals from the Human Genome Diversity Panel Project, 477 drawn from the NCI Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial(PLCO) Cohort, and 244 blood donors at the NIH Clinical Center; supplementary tables 1 and 2). DNA from peripheral blood leukocytes for telomere length measurement also was obtained from 175healthy volunteers ranging in age from 0 to 99 years (control group 3; median 35.8 years old; table 1).
Table 1.
Demographic Characteristics of 134 Patients with Hepatic Cirrhosis
| Cirrhotic Patients (n=134) | Healthy Controls (n=175) | |
|---|---|---|
| Number of Patients(%) | Number of Subjects (%) | |
| Gender | ||
| Male | 86 (64) | 89 (51) |
| Female | 48(36) | 86 (49) |
| Ethnicity | ||
| Caucasian | 84(63) | 101 (58) |
| Hispanic | 37(28) | 20 (11) |
| Native American | 4(3) | 0 |
| Asian | 4(3) | 6 (4) |
| Black | 0 | 21 (12) |
| Mixed-ethnicity | 5(4) | 27 (12) |
| Etiology of liver disease | ||
| Hepatitis C virus | 50 (37) | - |
| Alcohol | 33 (25) | - |
| NASH* | 12(9) | - |
| Hepatitis C and alcohol | 11(8) | - |
| Autoimmune hepatitis | 6(4) | - |
| Primary biliary cirrhosis | 7 (5) | - |
| Primary sclerosing cholangitis | 4(3) | - |
| Hepatitis B virus | 4(3) | - |
| Budd-Chiari | 1(1) | - |
| Congenital hepatic fibrosis | 1(1) | - |
| Hepatitis B + fatty liver | 1(1) | - |
| Hepatitis C + B cell lymphoma | 1(1) | - |
| Sarcoidosis | 1(1) | - |
| Wilson's disease | 1(1) | - |
| Cause unknown | 1 (1) | - |
| Age, median | 56 years (range, 21to 74 years) | 36 years (range, 0 to 99 years) |
Mutational Analysis
Bidirectional sequencing of TERC and TERT was performed as previously described.(11, 14)
Telomere Length Measurement
Mean telomere length was measured in peripheral blood leukocytes by quantitative polymerase chain reaction (qPCR), as previously described.(29, 30) PCR was conducted in triplicate in a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA), and analysis was completed using SDSv1.3. The telomere length for each sample was determined using the telomere to single copy gene ratio (T/S ratio) with the calculation of the ΔCt[Ct(telomere)/Ct(singlegene)]. The T/S ratio for each sample (x) was normalized to the mean T/S ratio of reference sample [2−(ΔCt_x_ − ΔCt_r_) = 2−ΔΔCt], which was used for the standard curve, both as a reference sample and as a validation sample.
Functional Analysis
Functional analysis was performed for the TERC mutation 37A→G and TERT mutations P529L and T882I, as previously described.(14, 25) In vitro mutagenesis was performed on the wild-type vector by Mutagenex (Somerset, New Jersey). Telomerase activity was measured using the fluorescent telomerase repeat amplification protocol (TRAPeze XL, Chemicon), as previously described.(14, 25)
Statistical Analysis
Fisher’s exact test was used to evaluate differences in the cumulative frequency of missense variations between patients and controls. For the comparison of TERT codon A1062T variant allele frequency (most common variant in patients) between patients and an extended number of controls (control group 2; n=1472), the χ2 test was employed, as it is not possible to calculate Fisher’s test with such large sample groups. For the analysis of differences between patients and controls, telomere length was corrected for age. A subject’s “predicted telomere length” was computed using (a + b × Age), where a and b were the least squares estimates of the slope and intercept for the linear model of telomere length versus age. Age-adjusted telomere length for each subject was computed by subtracting the subject’s “predicted telomere length” from his/her observed telomere length. The differences in telomere length between the two groups were evaluated using the Student’s t test. A P value <0.05 was considered as statistically significant.
Results
Patients
From May 2008 to April 2009, 149 patients with hepatic cirrhosis were enrolled in the study. Thirteen patients did not donate buccal mucosa and/or peripheral blood for DNA extraction; one patient withdrew from the study; and the DNA sample from one patient was not adequate for amplification. Thus, samples from 134 patients were available for analysis; their characteristics are described in Table 1. In 67 patients (50%), the diagnosis of cirrhosis was established by liver biopsy; in the remaining patients, cirrhosis was diagnosed based on clinical parameters for cirrhosis and portal hypertension.
Gene variants
Among the 134 patients with cirrhosis, one heterozygous mutation in TERC was found in one patient and four missense gene variants in TERT were identified in nine patients (cumulative carrier frequency for TERT missense variants, 7%). Eight patients were heterozygous, and one was homozygous for the TERT codon Ala1062Thr gene variant (Tables 2 and 3).
Table 2.
Functional Gene Variants in TERC and TERT in Patients with Hepatic Cirrhosis
| Gene | Location of Variation | Patients with Hepatic Cirrhosis (n = 134) | Controls (n = 528) |
|---|---|---|---|
| no. of heterozygotes; no. of homozygotes (allele frequency) | |||
| TERC | N. 37A→G | 1;0 (0.004) | 0;0 |
| Total | 1;0 (0.004) | 0;0 | |
| TERT | Exon 2, codon 441 (Glu) deletion† | 1;0 (0.004) | 1;0 (0.001) |
| Exon 3, codon 530 CCG/CTG (Pro/Leu) | 1;0 (0.004) | 0;0 | |
| Exon 10, codon 882 ACC/ATC (Thr/Ile) | 1;0 (0.004) | 0;0 | |
| Exon 15, codon 1062 GCC/ACC(Ala/Thr) | 5;1 (0.026)‡ | 7;0 (0.007) | |
| Total | 8;1 (0.037)* | 8;0 (0.008) |
Table 3.
Clinical Profile of Patients with Hepatic Cirrhosis Carrying TERC and TERT Gene Variants
| Patient | Gene Variant | Age (yr) | Sex | Ethnicity | Cause of Cirrhosis | Clinical Follow-up |
|---|---|---|---|---|---|---|
| A | TERC N. 37A→G | 54 | F | Caucasian | Hepatitis C | Died of head and neck cancer |
| B | TERT codon441E deletion | 56 | M | Caucasian | Alcohol | Died of progressive liver disease |
| C | TERT codon P530L | 62 | F | Caucasian | NASH* | - |
| D | TERT codon T882I | 48 | F | Native American | Alcohol | - |
| E | TERT codon A1062T | 51 | F | Caucasian | Alcohol | - |
| F | TERT codon A1062T (homozygous) | 64 | F | Caucasian | Primary biliary cirrhosis | - |
| G | TERT codon A1062T | 56 | M | Caucasian | Hepatitis C | - |
| H | TERT codon A1062T | 62 | M | Caucasian | Hepatitis C | - |
| I | TERT codon A1062T | 47 | M | Caucasian | Wilson’s disease | Liver transplantation |
| J | TERT codon A1062T | 59 | F | Caucasian | Hepatits C | - |
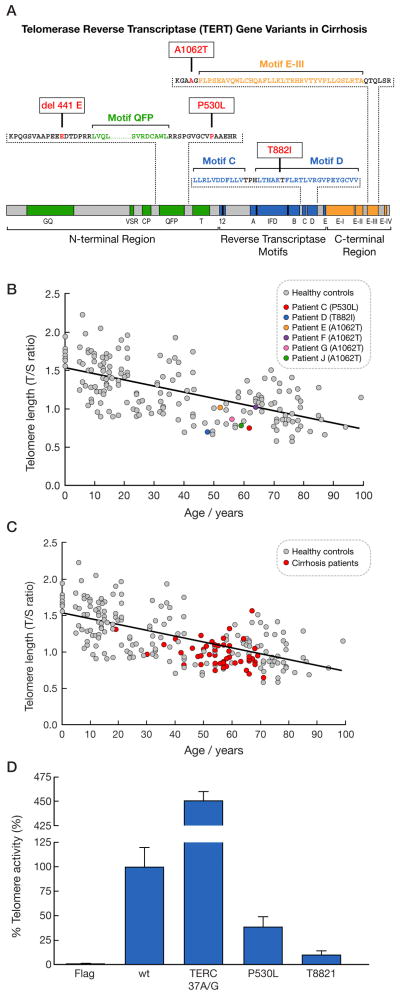
A 54-year-old female patient with hepatitis C virus-associated cirrhosis was heterozygous for the TERC n.37A→G mutation, which has been previously described in one patient with dyskeratosis congenita(31)and in one patient with idiopathic pulmonary fibrosis,(13)but not in healthy individuals in our study and in other series.(32–34) A 56-year-old male patient with alcoholic cirrhosis was heterozygous for a codon 441Gludeletion in the N-terminal region of TERT (Figure 1A), previously described in aplastic anemia,(11)acute myeloid leukemia (in homozygosity),(14)and in one healthy subject.(11) Two individuals carried novel TERT mutations: a 62-year-old woman with non-alcoholic steatohepatitis (NASH) who was heterozygous for a TERT codon Pro530Leu located in the N-terminal region, and a 48-year-old woman with alcoholic cirrhosis, heterozygous for a TERT codon Thr882Ilein the Reverse Transcriptase Motif D (Fig. 1A). These novel mutations were not found in 528 control subjects.
Figure 1.
(A) Schematic domain structure of TERT, indicating 3 major regions: N-terminal, reverse transcriptase motifs, and C-terminal. Mutation codon location and amino acid substitutions caused by mutations are shown. Abbreviations for aminoacid residues: A, alanine; E, glutamic acid; H, I, isoleucine; L, leucine; P, phenylalanine; T, threonine. (B) Leukocyte telomere length was measured by quantitative polymerase chain reaction (qPCR). Telomere length of 175healthy controls is shown in gray and patients with telomerase mutation and hepatic cirrhosisare depicted indifferent colors. (C) Telomere length of cirrhotic patients without identifiable mutations (red) was significantly shorter than in healthy controls (gray; _P_=0.0004). Eighty-two percent of cirrhotic patients had their telomere lengths below the median. (D)Telomerase activity of the empty (Flag), wild-type, or mutated TERT or TERC expression vectors in the telomerase-negative VA13 cell line was measured by the fluorescent telomeric repeat-amplification protocol (TRAP) assay. Telomerase activity was considered 100% for the wild-type. Telomerase activity in each experiment was corrected for TERT or TERC mRNA levels as measured by RT-PCR.
Five patients were heterozygous and one was homozygous for the TERT codon Ala1062Thr, located in the C-terminal region and adjacent to Motif E-III (Fig. 1A). The primary etiology for cirrhosis for these patients was chronic hepatitis C virus infection in three; alcoholic cirrhosis in one; primary biliary cirrhosis in one (homozygous); and Wilson’s disease in another. The TERT codon Ala1062Thr gene variant has been previously described in aplastic anemia,(11) acute myeloid leukemia,(14)idiopathic pulmonary fibrosis,(12)and healthy individuals.(11) As the TERT codon Ala1062Thr gene variant was observed in low frequency in the 528 control subjects (allele frequency, 0.007), we screened an additional 1472 controls for this gene variant (total number of controls, 2000); the allele frequency for the TERT codon Ala1062Thr variant was 3.7 times higher in cirrhosis patients than in healthy controls (0.026 vs. 0.007, respectively; _P_=0.001, χ2 test; Table 2). Silent single nucleotide polymorphisms (SNPs) and intronic SNPs showed similar allele frequencies in patients and healthy controls(35)(Supplementary Table 3). The overall cumulative frequency of TERT gene missense variants in patients with hepatic cirrhosis was significantly greater than in 528 healthy controls (P=0.0009, Fisher’s exact test; Table 2). Of note, none of the patients with mutations had hepatocellular carcinoma. One had undergone liver transplantation and two died during the study period(of head and neck cancer and one of progressive liver disease; Table 3).
Germ-line origin of gene variants was demonstrated by analysis of DNA obtained from peripheral blood leukocytes and buccal mucosa in all patients tested, except for two patients, one with the TERC n. 37A→G mutation and another with the TERT 441E deletion, who died before the study was complete.
Telomere Length
Leukocyte telomere length in the six patients with cirrhosis and mutations who were tested was below the median based on a reference group of 175healthy individuals varying in age from 0 to 99 years, as measured by qPCR (Figure 1B). The leukocyte telomere lengths of the two patients carrying novel TERT mutations (patients C and D) were in the shortest quartile for healthy controls.
Leukocyte telomere length also was measured for 44cirrhotic patients without identifiable telomerase missense mutations from whom peripheral blood leukocytes were collected; they had significantly shorter telomeres in comparison to controls (Fig. 1C; _P_=0.0004). The mean age-adjusted telomere length in cirrhotic patients was −0.114 (95% confidence interval, −0.162, −0.06), compared to 0.001 (95%, −0.04, 0.04)in controls. Eighty-two percent of cirrhotic patients had telomere lengths below the median for their age.
Telomerase Activity
To evaluate whether mutations in TERC and TERT decreased telomerase enzymatic activity(its ability to synthesize telomeric repeats), telomerase-deficient VA13 cells were transfected with plasmids containing wild-type or mutant TERT and TERC constructs(or transfected with an empty vector). Novel TERT codon Pro530Leu and codon Thr882Ile mutations produced significant reduction intelomerase activity as compared to wild-type TERT (Fig. 1D). In our transfection experiments, TERC 37A→G mutation resulted in increased telomerase enzymatic activity in comparison to wild-type TERC. However, previous studies indicated that this mutation modulates telomerase activity from 75%(13)to 100%(31)of wild-type function. TERT 441Glu deletion has been previously found to generate approximately 40% of wild-type telomerase activity, whereas the telomerase activity produced by the TERT codon Ala1062Thr variant is 60% of wild-type TERT.(14)
Discussion
In this study, we found that missense variants in genes encoding components of the telomerase complex occurred at increased frequency in sporadic cirrhosis, suggesting that telomerase deficiency causing accelerated telomere shortening may predispose to cirrhosis and that the clinical spectrum of “telomere diseases”(3)may be broader and more common than previously suspected. We also confirmed that cirrhotic patients have shorter telomeres of peripheral blood leukocytes than age-matched controls, further implicating telomere dysfunction as a molecular event in the pathophysiology of cirrhosis.
Mutations in telomerase complex genes have been associated with the inherited bone marrow failure syndrome dyskeratosis congenita, apparently acquired aplastic anemia, and familial idiopathic pulmonary fibrosis.(3) Less than ten percent of patients with dyskeratosis congenita eventually develop severe liver disease with several histopathologic findings, especially after hematopoietic stem-cell transplant. In pedigrees of patients with bone marrow failure and telomerase deficiency, loss-of-function mutations correlate with an unusual high prevalence of severe hepatic disease, mainly represented by cirrhosis and nodular regenerative hyperplasia.(25) In the present work, we determined that telomerase mutations also are associated with non-familial cirrhosis with an identifiable etiologic factor, and that telomerase mutations might contribute to cirrhosis development in these patients. That mutations may contribute to fibrosis progression is further indicated by the recent observation by others of an absence of telomerase mutations in 200 individuals with chronic hepatitis C virus infection who did not progress to cirrhosis (K.L. Rudolph, personal communication).
Hepatic fibrosis in combination with the formation of regenerative nodules is the pathologic hallmark of cirrhosis.(36) The most common causes of cirrhosis in the developed world are hepatitis C virus infection and chronic alcohol abuse. However, only a portion of patients with chronic hepatitis C or who abuse alcohol eventually develops cirrhosis, suggesting host factors play a critical role in disease progression.(37) Numerous attempts to identify genetic risk factors for the development of cirrhosis have had limited success. Most reports have focused on candidate variants that might alter the primary pathologic process, such as oxidant stress and immunologic response, with inconsistent results.(38–42) One of the better studied risk factors are mutations in keratins as susceptibility markers for cirrhosis. Mutations in keratins 8 and 18 have been found in patients with cirrhosis due to a variety of causes. A 3.35 fold increase in frequency of mutations in the keratin genes was found relative to controls,(43)which is somewhat less than the 4.63 fold increase found in TERT in the current study. It is important to note that in contrast to the studies of keratins and other genes where many of the mutations were of uncertain functional significance, in our study all of the mutations in TERT were shown to reduce telomerase activity leading to shorten telomeres.
Telomerase-deficient murine models have provided some insights into possible mechanisms that might explain the current observations. Short telomeres rather than telomerase insufficiency causes impairment of regeneration and pathological phenotypes in the mouse.(44) Also in the telomerase “knockout” model, excessively short and dysfunctional telomeres predispose the mouse to chemically induced cirrhosis, and exogenous telomerase expression in hepatocytes ameliorates hepatic function and fibrosis in response to liver chemical injury, indicating a role of telomeres in pathogenesis of cirrhosis.(26) In addition, human cirrhosis due to chronic liver injury may improve once liver injury is eliminated (45–49) and hepatocyte regenerative capacity and reduced synthesis of collagen are critical in this process. Excessive telomere shorting may impair this repair process.
Telomere length was measured in peripheral blood samples from 50 cirrhotic patients (37% of patients) and was significantly shorter than in healthy controls (Fig. 1C). It has been shown previously that telomeres are shorter in cirrhotic than in non-cirrhotic hepatocytes regardless of disease etiology.(27, 28) Short telomeres in both hepatocytes and peripheral blood leukocytes indicate the constitutive essence of telomere attrition in cirrhosis and implicate short and dysfunctional telomeres as a molecular mechanism for cirrhosis. Excessive telomere shortening (caused by telomerase gene mutations or other factors) may impair the hepatocyte regenerative ability in response to chronic injury, thus facilitating fibrosis progression. For example, as telomeres are eroded with aging (Fig. 1B), shorter telomeres in older humans may contribute to the more rapid rate of progression to cirrhosis with hepatitis C virus infection in the more elderly.(50) In agreement with our findings, in families with idiopathic pulmonary fibrosis and telomerase mutations, short telomeres have been hypothesized to limit pneumocyte proliferation, causing loss of alveolar cells and, secondarily, fibrosis.(12, 13) Alternatively, in cirrhosis, short telomeres may affect stellate cell differentiation into myofibroblasts upon injury thereby affecting the severity of fibrosis. Additionally, telomere attrition in inflammatory cells may induce a profibrotic response or contribute to the myofibroblast differentiation of cells of bone marrow origin.
Environmental factors may influence disease expression. For example, patients with X-linked dyskeratosis congenita, caused by DKC1 mutations, have extremely short telomeres due to DKC1 gene hemizigosity and present a severe and multi-organ phenotype, including mucocutaneous anomalies, bone marrow failure, and pulmonary and hepatic fibrosis.(3) In patients with telomerase deficiency due to telomerase mutations_,_ enzyme function is reduced by haploinsufficiency and telomere shortening may be less intense and clinical phenotype may be less pronounced.(51) In patients with TERT or TERC mutations, aplastic anemia or pulmonary fibrosis may be the only clinical presentation.(11, 12) Most patients with telomerase mutations and aplastic anemia do not have respiratory failure, and most patients with pulmonary fibrosis do not have cytopenias, suggesting that environmental factors contribute to disease development in a susceptible patient; for example, most patients with telomerase mutations and pulmonary fibrosis are smokers.(12, 13) In pedigrees of telomerase mutations, liver disease and aplastic anemia presented alone in different affected individuals, further suggesting a role for environmental factors. In one study, approximately three percent of patients with idiopathic pulmonary fibrosis also had cryptogenic cirrhosis, indicating some overlap between clinical features.(52)
In conclusion, telomerase mutations resulting intelomere erosion appear to be a genetic risk factor for human cirrhosis and may predispose affected subjects to disease progression in combination with environmental injury, further supporting telomere attrition as a causal event in cirrhosis pathophysiology. Establishing how shortened telomeres increase the risk of cirrhosis may allow for the design of future therapies to reduce the risk of hepatic fibrosis in susceptible populations. Patients with mutations also may be appropriate targets for more aggressive forms of therapy to treat their primary disease given their increased risk of cirrhosis.
Supplementary Material
Supp Table S1-S3
Acknowledgments
Financial support: This work was supported by the NIH Intramural Research Program. JB and PM’s research years were made possible through the Clinical Research Training Program (CRTP), a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc).
Contributor Information
Rodrigo T. Calado, Email: calador@nhlbi.nih.gov.
Jennifer Brudno, Email: jbrudno@gmail.com.
Paulomi Mehta, Email: Mehta.paulomi@gmail.com.
Joseph J. Kovacs, Email: kovacsj2@mail.nih.gov.
Colin Wu, Email: wuco@mail.nih.gov.
Marco A. Zago, Email: marazago@usp.br.
Stephen J. Chanock, Email: chanocks@mail.nih.gov.
Thomas D. Boyer, Email: tboyer@deptofmed.arizona.edu.
Neal S. Young, Email: youngns@mail.nih.gov.
Reference List
- 1.Blackburn EH. Switching and signaling at the telomere. Cell. 2001 Sep 21;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201(6):1496–1499. [PubMed] [Google Scholar]
- 3.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009 Dec 10;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco MA. Telomere length, stem cells and aging. Nature Chemical Biology. 2007;3:640–646. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 5.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 6.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007 Mar 30;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 8.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 9.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason P, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 10.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand EM, Zeng W, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi H, Calado RT, Ly H, Baerlocher GM, Kajigaya S, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Eng J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 12.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll SG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Eng J Med. 2007 Mar 29;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 13.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Nat Acad Sci USA. 2007 May 1;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Nat Acad Sci USA. 2009 Jan 15;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirwan M, Vulliamy T, Marrone A, Walne AJ, Beswick R, Hillmen P, et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum Mutat. 2009 Sep 2;30:1567–1573. doi: 10.1002/humu.21115. [DOI] [PubMed] [Google Scholar]
- 16.Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009 Feb;41(2):221–227. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008 Dec;40(12):1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009 Jul 5; doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosgood HD, 3, Cawthon RM, He X, Chanock SJ, Lan Q. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer. 2009 Mar 12; doi: 10.1016/j.lungcan.2009.02.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen GM, Amundadottir L, Fuchs CS, Fraft P, Stolzenberg-Solomon RZ, Jacobs KB, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010 doi: 10.1038/ng.522. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 22.Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009 Apr;23(2):215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008 Jan 5;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha V, Devergie A, Socie G, Ribaud P, Esperou H, Parquet N, et al. Unusual complications after bone marrow transplantation for dyskeratosis congenita. Brit J Haem. 1998;103:243–248. doi: 10.1046/j.1365-2141.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 25.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, et al. A spectrum of severe familal liver disorders associate with telomerase mutations. PLoS one. 2009 Nov 20;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000 Feb 18;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 27.Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002 Jul;16(9):935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 28.Kitada T, Seki S, Kawakita N, Kuroki T, Monna T. Telomere shortening in chronic liver diseases. Biochem Biophys Res Commun. 1995 Jun 6;211(1):33–39. doi: 10.1006/bbrc.1995.1774. [DOI] [PubMed] [Google Scholar]
- 29.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002 May 15;:30. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007 Jan 13;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 31.Ly H, Schertzer M, Jastaniah W, Davis J, Yong SL, Ouyang Q, et al. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood. 2005 Aug 15;106:1246–1252. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002 Jan 9;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 33.Wilson DB, Ivanovich J, Whelan A, Goodfellow PJ, Bessler M. Human telomerase RNA mutations and bone marrow failure. The Lancet. 2003;361:1993–1994. doi: 10.1016/S0140-6736(03)13575-1. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Yagasaki H, Kamachi Y, Hama A, Matsumoto K, Kato K, et al. Mutations in telomerase catalytic protein in Japanese children with aplastic anemia. Haematologica. 2006;91:656–658. [PubMed] [Google Scholar]
- 35.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005 Apr 7;352(14):1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 36.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008 Mar 8;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hepatology; National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002; June 10-12, 2002; 2002. Nov, pp. S3–20. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Shiffman ML, Cheung RC, Layden TJ, Friedman S, Abar OT, et al. Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology. 2006 May;130(6):1679–1687. doi: 10.1053/j.gastro.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Muhlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, et al. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003 Oct;125(4):1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 40.Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, et al. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003 Dec;38(6):1468–1476. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Satsangi J, Chapman RW, Haldar N, Donaldson P, Mitchell S, Simmons J, et al. A functional polymorphism of the stromelysin gene (MMP-3) influences susceptibility to primary sclerosing cholangitis. Gastroenterology. 2001 Jul;121(1):124–130. doi: 10.1053/gast.2001.25527. [DOI] [PubMed] [Google Scholar]
- 42.Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003 Mar;37(3):493–503. doi: 10.1053/jhep.2003.50127. [DOI] [PubMed] [Google Scholar]
- 43.Ku NO, Lim JK, Krams SM, Esquivel CO, Keeffe EB, Wright TL, et al. Keratins as susceptibility genes for end-stage liver disease. Gastroenterology. 2005 Sep;129(3):885–893. doi: 10.1053/j.gastro.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 44.Hao L-Y, Armanios M, Strong MD, Karim B, Feldser DM, Huso D, et al. Short Telomeres, even in the Presence of Telomerase, Limit Tissue Renewal Capacity. Cell. 2005 Dec 16;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997 Dec 1;127(11):981–985. doi: 10.7326/0003-4819-127-11-199712010-00006. [DOI] [PubMed] [Google Scholar]
- 46.Dufour JF, DeLellis R, Kaplan MM. Regression of hepatic fibrosis in hepatitis C with long-term interferon treatment. Dig Dis Sci. 1998 Dec;43(12):2573–2576. doi: 10.1023/a:1026601904609. [DOI] [PubMed] [Google Scholar]
- 47.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, et al. Histologic improvementof fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000 Apr 4;132(7):517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 48.Poynard T, McHutchison J, Davis GL, Esteban-Mur R, Goodman Z, Bedossa P, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology. 2000 Nov;32(5):1131–1137. doi: 10.1053/jhep.2000.19347. [DOI] [PubMed] [Google Scholar]
- 49.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Flejou JF, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001 Feb 8;344(6):418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 50.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001 May 31;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 51.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004 May;36(5):447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 52.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Nat Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Table S1-S3