HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies (original) (raw)
. Author manuscript; available in PMC: 2011 Dec 1.
Published in final edited form as: Expert Rev Anticancer Ther. 2011 Feb;11(2):263–275. doi: 10.1586/era.10.226
Abstract
HER2 amplification is seen in up to 20% of breast cancers and is associated with an aggressive phenotype. Trastuzumab, a monoclonal antibody to HER2, accrues significant clinical benefit in the metastatic and adjuvant settings. However, some patients suffer disease recurrence despite adjuvant trastuzumab therapy, and many patients with metastatic disease do not respond to therapy or develop refractory disease within 1 year of treatment. Given the increased recognition of de novo and acquired resistance to therapy, considerable research has been dedicated to understanding the molecular mechanisms of trastuzumab resistance. Here, we highlight putative models of resistance, including activation of the downstream PI3K-signaling pathway, accumulation of a constitutively active form of HER2, and crosstalk of HER2 with other growth factor receptors. The identification of these specific mechanisms of trastuzumab resistance has provided a rationale for the development of several novel HER2-targeted agents as the mechanisms have largely suggested a continued tumor dependence on HER2 signaling. We explore the emerging data for the treatment of trastuzumab-refractory disease with novel agents including lapatinib, neratinib, pertuzumab, trastuzumab-DM1, HSP90 and PI3K pathway inhibitors, and the future potential for these inhibitors which, if combined with reliable biomarkers of resistance, may ultimately usher in a new era of personalized medicine for this disease.
Keywords: 17-AAG, breast cancer, HER2, lapatinib, p95-HER2, pertuzumab, resistance, targeted therapy, trastuzumab
Breast cancer is the most common malignancy and the second leading cause of cancer death in women in the USA. Despite advances in detection, local therapy and adjuvant systemic therapies, approximately 40,000 women die from this disease annually in the USA. Breast cancer is a heterogeneous disease that can be classified by microscopic appearance and molecular profiles that includes the expression of estrogen receptor (ER), and amplification of HER2. The resulting subgroups not only classify clinical behavior with respect to pathogenesis, natural history and prognosis, but are also predictive of response to targeted systemic therapies against these receptors and the pathways they activate.
Amplification of HER2 is observed in approximately 20% of invasive breast carcinomas, and portends a poor prognosis with an increased risk for disease progression and a decreased overall survival [1–3]. The HER2 gene encodes a transmembrane tyrosine kinase receptor that belongs to the EGF receptor (EGFR) family. This family of receptors includes four members (EGFR/HER1, HER2, HER3 and HER4) that function by stimulating growth factor signaling pathways such as the PI3K–AKT–mTOR pathway [4]. Receptors of this family contain an extracellular ligand-binding domain, a lipophilic transmembrane domain, and an intracellular tyrosine kinase domain. Activation of receptor kinase function occurs predominantly via ligand-mediated hetero- or homo-dimerization. In the case of HER2, activation is also thought to occur in a ligand-independent manner, particularly when the receptor is found to be mutated or overexpressed [5].
Overexpression of HER2 enables constitutive activation of growth factor signaling pathways and thereby serves as an oncogenic driver in breast cancer. Through both genetic and pharmacologic approaches it was determined that HER2 was both necessary and sufficient for tumor formation and maintenance in models of HER2-amplified breast cancer. Given that HER2 amplification mediates the transformed phenotype, direct pharmacologic targeting of HER2 was proposed. Trastuzumab (herceptin), a humanized, recombinant monoclonal antibody that binds to the extracellular domain of HER2, has been shown to selectively exert anti-tumor effects in cancer models and patients with HER2-amplified breast cancer, and not in tumors with normal HER2 expression [6–8]. Although an unconfirmed analysis has suggested possible added benefits of trastuzumab for adjuvant patients with HER2 normal disease, the wealth of pre-clinical and clinical data point to the benefits of this drug exclusively in HER2-amplified disease [9]. Trastuzumab improves overall survival when given in combination with chemotherapy for metastatic disease and reduces the risk of disease recurrence and death when given in the adjuvant setting, making the drug the foundation for systemic therapy of HER2-overexpressing tumors [7,10–16].
Mechanisms of action
Trastuzumab has been demonstrated to exert a variety of anti-tumor effects selectively in HER2-overexpressing tumor cells (Figure 1A). Trastuzumab binds to the juxtamembrane domain of HER2 and upon receptor binding, the antibody downregulates the expression of HER2 [17]. More recent work has demonstrated that trastuzumab selectively blocks ligand-independent HER2–HER3 dimerization [18]. In addition, trastuzumab binding to HER2 blocks proteolytic cleavage of the extracellular domain of HER2, resulting in diminished levels of the more active p95–HER2 form of HER2 [19]. As a result of these effects on the HER2 receptor, trastuzumab causes downregulation of PI3K pathway signaling and downstream mediators of cell cycle progression such as cyclin D1 [20]. Trastuzumab not only inhibits HER2 signaling pathways but also triggers immune-mediated responses against HER2-overexpressing cells. Trastuzumab binding engages Fc receptors on immune effector cells leading to antibody-dependent cellular cytotoxicity [21,22]. Beyond these effects, trastuzumab has been shown to have antiangiogenic effects and to lower the proapoptotic threshold for chemotherapy [23]. Combinations of trastuzumab with several different chemo-therapeutic agents have been tested in HER2-amplifed cell lines and xenograft models, and demonstrate additive or synergistic interactions for doxorubicin, epirubicin, paclitaxel carboplatin, docetaxel and gemcitabine [24–26]. As a result of these actions, the drug yields a clinical benefit for patients with all stages of HER2-positive breast cancer.
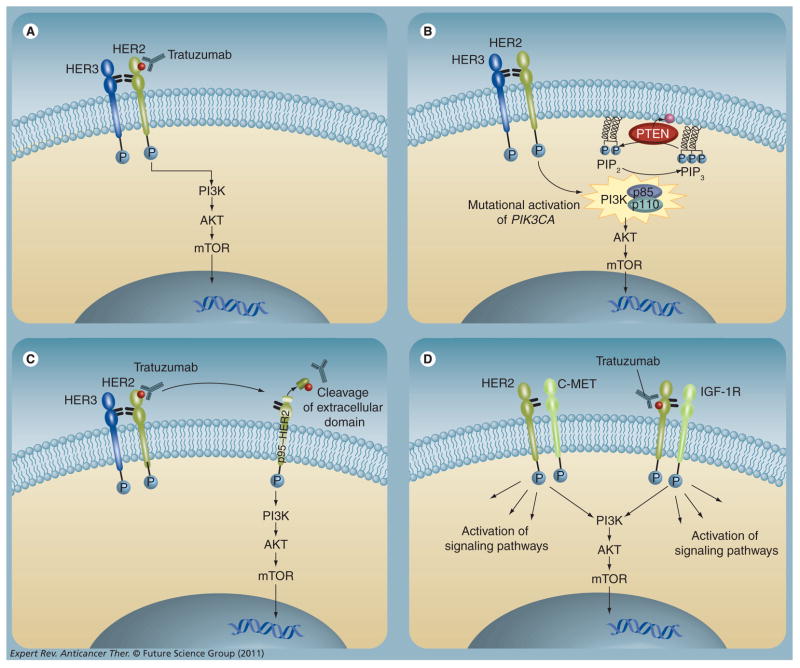
Figure 1. Proposed mechanisms of resistance to trastuzumab.
(A) HER2 signal transduction. Activation of the receptor tyrosine kinase occurs by homodimerization or heterodimerization with other HER family members. Activated HER2 initiates downstream signaling through the PI3K–AKT–mTOR pathway, promoting cell proliferation and survival. (B) Downstream activation of the PI3K pathway. PI3K is composed of an 85-kD regulatory subunit and a 110-kD catalytic subunit (PIK3CA), and upon subunit catalyzes phosphorylation of phosphatidylinositol bisphosphate at the membrane (PIP2) to phosphatidylinositol triphosphate promotes membrane localization and activation of downstream effector proteins such as AKT that stimulate cell proliferation. (PIP3). PIP3 or loss of PTEN result PTEN is a negative regulator of PI3K signaling that dephosphorylates PIP3 into PIP2. Activating mutations in PIK3CA in constitutive activation of the PI3K pathway and clinical resistance to trastuzumab therapy. (C) Accumulation of p95-HER2. The constitutively active truncated form of HER2, p95-HER2, lacks the trastuzumab-binding site. The intracellular kinase downstream signaling in the presence of trastuzumab, leading to increased cell proliferation. (D) Increased signaling from alternative receptors. Overexpression or activation of other receptors may drive growth factor signaling, either through trastuzumab-insensitive dimers with HER2 or in a HER2-independent fashion.
Mechanisms of resistance
Despite the clinical benefit seen with trastuzumab administration, both de novo and acquired clinical resistance have been increasingly recognized. Trastuzumab monotherapy in the meta-static setting results in response rates of 11–26% (clinical benefit rate: 48%), implying that many tumors with HER2-amplified metastatic breast cancer will not respond to monotherapy. In addition, the duration of response to trastuzumab-based therapy ranges from 5 to 9 months, suggesting that acquired resistance often develops [7,11,27]. Elucidating the molecular mechanisms of trastuzumab resistance has been difficult given the number of mechanisms of action of trastuzumab. Nevertheless, a detailed molecular understanding of clinical resistance to trastuzumab might greatly aid in the development of more effective targeted therapies, and has thus gained significant attention. Recently, several models of resistance have been described although final validation with analyses of human tumor samples has been limited [28,29].
Upregulation of the PI3K pathway
Persistent activation of the PI3K–AKT–mTOR signaling pathway drives aberrant cell growth and proliferation in a variety of tumor types. Recent work has demonstrated a strong association between mutational activation of this pathway and resistance to therapies targeted against the HER kinases such as trastuzumab (Figure 1B). Constitutive activation of PI3K most frequently occurs via two mechanisms: loss of function of the phosphatase and ten-sin homolog (PTEN), or activating mutations in the gene encoding the catalytic subunit of PI3K (PIK3CA) [30]. As a negative regulator of the PI3K pathway, loss of PTEN function through mutational inactivation or downregulation of expression results in activation of PI3K–AKT signaling and prevents trastuzumab-mediated growth arrest in HER2-amplified breast cancer cells. Nagata and colleagues conducted a retrospective analysis of 47 HER2-amplified primary breast tumors from stage IV patients treated with trastuzumab plus taxane, in which they correlated the presence of PTEN expression with tumor response to therapy using immunohistochemistry [31]. PTEN expression was absent or reduced in 36% of tumors, consistent with prior reports of PTEN loss in up to 40% of breast cancers [32]. Patients with PTEN-deficient tumors had significantly lower overall response rates to trastuzumab plus taxane therapy than patients with PTEN-positive tumors (35.7 vs 66.7%); a statistically significant trend was identified such that the probability of response to trastuzumab decreased as PTEN expression decreased. While studies such as these on PTEN expression by immunohistochemistry have been difficult to generalize due to the lack of standard assay, when coupled with the data on the trastuzumab responsiveness of laboratory models of HER2-amplified PTEN-null breast cancers, these results suggest that PTEN status may ultimately serve as a predictive biomarker in this setting.
Oncogenic activating PI3K mutations have also been implicated in trastuzumab resistance. Berns and colleagues examined 55 HER2-amplified primary tumor samples from trastuzumab-refractory patients for activating PIK3CA mutations in hotspot regions of the gene. Mutations were seen in 25% of tumors [33], in agreement with prior studies on rates of mutations in HER2-amplified breast cancer [34,35]. PTEN expression was also analyzed in this set of tumors and reduced PTEN expression was demonstrated in 22% of tumors. Kaplan–Meier survival curves demonstrated that patients with activated PI3K (defined as diminished PTEN expression or PIK3CA mutation) had a significantly shorter progression-free survival (PFS) on trastuzumab-based therapy than patients without evidence of PI3K pathway activation. Multivariate analysis identified PI3K pathway status as a significant independent risk factor for progression on trastuzumab-based therapy (hazard ratio [HR]: 1.9; p = 0.048). A more recent study featuring a larger cohort of tumors from patients with HER2-amplified metastatic breast cancer investigated PI3K–AKT pathway changes as predictive biomarkers of trastuzumab resistance and found that the individual biomarkers alone were not sufficient to predict diminished response to trastuzumab-based therapy; however, combinations (e.g., PI3K mutation or PTEN loss) did [36]. These and other studies highlight important caveats to translating the findings of resistance from preclinical studies into the clinical realm: reproducible assays for the biomarkers themselves (e.g., immunohistochemistry of P-AKT S473 or PTEN) can be difficult to develop, trastuzumab is commonly given with various chemotherapies complicating the interpretation of resistance, the PI3K–AKT pathway is tightly regulated so that static measurements of biomarkers such as P-AKT may not reflect actual flux through the pathway, and most assays of the biomarkers have been performed on pretreatment samples, which may not refect the status of the tumor at progression on trastuzumab.
In order to determine if these changes in the PI3K pathway are present in tumors after exposure to trastuzumab, a more recent study has analyzed of a series of pre- and post-trastuzumab treated tumor samples [37]. In preliminary reports of this work, tumor samples (n = 45) collected after progression on trastuzumab demonstrated that PTEN loss (immunohistochemistry [38]) and PIK3CA mutations (sequenom assay [39]) were frequently identified even among a cohort of patients who had initially derived a benefit from trastuzumab. In addition, reduced PTEN expression was identified in several post-treatment resistant samples while not appearing in the primary pretrastuzumab tumor, suggesting that in some cases these molecular lesions may arise in response to trastuzumab exposure. Increasingly, analysis of PIK3CA and PTEN are being performed in the context of clinical trials of HER2-targeted agents, in some cases with fresh metastatic tumor biopsies, and the ability to use these tests as predictive biomarkers will be more rigorously defined.
Accumulation of p95-HER2
Another mechanism proposed to mediate resistance to trastuzumab is the accumulation of a truncated form of the HER2 receptor, p95-HER2 (Figure 1C). The aminoterminal-truncated p95-HER2 is a constitutively active kinase that can participate in dimers with other HER family members and activate downstream signaling pathways. This form of HER2 lacks the trastuzumab binding site, enabling activated signaling in the presence of trastuzumab. p95-HER2 expression has been observed in up to 30% of HER2-amplified breast cancers and is associated with shorter disease-free survival when compared with tumors that overexpress full-length HER2 [40]. A retrospective analysis of 46 HER2-amplified tumors confirmed that p95-HER2 expression (by immunofluorescence) was strongly associated with trastuzumab resistance, while tumors expressing full-length HER2 maintained sensitivity to trastuzumab. Cell lines transfected with p95-HER2 and p95-HER2-expressing xenograft models are resistant to trastuzumab but maintain sensitivity to the HER2 kinase inhibitor lapatinib – with diminished p95-HER2 phosphorylation, reduced downstream phosphorylation of AKT, and cell growth inhibition [41]. These data suggest that p95-HER2-expressing tumors retain their dependence on HER2 function and may respond to alternative approaches to inhibiting HER2. Full confirmation for the utilization of p95-HER2 as a biomarker of resistance or sensitivity awaits larger studies using newer and more facile methodologies for evaluating p95-HER2 expression.
Increased signaling from HER family receptors & IGF-1R
The HER family homo- and heterodimers have different signaling potencies, and it is thought that the HER2–HER3 heterodimer is most active with respect to ligand-induced phosphorylation and activation of downstream signaling [42]. The PI3K pathway can be activated by HER2 through adapter proteins or through direct interaction with HER3 [42,43]. HER3-knockdown studies demonstrate that HER3 is essential in HER2-driven tumorigenesis [44]. Similarly, activation of HER2–HER3 dimers through additional ligand stimulation can overcome the antisignaling effects of trastuzumab, although it is unknown how often the ligand is upregulated in vivo [45,46]. Given the evidence that trastuzumab does not effectively disrupt the formation of ligand-induced HER2–HER3 heterodimers, overexpression of HER3 and generation of high levels of ligand-stimulated HER2–HER3 heterodimers may also contribute to trastuzumab resistance [47,48]. The activity of the HER2–HER3 heterodimer to activate the PI3K pathway has led to the development of pertuzumab, a monoclonal antibody that inhibits HER2–HER3 [44,49]. In addition to HER3, EGFR may play a role in trastuzumab resistance. Work by Ritter and colleagues demonstrated that trastuzumab-resistant cell lines and xenograft models overexpress phosphorylated EGFR, EGFR, EGFR/HER2 heterodimers, and HER family ligands EGF, heparin-binding EGF and heregulin. Furthermore, the addition of dual EGFR/HER2 tyrosine kinase inhibitors led to diminished HER2 phosphorylation and cellular proliferation [50]. Further validation of the role of EGFR and HER3 in mediating trastuzumab resistance is underway.
Apart from changes in the HER2 receptor and the pathways it activates, alterations in other receptor tyrosine kinases that help drive growth factor signaling may also mediate trastuzumab resistance (Figure 1D). For instance, expression of high levels of IGF-1 receptor (IGF-1R) in HER2-amplified cell lines is correlated with diminished response to trastuzumab [51]. This is speculated to be due to crosstalk between HER2 and IGF-1R with IGF-1 stimulation leading to phosphorylation of HER2 and activation of PI3K. Furthermore, inhibition of IGF-1R signaling blocks HER2 phosphorylation and restores sensitivity to trastuzumab in selected laboratory models [52]. In further support of a role for IGF-1R in mediating trastuzumab resistance, analysis of primary tumor samples from a neoadjuvant study of vinorelbine and trastuzumab demonstrated that the presence of membrane expression of IGF-1R was associated with a lower response rate to therapy compared with tumors lacking IGF-1R expression (50 vs 97%; p = 0.001). Apart from IGF-1R, expression of other receptor tyro-sine kinases including c-MET, EphA2 and AXL have also been associated with resistance to trastuzumab in HER2-amplified breast cancer models [50,53–55].
To date, analysis of these mechanisms of resistance has been almost entirely through laboratory models and very small retrospective studies from archived primary tumor samples. The true incidence and role of each of these putative mechanisms of trastuzumab resistance awaits analysis from pre- and post-treatment tumor samples, and validation of reproducible assays on biomarkers of resistance. Indeed, commercially available assays for these specific mechanisms are not available and are presently in various phases of testing and validation. Moreover, full confirmation of the relevance of these mechanisms should be validated by the demonstration that targeting the specific molecular determinants of resistance in the relevant cancer results in meaningful clinical benefit to those selected patients.
Therapeutics
HER2 tyrosine kinase inhibitors
Lapatinib
Lapatinib (Tykerb) is a reversible, ATP-competitive inhibitor of the HER2 and EGFR tyrosine kinases, which was hypothesized to have efficacy in trastuzumab-resistant, HER2-amplified tumors based on having a distinct mechanism of anti-HER2 action from trastuzumab (Figure 2) [56]. In preclinical studies of trastuzumab-refractory cell lines characterized by mutational activation of PI3K, lapatinib was shown to downregulate AKT signaling and potently block tumor cell proliferation [57]. In addition, although PTEN loss predicts resistance to trastuzumab, lapatinib retains anti-tumor activity in PTEN null HER2-overexpressing cell lines [58]. Furthermore, trastuzumab-resistant, p95-HER2-expressing tumors also remain sensitive to lapatinib. Lapatinib inhibited p95-HER2 phosphorylation, reduced downstream phosphorylation of AKT, and inhibited cell growth of p95-HER2-expressing cell lines and xenograft models [41,47]. Taken together, the preclinical evidence suggests lapatinib ought to have activity in trastuzumab-refractory breast cancer.
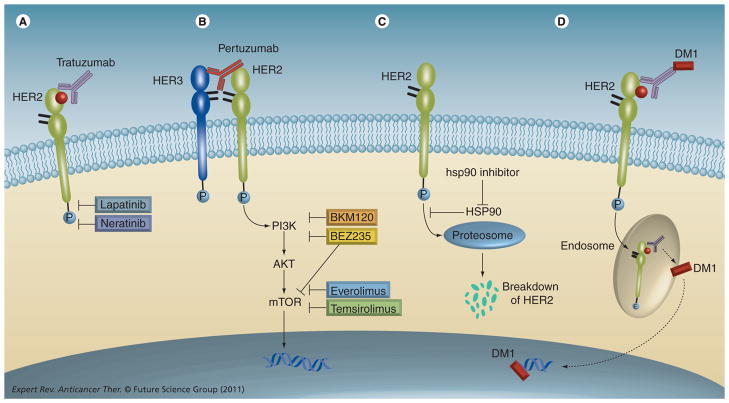
Figure 2. Sites of action of novel agents for HER2-amplified breast cancer.
(A) Lapatinib is a dual EGF receptor (EGFR)/HER2 tyrosine kinase inhibitor approved for use in trastuzumab-refractory patients. Neratinib is an irreversible tyrosine kinase inhibitor of EGFR/HER2. (B) Pertuzumab, a monoclonal antibody to HER2, binds to HER2 at a distinct epitope from where trastuzumab binds and prevents ligand-induced heterodimerization with HER3. Dysregulated activation of the PI3K–AKT–mTOR pathway can mediate trastuzumab resistance, and molecular therapies aimed at directly inhibiting PI3K, AKT and mTOR are therefore in development. (C) HSP90 inhibitors promote HER2 degradation by blocking the activity of HSP90, a chaperone protein that protects HER2 from proteasomal degradation. (D) TDM-1, the antibody–drug conjugate of trastuzumab and maytansine, allows for delivery of a potent microtubule inhibitor selectively into HER2-overexpressing cells.
In a Phase I clinical trial, lapatinib proved tolerable to patients with promising anti-tumor effects noted in patients with tumors overexpressing HER2. Lapatinib doses ranging from 500 to 1600 mg daily were well tolerated, with grade 1 or 2 diarrhea and rash being the most common drug-related toxicities. First-line therapy with lapatinib for advanced HER2-amplified breast cancer confirmed clinical activity at either 1500 mg once daily or 500 mg twice daily with a response rate of 24% [59]. Lapatinib monotherapy in the trastuzumab-refractory, HER2-amplified setting demonstrated modest activity with response rates ranging from 1.4 to 5.1% [60,61]. The efficacy of lapatinib in the trastuzumab-refractory subpopulation was fully realized when given in combination with chemotherapy.
The benefit of lapatinib in the trastuzumab-refractory setting was confirmed in a randomized Phase III clinical trial in which patients either received capecitabine monotherapy (2500 mg/m2) [2] on days 1–14 of a 21-day cycle) or combination therapy with lapatinib (capecitabine 2000 mg/m2 on days 1–14 of a 21-day cycle plus continuous lapatinib 1250 mg daily) [62]. Study accrual was halted as the interim analysis and demonstrated that the addition of lapatinib to capecitabine was associated with a 51% reduction in the risk of disease progression (HR: 0.49; 95% CI: 0.34–0.71; p < 0.001) and a doubling of time to progression. As a result, lapatinib has been approved in combination with capecitabine for patients with HER2-amplified advanced or metastatic breast cancer with progression after prior anthracycline, taxane and trastuzumab therapy. Given the approximately 4% incidence of heart failure among patients receiving trastuzumab therapy, cardiac safety of lapatinib has been evaluated in a pooled analysis of over 3000 patients with a low rate of symptomatic congestive heart failure (0.2%) or asymptomatic cardiac events (1.4%) reported, and partial or full recovery observed in most patients [63].
The modest activity seen for lapatinib therapy in trastuzumab-refractory patients has been speculated to be attributed to the dose and schedule of lapatinib administration. Intermittent lapatinib dosing (5 days on, 9 days rest) achieved higher maximum tolerated doses, improved tumor regression and increased duration of anti-tumor activity over continuous dosing in mice models [64]. Intermittent lapatinib dosing will be tested prospectively to determine if this schedule can improve the efficacy of lapatinib therapy without prohibitive toxicity. In addition, molecular lesions that predict resistance to trastuzumab may be predictive of subsets with particular sensitivity or resistance to lapatinib. Scaltriti and colleagues analyzed the relationship between p95-HER2 expression and response to lapatinib as monotherapy or in combination with capecitabine [65]. In the first-line lapatinib study, 20.5% of tumors were p95-HER2-positive, while 28.5% of tumors in the lapatinib and capecitabine study were p95-HER2-positive, consistent with rates in previous reports [40,41]. PFS and response rate were analyzed by p95-HER2 status and no statistically significant difference was observed between the p95-HER2-positive or -negative subgroups in either study. Given the data that p95-HER2 tumors may be resistant to trastuzumab while retaining sensitivity to lapatinib, this work suggests that lapatinib and other tyrosine kinase inhibitors may be the preferred agent for these patients. Furthermore, analyses from a neoadjuvant study and a trial in advanced inflammatory breast cancer both demonstrate that PTEN loss or PIK3CA mutations did not preclude response to lapatinib monotherapy [66,67].
Neratinib
Like lapatinib, neratinib is a potent EGFR/HER2 kinase inhibitor that binds the ATP pocket and has anti-tumor effects in cell lines with HER2 overexpression. Unlike lapatinib, neratinib is an irreversible kinase inhibitor that covalently binds the target kinase as part of its mechanism of action (Figure 2). A Phase I dose–escalation study in patients with advanced solid tumors determined the maximum tolerated dose to be 320 mg; common adverse events included nausea, fatigue, vomiting and anorexia, and the dose-limiting toxicity was grade 3 diarrhea. Among the 25 evaluable patients with HER2-amplified metastatic breast cancer, all who had progression on prior anthracycline, taxane and trastuzumab, a response rate of 24% and clinical benefit rate of 38% was observed [68].
Given the promising activity seen for neratinib, a large Phase II trial examined the efficacy of neratinib in patients with HER2- amplified breast cancer [69]. The study had one cohort of patients with prior trastuzumab exposure and another cohort of patients with no prior trastuzumab treatment. Patients had an average of two prior chemotherapeutic regimens and 65% had both prior taxane and anthracycline therapy. The major toxicity seen with neratinib at 240 mg oral daily was diarrhea; no neratinib-related grade 3–4 cardiotoxicity was reported. Neratinib therapy resulted in a response rate of 24% (95% CI: 14–36%) for patients with prior trastuzumab and a response rate of 56% (95% CI: 43–69%) for patients with no prior trastuzumab (TABle 1). Durable benefit of neratinib monotherapy was reported with a median PFS of 22.3 and 39.6 weeks for patients with prior trastuzumab and no prior trastuzumab, respectively [69].
Table 1.
Efficacy results for trials of novel HER2-targeted agents given to patients with trastuzumab-resistant, HER2-amplified breast cancer
| Agent | Treatment regimen | Patients prior therapy (n) | Objective response rate (%) (95% CI) | Clinical benefit rate (%)(95% CI) | Ref. |
|---|---|---|---|---|---|
| Lapatinib | 1500 mg daily | 148; prior T | 6.9 (5.9–16.4) | 12.4 (7.5–18.9) | [78] |
| 1000 mg daily with weekly T | 148; prior T | 10.3 (3.4–12.3) | 24.7 (17.9–32.5) | ||
| Neratinib | 240 mg daily | 66; prior T | 24 (14–36) | 33 (22–46) | [69] |
| Pertuzumab | 840 mg loading dose, then 420 mg every 3 weeks with weekly T | 66; prior T | 24.2 (14.5–36.4) | 50 (37.4–62.6) | [86] |
| TDM-1 | 3.6 mg/kg every 3 weeks | 110; prior T and L | 32.7 (24.1–42.1) | 44.5 (35.1–54.3) | [92] |
| Tanespimycin | 450 mg/m 2 with weekly T | 27; prior T | 22.2 (8.6–42.2) | 59.3 (38.8–77.6) | [100] |
The clinical activity of neratinib in HER2-amplified patients has led to testing of the agent in combination with chemotherapy for refractory metastatic HER2-amplified breast cancer. Several ongoing Phase I/II trials of neratinib combinations are in progress, and preliminary safety and efficacy data have been presented. Neratinib (240 mg) with paclitaxel (80 mg/m2 on days 1, 8, 15 of a 28-day cycle) was well tolerated and demonstrated a clinical activity with a response rate of 69% including patients with prior taxane, trastuzumab and lapatinib therapy [70]. A Phase III trial is planned to study this combination in the first-line setting compared with paclitaxel plus trastuzumab. Other combinations with vinorelbine, capecitabine and trastuzumab have also been performed in the metastatic setting and demonstrated drug tolerability with additive clinical activity [71–73].
The activity of lapatinib and neratinib in trastuzumab-refractory patients suggests that HER2 suppression continues to be an effective treatment strategy. In addition, tyrosine kinase inhibitors, by virtue of a different mechanism of HER2 targeting, overcome some of the mechanisms thought to mediate trastuzumab resistance leading to a clinical benefit for trastuzumab-refractory HER2-amplified patients.
Continuing trastuzumab beyond progression
Data on the effect of trastuzumab in xenograft models of HER2 breast cancer demonstrate that it exerts a suppressive effect against rapid tumor growth as long as it is present. Withdrawal of the drug results in an acceleration of tumor growth, suggesting that some of the anti-tumor mechanisms of the drug may persist even after resistance develops. In addition, a number of groups have demonstrated that trastuzumab administered in combination with various chemotherapies has additive or synergistic anti-tumor effects, which occur despite the distinct mechanisms of action of the chemotherapeutics [24–26,74]. These data have influenced the use of trastuzumab beyond progression in combination with a different chemotherapeutic agent. Retrospective studies on patients receiving trastuzumab therapy after progression on a trastuzumab-containing regimen suggested the possibility of a continued benefit from the drug [75–78]. This concept was proven in a prospective randomized study of capecitabine versus capecitabine plus trastuzumab as second-line therapy after trastuzumab [79]. Accrual was halted at 156 patients when the interim analysis documented an improvement in PFS from 5.6 to 8.2 months (HR: 0.69; 95% CI: 0.48–0.97; p = 0.0338) for patients who continued trastuzumab therapy.
In addition to additive or synergistic anti-tumor properties in combination with chemotherapies, trastuzumab has demonstrated synergistic anti-tumor effects when given in combination with lapatinib. The combination of lapatinib and trastuzumab markedly enhanced the rate of apoptosis seen in HER2-amplified cell lines and xenograft models [80]. Furthermore, combined inhibition of HER2 with the antibody and kinase inhibitor resulted in complete tumor regression within 10 days of therapy [81]. A Phase I dose–escalation study of the combination determined the optimal regimen to be 1000 mg of daily lapatinib with standard weekly trastuzumab [82]. The most frequent adverse drug-related events were diarrhea, fatigue and rash. In total, eight out of 54 patients experienced an objective response (complete or partial) despite having progressed on prior trastuzumab-based therapies.
The efficacy of this combination was tested in a randomized study of lapatinib monotherapy (1500 mg daily) versus lapatinib (1000 mg daily) in combination with weekly trastuzumab [78]. The combination of trastuzumab and lapatinib significantly improved PFS compared with lapatinib alone (HR: 0.73; 95% CI: 0.57–0.93; p = 0.008) for patients with HER2-amplified breast cancer and a median of three prior trastuzumab-based regimens for metastatic disease. A doubling in clinical benefit rate (CBR; complete response, partial response and stable disease for ≥24 weeks) was observed for the continuation of trastuzumab (24.7 vs 12.4%) (TABle 1). The improvements in PFS and CBR were seen despite a high crossover rate, with approximately 50% of patients in the lapatinib monotherapy arm crossing over to combination therapy upon progression. The incidence of left ventricular ejection fraction decline with the combination was not greater than that previously reported for each agent [63,83]; 2 and 3.4% of patients experienced symptomatic and asymptomatic declines, respectively.
These results provide evidence that combined HER2 blockade is beneficial for HER2-amplified trastuzumab-refractory metastatic breast cancer, and this strategy has been employed in evaluating other HER2-targeted therapies. The efficacy seen for trastuzumab and lapatinib combination therapy provides an alternative to chemotherapy for patients with refractory disease – one that is well tolerated and easy to administer. The safety and efficacy of dual HER2 blockade with trastuzumab and lapatinib combined with standard chemotherapy is currently being evaluated.
HER2-targeted agents
Pertuzumab
Pertuzumab is a monoclonal antibody binding to a different epitope on the extracellular domain of HER2 than trastuzumab, which blocks ligand-induced dimerization of HER2 and HER3 (Figure 2) [18,49]. Preclinical experiments show that in both HER2-amplified cell lines and xenograft models, pertuzumab is effective in disrupting HER2–HER3 heterodimers, leading to inhibition of downstream MAPK and PI3K signaling, and anti-tumor activity [84]. The use of combined HER2 blockade with trastuzumab and pertuzumab is supported by xenograft models of HER2-amplified breast cancer that show enhanced tumor regression for the combination over monotherapy. In addition, the combination also had anti-tumor activity in models of trastuzumab resistance, suggesting that trastuzumab and pertuzumab have complementary mechanisms of action [85].
Inhibition of HER2 signaling with combined HER2-targeting antibodies has shown clinical activity in patients with metastatic breast cancer with progression on trastuzumab-based therapies. An overall response rate of 24.2% and CBR of 50% was observed for 66 patients with prior trastuzumab treatment for the combination of trastuzumab and pertuzumab (TABle 1). The most common drug-related adverse events were diarrhea, nausea and rash [86]. An additional arm added to this trial allowed for patients with prior trastuzumab therapy to receive pertuzumab monotherapy; trastuzumab could be added if no response or progression was seen with pertuzumab monotherapy. The response rate for per-tuzumab monotherapy was 3.4%, and a response rate of 21.4% was seen for the combination after progression on trastuzumab or pertuzumab alone; this validates preclinical modeling of the combined efficacy of these antibodies [87]. A second Phase II trial of combined trastuzumab and pertuzumab therapy demonstrated a response rate of 18% with a median time to progression of 6 weeks [88]. Cardiac safety was monitored during these trials with repeat echocardiograms; the majority of toxicity observed was asymptomatic left ventricular function decline. Based on this activity and tolerability, pertuzumab in combination with trastuzumab and docetaxel is now being evaluated as part of a large, randomized trial for first-line therapy in HER2-amplified metastatic breast cancer patients.
TDM-1
In an attempt to improve the potency of trastuzumab therapy, an antibody–drug conjugate has been designed to utilize the antibody to deliver cytotoxic therapy to antigen-expressing tumors. Trastuzumab has been conjugated to DM1, a derivative of maytansine 1, a potent microtubule inhibitor, to create the trastuzumab–maytansine conjugate (TDM-1). Potent anti-tumor activity of TDM-1 was seen in trastuzumab-refractory HER2-amplified cell lines and xenograft models [89]. An exploration of mechanisms of action demonstrated that TDM-1 disrupts the HER2–HER3 complex and results in inhibition of PI3K signaling, prevents shedding of the extracellular domain of HER2 and the formation of p95-HER2, and activates antibody-dependent cellular cytotoxicity (Figure 2) [90].
The Phase I trial of TDM-1 monotherapy in patients with HER2-amplified metastatic breast cancer with prior trastuzumab-based therapy described the maximum tolerated dose to be 3.6 mg/kg every 3 weeks, with five patients having a partial response at this dose. Dose-limiting toxicity was grade 3–4 thrombocytopenia; common drug-related adverse events included fatigue, nausea and elevated transaminases, while no significant cardiotoxicity was noted [91]. Phase II evaluation of TDM-1 demonstrated a response rate of 32.7% in patients with metastatic HER2-amplified breast cancer with prior anthracycline, taxane, capecitabine, trastuzumab and lapatinib therapy (TABle 1) [92]. A second Phase II trial in patients with heavily pretreated HER2-amplfied disease documented a clinical benefit for TDM-1 mono-therapy with a response rate of 27.1% [93]. Toxicities of these studies were similar to those documented in the Phase I trial. TDM-1 monotherapy and in combination with chemotherapeutic agents is currently being explored for the first-line treatment of metastatic HER2-amplified breast cancer. The efficacy of the antibody–drug conjugate TDM-1 in trastuzumab-refractory patients provides evidence that HER2 remains a viable target and this agent, with a favorable toxicity profile, is a strong candidate for future approval in this setting.
Heat shock protein 90 inhibitors
Heat shock protein 90 (HSP90) is a molecular chaperone that plays an essential role in the maturation and stabilization of several oncogenic proteins including HER2 [94]. Inhibition of HSP90 function results in ubiquitination and proteasomal degradation of HSP90 client proteins (Figure 2) [95]. HER2-amplified breast cancer cell lines and xenograft models exposed to HSP90 inhibitors demonstrate HER2 degradation, inhibition of PI3K signaling and significant growth inhibition [94,96,97]. One basis for studying HSP90 inhibitors in trastuzumab-refractory disease comes from the reported ability of HSP90 inhibitors to target p95-HER2 in addition to full-length HER2. In vitro models show degradation of HER2 and p95-HER2, and xenograft models of p95-HER2 expression recapitulate these findings, suggesting that, in trastuzumab-refractory disease where resistance is mediated by p95-HER2, HSP90 inhibitors are effective [98]. The unique way in which HSP90 inhibitors target HER2 and downstream signaling through alternative HSP90 client proteins such as HER3 or p95-HER2 that may mediate trastuzumab resistance makes a strong case for the evaluation of these inhibitors specifically in this disease setting.
Phase I evaluation of the HSP90 inhibitor tanespimycin in combination with trastuzumab demonstrated the clinical ben-efit for HER2-amplified trastuzumab-refractory breast cancer patients, with one partial response and four minor responses observed. Diarrhea, fatigue and nausea were the most frequent adverse effects of therapy, and no cardiotoxicity was reported [99]. In a Phase II evaluation of the combination of tanespimycin and trastuzumab, a response rate of 24% and a CBR of 57% were seen for patients with HER2-amplified metastatic breast cancer with prior progression on trastuzumab therapy (TABle 1) [100]. Second-generation HSP90 inhibitors are now in clinical trial development [101,102]. Whereas HSP90 inhibitors have been developed in the hopes of treating most tumor types, it is notable that actual clinical activity has thus far been most prominently observed in HER2-amplified breast cancer.
PI3K inhibitors
PI3K pathway activation through PTEN loss or PIK3CA mutation has been described in 40 and 25% of breast tumors, respectively, implicating the importance of this pathway in breast cancer tumorigenesis [31–34]. In addition, assessment of biopsy samples after trastuzumab progression demonstrated PI3K pathway activation as a common mediator of trastuzumab resistance [37], providing a rational basis for the evaluation of PI3K, AKT and mTOR inhibitors in HER2-amplified breast cancer.
At the 2010 Annual American Society of Clinical Oncology (ASCO) Meeting, Phase I data of several PI3K inhibitors was presented. BEZ235 is an oral, selective PI3K and mTOR inhibitor with antiproliferative and apoptotic activity in xenograft models of PI3K pathway dysregulation. The Phase I study demonstrated tolerability with no dose-limiting toxicities and adverse events of nausea, emesis, diarrhea and fatigue. Promising clinical activity was seen with two partial responses and 14 patients with stable disease of 4 months and over, including disease stabilization in six patients with PI3K dysregulation [103]. BKM120, a highly selective PI3K inhibitor, has also shown tolerability and activity in patients with refractory solid tumors (Figure 2) [104].
Given that AKT is an important downstream target of PI3K, several inhibitors of AKT are in development. A dose–escalation trial of the allosteric AKT inhibitor MK-2206 found the maximum tolerated dose to be 60 mg every other day and stabilization of disease was reported for five patients at this dose level [105]. Two mTOR inhibitors – everolimus, with better oral availability than sirolimus and temsirolimus, an ester derivative of sirolimus – are under investigation in clinical trials with refractory breast cancer patients (Figure 2). Everolimus administered with weekly paclitaxel and trastuzumab demonstrated a favorable toxicity profile; Phase II evaluation of the combination demonstrated a response rate of 20% and a CBR of 76% in HER2-amplified breast cancer patients refractory to taxanes and trastuzumab [106,107]. The safety and tolerability of temsirolimus administered weekly was documented in a Phase I dose–escalation study, with a dose-limiting toxicity of thrombocytopenia [108]. In a Phase II study of locally advanced or metastatic breast cancer, temsirolimus (75 or 250 mg weekly) resulted in a response rate of 9.2% [109].
Given that PI3K pathway dysregulation is a mediator of trastuzumab resistance, inhibitors of this pathway may restore sensitivity to trastuzumab or improve the efficacy of HER2-targeted therapies. For instance, the combination of the mTOR inhibitor temsirolimus and the HER2 tyrosine kinase inhibitor, neratinib, is under investigation in a Phase I/II clinical trial in trastuzumab-refractory HER2-amplified metastatic breast cancer patients. Indeed, a number of trials are now underway testing the combination of inhibitors of the PI3K–AKT–mTOR pathway with inhibitors of HER2. These trials will not only assess the tolerability and efficacy of such combinations, but in several cases the trials are examining tumor biopsies to determine if activating lesions in the pathway are present along with amplified HER2 and predict resistance to trastuzumab and sensitivity to the combination.
Expert commentary & five-year view
In the past 10 years we have seen the evolution of molecular-targeted therapies in the treatment of HER2-amplified breast cancer. Trastuzumab is a model of targeted therapy that offers significant clinical benefit selectively in patients with HER2-overexpressing cancers; however, trastuzumab alone or in combination with cytotoxic therapy yields no response for many patients, and indeed, the majority of patients with advanced breast cancer develop resistance to therapy. Elucidation of the mechanisms of de novo and acquired resistance to trastuzumab has been difficult given the drug’s multiple mechanisms of action. Research has identified dysregulation of the downstream PI3K–AKT–mTOR pathway, accumulation of the truncated kinase active p95-HER2, and alternative receptor tyrosine kinase signaling as mediators of trastuzumab resistance. This work has led to the identification of potential predictors of response to HER2-targeted agents and aided in the development of rational second- and third-line therapies by promoting the concept that HER2 remains the active oncogenic driver in this disease, and pointing to the continued relevance of targeting HER2–HER3–PI3K–AKT–mTOR signaling. Clinical trials of novel therapies targeted against this pathway have yielded a number of promising new candidate compounds including pertuzumab, neratinib, TDM-1 and tanespimycin, which together may lead to an improvement in overall survival for patients with refractory HER2-amplifed disease.
By continuing to expand on our knowledge of the mechanisms of trastuzumab resistance, we may find additional oncogenic targets to guide the development of effective therapies. Furthermore, the discovery of specific molecular predictors of response to emerging therapies will allow a more personalized approach to the treatment of HER2-amplified breast cancer. Recognizing that activation of the PI3K pathway, through loss of PTEN or mutational activation of PIK3CA, is a common mechanism of trastuzumab resistance, facile and reliable assays to detect these molecular lesions are actively being developed to identify patients most likely to benefit from PI3K-based treatment options. This model may be applied to other predictors of response to HER2-targeted agents to enable a more rational approach to treatment selection. As we continue to strive towards a highly personalized approach to the treatment of breast cancer, we should employ one that relies on more than the HER2 expression status alone, and incorporates molecular mechanisms of trastuzumab sensitivity and resistance to improve patient outcomes in this disease.
Footnotes
Financial & competing interests disclosure
Sarat Chandarlapaty has received a grant from Pfizer to fund preclinical research. He is also NIH funded (grant no. 1K08CA134833-01). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/Neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Andrulis IL, Bull SB, Blackstein ME, et al. Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16:1340–1349. doi: 10.1200/JCO.1998.16.4.1340. [DOI] [PubMed] [Google Scholar]
- 3.Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 4.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 5.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 6.Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41:697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 7.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 8.Seidman AD, Berry D, Cirrincione C, et al. Randomized Phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of cancer and leukemia group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 9.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–1411. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 10.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobleigh M, Vogel C, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER 2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 13.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 15.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Presented at: San Antonio Breast Cancer Symposium; San Antonio, TX, USA. 8–11 December 2005. [Google Scholar]
- 16.Perez EA, Reinholz MM, Hillman DW, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28:4307–4315. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuello M, Ettenberg SA, Clark AS, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61:4892–4900. [PubMed] [Google Scholar]
- 18•.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. Discriminates mechanisms whereby trastuzumab and pertuzumab antagonize HER2 signaling. [DOI] [PubMed] [Google Scholar]
- 19.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 20.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 21.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 22.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Yarmand-Bagheri R. The role of HER2 in angiogenesis. Semin Oncol. 2001;28:27–32. doi: 10.1016/s0093-7754(01)90279-9. [DOI] [PubMed] [Google Scholar]
- 24•.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/Neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. Pivotal preclinical work demonstrating the combined efficacy of chemotherapy with trastuzumab. [PubMed] [Google Scholar]
- 25.Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/Neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 26.Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 27.Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 28.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pegram M. Can we circumvent resistance to ErbB2-targeted agents by targeting novel pathways? Clin Breast Cancer. 2008;8(Suppl 3):S121–S130. doi: 10.3816/cbc.2008.s.008. [DOI] [PubMed] [Google Scholar]
- 30.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 31•.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. Key demonstration that loss of PTEN mediates resistance to trastuzumab. [DOI] [PubMed] [Google Scholar]
- 32.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–676. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 33•.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. Demonstrated that the PI3K pathway activation status is the predictive biomarker able to identify patients at risk for progression after trastuzumab therapy. [DOI] [PubMed] [Google Scholar]
- 34•.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. Identification of a significant subset of HER2-amplified tumors with coincident PIK3CA mutations. [DOI] [PubMed] [Google Scholar]
- 35.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandarlapaty S, King T, Sakr R, et al. Hyperactivation of the PI3K–AKT pathway commonly underlies resistance to trastuzumab in HER2 amplified breast cancer. Cancer Res (Meeting Abstracts) 2009;69:S709. [Google Scholar]
- 38.Sakr RA, Barbashina V, Morrogh M, et al. Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol. 2010;18:371–374. doi: 10.1097/PAI.0b013e3181d50bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalinsky K, Jacks LM, Heguy A, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5049–5059. doi: 10.1158/1078-0432.CCR-09-0632. [DOI] [PubMed] [Google Scholar]
- 40.Saez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12:424–431. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- 41•.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. Demonstration of the association between p95-HER2 expression and trastuzumab resistance in human tumor samples. [DOI] [PubMed] [Google Scholar]
- 42•.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. Experiments demonstrate the essential functions for HER3 in HER2-overexpressing tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 44.Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 45.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62:3151–3158. [PubMed] [Google Scholar]
- 46.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 48.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 51.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 52.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 53.Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of HER2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Greger J, Shi H, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang G, Brantley-Sieders DM, Vaught D, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 2010;70:299–308. doi: 10.1158/0008-5472.CAN-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 57•.O’Brien NA, Browne BC, Chow L, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. Demonstrates that PI3K pathway activation does not predict resistance to lapatinib therapy. [DOI] [PubMed] [Google Scholar]
- 58.Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 59.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 60.Burstein HJ, Storniolo AM, Franco S, et al. A Phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 61.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 62.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 63.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 64.Amin DN, Sergina N, Ahuja D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2010;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. Shows that lapatinib therapy is effective for patients with p95-HER2 expressing breast tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston S, Trudeau M, Kaufman B, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 67.Migliaccio I, Gutierrez M, Wu M-F, et al. PI3 kinase activation and response to trastuzumab or lapatinib in HER-2 overexpressing locally advanced breast cancer. Cancer Res (Meeting Abstracts) 2009;69:S34. [Google Scholar]
- 68.Wong KK, Fracasso PM, Bukowski RM, et al. A Phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 69.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 70.Chow L, Gupta S, Hershman D, et al. Safety and efficacy of neratinib (HKI-272) in combination with paclitaxel in ErbB2+ metastatic breast cancer. Cancer Res (Meeting Abstracts) 2009;69:S5081. [Google Scholar]
- 71.Awada A, Dirix L, Beck J, et al. Safety and efficacy of neratinib (HKI-272) in combination with vinorelbine in ErbB2+ metastatic breast cancer. Cancer Res (Meeting Abstracts) 2009;69:S5095. [Google Scholar]
- 72.Saura C, Martin M, Moroose R, et al. benefits. Safety of neratinib (HKI-272) in combination with capecitabine in patients with solid tumors: a Phase 1/2 study. Cancer Res (Meeting Abstracts) 2009;69:S5108. [Google Scholar]
- 73.Swaby R, Blackwell K, Jiang Z, et al. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a Phase 1/2 study. Cancer Res (Meeting Abstracts) 2009;69:S243. [Google Scholar]
- 74.Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/Neu monoclonal antibody plus cisplatin in patients with HER2/Neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 75.Fountzilas G, Razis E, Tsavdaridis D, et al. Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the Hellenic Cooperative Oncology Group. Clin Breast Cancer. 2003;4:120–125. doi: 10.3816/cbc.2003.n.017. [DOI] [PubMed] [Google Scholar]
- 76.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer. 2004;5:52–58. doi: 10.3816/cbc.2004.n.010. discussion 59–62. [DOI] [PubMed] [Google Scholar]
- 77.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22:1063–1070. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 78.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. Clinical trial demonstrating that combined HER2 blockade with trastuzumab and lapatinib improves clinical outcomes after progression on trastuzumab. [DOI] [PubMed] [Google Scholar]
- 79•.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. Clinical trial demonstrating that continuing trastuzumab even after cancer progression on trastuzumab has clear benefits. [DOI] [PubMed] [Google Scholar]
- 80.O’Donovan N, Byrne AT, O’Connor AE, McGee S, Gallagher WM, Crown J. Synergistic interaction between trastuzumab and EGFR/HER-2 tyrosine kinase inhibitors in HER-2 positive breast cancer cells. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9415-5. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 81.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 82.Storniolo AM, Pegram MD, Overmoyer B, et al. Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2008;26:3317–3323. doi: 10.1200/JCO.2007.13.5202. [DOI] [PubMed] [Google Scholar]
- 83.Smith KL, Dang C, Seidman AD. Cardiac dysfunction associated with trastuzumab. Expert Opin Drug Saf. 2006;5:619–629. doi: 10.1517/14740338.5.5.619. [DOI] [PubMed] [Google Scholar]
- 84.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 85.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 86.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baselga J, Cortes J, Fumoleau P, et al. Pertuzumab and trastuzumab: re-responses to 2 biological agents in patients with HER2-positive breast cancer which had previously progressed during therapy with each agent given separately: a new biological and clinical observation. Cancer Res (Meeting Abstracts) 2009;69:s5114. [Google Scholar]
- 88.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 90.Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (TDM-1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1090-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 91.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 92.Krop IE, LoRusso P, Miller KD, et al. A Phase 2 study of the HER2 antibody-drug conjugate trastuzumab-DM1 (TDM-1) in patients (PTS) with HER2-positive metastatic breast cancer (MBC) previously treated with trastuzumab, lapatinib, and chemotherapy. Ann Oncol. 2010:2770. [Google Scholar]
- 93.Vogel C, Burris HA, Limentani S, et al. A Phase II study of trastuzumab-DM1 (TDM-1), a HER2 antibody-drug conjugate in patients with HER2+ metastatic breast cancer: final results. J Clin Oncol. 2009;27:s1017. [Google Scholar]
- 94.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther. 2002;2:3–24. doi: 10.1517/14712598.2.1.3. [DOI] [PubMed] [Google Scholar]
- 96.Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase–AKT-dependent pathway. Cancer Res. 2002;62:3132–3137. [PubMed] [Google Scholar]
- 97.Chandarlapaty S, Sawai A, Ye Q, et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008;14:240–248. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–334. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Modi S, Stopeck AT, Gordon MS, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a Phase I dose–escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 100.Modi S, Sugarman S, Stopeck AT, et al. Phase II trial of the Hsp90 inhibitor tanespimycin + trastuzumab in patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2008;26:s1027. [Google Scholar]
- 101.Miller K, Rosen L, Modi S, et al. Phase I trial of alvespimycin (KOS-1022; 17-DMAG) and trastuzumab. J Clin Oncol. 2007;25:s1115. doi: 10.1158/1078-0432.CCR-11-3200. [DOI] [PubMed] [Google Scholar]
- 102.Solit DB, Chiosis G. Development and application of Hsp90 inhibitors. Drug Discov Today. 2008;13:38–43. doi: 10.1016/j.drudis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Burris H, Rodon J, Sharma S, et al. First-in-human Phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol (Meeting Abstracts) 2010;28:3005. [Google Scholar]
- 104.Baselga J, De Jonge MJ, Rodon J, et al. A first-in-human Phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol (Meeting Abstracts) 2010;28:3003. [Google Scholar]
- 105.Tolcher AW, Yap TA, Fearen I, et al. A Phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST) J Clin Oncol (Meeting Abstracts) 2009;27:3503. [Google Scholar]
- 106.Andre F, Campone M, Hurvitz SA, et al. Multicenter Phase I clinical trial of daily and weekly RAD001 in combination with weekly paclitaxel and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab. J Clin Oncol (Meeting Abstracts) 2008;26:1003. [Google Scholar]
- 107.Dalenc F, Campone M, Hupperets P, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: a multicenter Phase II clinical trial. J Clin Oncol (Meeting Abstracts) 2010;28:1013. [Google Scholar]
- 108.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 109.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]