Cushing’s Syndrome: All variants, detection, and treatment (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 1.
Published in final edited form as: Endocrinol Metab Clin North Am. 2011 Jun;40(2):379–391. doi: 10.1016/j.ecl.2011.01.006
Synopsis
Cushing’s syndrome is caused by prolonged exposure to excess glucocorticoids. Diagnosis of Cushing’s syndrome involves a step-wise approach and establishing the cause can be challenging in some cases. Hypertension is present in about 80% of patients with Cushing’s syndrome and can lead to significant morbidity and mortality. Several pathogenic mechanisms have been proposed for glucocorticoid-induced hypertension including a functional mineralocorticoid excess state, up-regulation of the renin angiotensin system and deleterious effects of cortisol on the vasculature. Surgical excision of the cause of excess glucocorticoids remains the optimal treatment for Cushing’s syndrome. Anti-glucocorticoid and antihypertensive agents and steroidogenesis inhibitors can be used as adjunctive treatment modalities in preparation for surgery, and in cases where surgery is contraindicated or has not led to cure.
Keywords: Cushing’s syndrome, hypertension, glucocorticoids, ectopic ACTH secretion, 11-beta hydroxysteroid dehydrogenase, hypercortisolemia
Introduction
Cushing’s syndrome is a rare disorder that results from prolonged and pathological exposure to excess glucocorticoids. The incidence of Cushing’s syndrome varies depending on the population studied, anywhere from 2 to 3 cases per million population per year (1 – 3). More recent data suggests that this is probably an underestimate and Cushing’s syndrome may be more common than previously thought (4 – 5). Iatrogenic Cushing’s syndrome caused by the administration of supraphysiologic doses of glucocorticoids is probably much more common (although underreported) than endogenous causes.
The clinical presentation of Cushing’s syndrome varies widely. Although the diagnosis is straightforward in full-blown cases, establishing the diagnosis can be difficult in mild hypercortisolism especially as none of the signs or symptoms is pathognomonic of the syndrome (Table 1). However, some of the signs that have been reported to better distinguish Cushing’s syndrome from simple obesity include proximal muscle weakness, easy bruising, violaceous striae greater than 1 cm, and hypertension (6 - 7). The clinical presentation differs in children, in whom weight gain and growth retardation are more prominent (8).
Table 1.
Signs and symptoms of Cushing’s syndrome
| Hypertension |
|---|
| Adipose |
| Weight gain |
| Increased centripetal, supraclavicular, temporal, and/ordorsocervical fat |
| Skin |
| Hirsutism |
| Striae (especially is > 1 cm diameter and purple) |
| Easy bruising |
| Plethora |
| Reproductive System |
| Menstrual irregularity |
| Amenorrhea |
| Decreased libido |
| Psychiatric and Cognitive |
| Depression |
| Emotional lability |
| Irritability |
| Decreased memory |
| Decreased concentration |
| Skeleton and Muscle |
| Proximal muscle weakness |
| Reduced bone mineral density |
| Fractures |
| Metabolism |
| Impaired glucose tolerance |
| Diabetes |
When the clinical presentation suggests Cushing’s syndrome, biochemical confirmation of hypercortisolism is necessary. According to the 2008 Endocrine Society guidelines, any of the following tests can be used for the initial diagnosis of Cushing’s syndrome: 24 hour urinary free cortisol, late-night salivary cortisol, or a dexamethasone suppression test (DST, either as a 1 mg overnight test or the longer low-dose test using 2mg/day over 48 hours) (9).
After two different abnormal tests establish the diagnosis, the cause of Cushing’s syndrome must be determined. Endogenous Cushing’s syndrome can be divided into adrenocorticotropin (ACTH) dependent and independent forms. ACTH-independent Cushing’s syndrome (15%) results from increased autonomous production of cortisol from adrenal tumors (adenoma or carcinoma) or hyperplasia. Corticotropin-dependent causes of Cushing’s syndrome include ACTH production from pituitary (Cushing’s disease, 70%) or other tumors (ectopic Cushing’s syndrome, 15%) and rarely, corticotropin-releasing hormone (CRH)-producing tumors (10 - 11).
In all forms of Cushing’s syndrome the normal secretion of CRH and ACTH are suppressed by the excessive cortisol levels. Thus, the first step in the differential diagnosis strategy is measurement of a plasma ACTH level. A low normal or undetectable value points to an adrenal source of hypercortisolemia, the so-called ACTH-independent forms. Imaging of the adrenal glands with computerized tomography (CT) or magnetic resonance imaging (MRI) should be performed to identify the site(s) of abnormality.
An elevated or inappropriately normal ACTH level reflects a pituitary or an ectopic source of ACTH as a cause of excessive cortisol. Differentiating between these two etiologies can be challenging. Pituitary MRI should be obtained first. If a pituitary mass larger than 6mm is found, and biochemical testing with CRH stimulation test and high-dose 8 mg DST are consistent with Cushing’s disease, no further testing is necessary. If the biochemical testing is discordant and the pituitary MRI is normal or equivocal (mass less than 6 mm), bilateral inferior petrosal sinus sampling (IPSS) should be strongly considered. Alternatively, IPSS may be obtained without additional biochemical testing, as it has the highest diagnostic accuracy. A significant central-to-peripheral ACTH gradient during IPSS (more than 2 before, and more than 3 after CRH administration) indicates Cushing’s disease. In the absence of a gradient, a search for an ectopic source should be performed using various imaging modalities (3, 10).
Hypertension in Cushing’s syndrome
The increased mortality of Cushing’s syndrome is caused in part by an increased risk of vascular disease up to five times the population average (2, 12). In one prospective study, ultrasound identified atherosclerotic plaques in both carotid arteries in eight of 25 patients with active Cushing’s disease and two of 32 age, sex, and BMI matched controls (13).
Hypertension, impaired glucose tolerance, diabetes, dyslipidemia and visceral obesity are common cardiovascular risk factors in patients with Cushing’s syndrome. Hypertension, although not invariable, is a frequent feature of endogenous Cushing’s syndrome with a prevalence of approximately 80% in adults. It is even more common (95%) in ectopic Cushing’s syndrome (14), whereas in children and adolescents, it is less common (about 47%) (15). By contrast, hypertension is present in only about 20% of patients with iatrogenic Cushing’s syndrome, where it correlates with the daily dose of glucocorticoid (16 – 17).
Studies have clearly shown that in the general population, the chance of myocardial infarction, heart failure, stroke, and kidney disease increases as blood pressure increases (18). In a meta-analysis of 61 prospective studies of individuals aged 40 – 69 years, Lewington et al. reported that for every 20 mmHg systolic or 10 mm Hg diastolic increase in blood pressure, there is a two-fold increase in mortality from ischemic heart disease and stroke (19).
Because of the potential impact of hypertension on morbidity and mortality, it is important to understand its cause(s) and to treat it. The purpose of this article is to review the various possible mechanisms leading to hypertension in Cushing’s syndrome and its treatment.
Physiology of Blood Pressure in Cushing’s Syndrome
Healthy adults have a diurnal variation in blood pressure, with a decrease in blood pressure during sleep. This decrease parallels that of cortisol, and may reflect decreased sensitivity to catecholamines. The absence of sleep-related decrease in blood pressure is found in a number of pathological conditions including glucocorticoid-induced hypertension (20 -21).
In 2004, Zacharieva and colleagues performed 24-hour ambulatory blood pressure monitoring in 100 patients with Cushing’s syndrome (80 with Cushing’s disease and 20 with adrenal causes) and 40 patients with essential hypertension. They found that the nighttime decrease in blood pressure in patients with each type of Cushing’s syndrome was significantly reduced compared to patients with essential hypertension. However, the nocturnal fall in heart rate was preserved in both groups of patients. The blunted decrease in nighttime blood pressure improved with treatment of Cushing’s syndrome and the degree of improvement was negatively correlated with the duration of hypercortisolism (22). Administration of supra-physiologic doses of exogenous glucocorticoids leads to a similar loss of the nocturnal fall in blood pressure (23).
Pathogenesis of Hypertension in Cushing’s Syndrome
Glucocorticoids play an important role in blood pressure regulation. Several mechanisms have been postulated to explain how hypercortisolism leads to hypertension in Cushing’s syndrome. These include mineralocorticoid effects of cortisol, activation of the renin angiotensin system and the action of cortisol on peripheral and systemic vasculature (17, 24).
Mineralocorticoid Effects of Cortisol: Role of 11-betahydroxysteroid dehydrogenase
Cortisol binds to both type 1 (mineralocorticoid) and type 2 (glucocorticoid) receptors. It is likely that glucocorticoid induced hypertension is mediated through effects of cortisol on both receptors. At the cellular level, cortisol availability is modulated by the two isoforms of the enzyme 11-betahydroxysteroid dehydrogenase (II-β HSD) (25).
11-β HSD-1 is a nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzyme that is most abundantly expressed in liver and adipose tissue. It has bidirectional activity and catalyzes both dehydrogenation (conversion of cortisol to cortisone) and reduction (conversion of cortisone to cortisol) reactions. In vivo, it predominantly functions as a reductase, converting inactive cortisone to active cortisol. This reductase activity relies on high NADPH concentrations, which in turn are dependent on the activity of hexose-6-phosphate dehydrogenase within the endoplasmic reticulum.
11-β HSD-2 is an NAD-dependent enzyme and predominantly acts as a dehydrogenase to convert cortisol into inactive cortisone. It is abundantly expressed in the classical mineralocorticoid target tissues including the renal cortex, colon, and salivary glands (17, 25). Both 11-β HSD-1 and -2 are present in vascular endothelial cells, coronary artery cells and vascular smooth muscles thereby modulating local access of cortisol to the vasculature (26, 27).
In vitro, the mineralocorticoid receptor has similar binding affinity for both cortisol and aldosterone. However, the circulating levels of cortisol in healthy individuals are about 100- to 1000- times higher than aldosterone levels. The in vivo selectivity of the renal mineralocorticoid receptor for aldosterone requires conversion of cortisol to cortisone by 11-β HSD-2, rendering it unable to bind to the mineralocorticoid receptor (28 – 29).
The global activity of 11-β HSD can be assessed by measurement of urinary steroid metabolites. Both cortisol and cortisone undergo A-ring reduction by 5 α- and 5 β-reductases and 3 α-hydroxysteroid dehydrogenase, yielding 5 β-tetrahydrocortisol (THF), 5 α-tetrahydrocortisol (allo-THF) and 5 β-tetrahydrocortisone (THE). The overall 11-β HSD-1 and 2 activity in the body is reflected in the ratio of urinary cortisol and cortisone metabolites (THF + allo-THF/THE). Alternatively, urine free cortisol-to-cortisone ratio or plasma cortisol-to-cortisone ratio can also help in the evaluation (25, 30).
One potential etiology for hypertension in Cushing’s syndrome is decreased renal conversion of cortisol to cortisone, which would increase mineralocorticoid action. In Cushing’s syndrome, the urinary THF + allo-THF/THE ratio is elevated, especially in patients with the highest UFC. These findings suggest that high cortisol levels can overwhelm the 11-β HSD-2 enzyme due to substrate saturation leading to spillover of cortisol to the mineralocorticoid receptor. This may cause a functional mineralocorticoid excess state with hypokalemia, increased renal tubular sodium reabsorption, intravascular volume expansion, and hypertension (14, 31). These findings are similar to those in patients with inactivating mutations of 11-β HSD-2 who have a “syndrome of apparent mineralocorticoid excess” (SAME) (25). However, in SAME, THE, aldosterone and renin levels are suppressed in comparison to those seen in Cushing’s syndrome.
Other studies suggest that cortisol induced hypertension is not primarily mediated via sodium retention. Connell and colleagues gave ACTH (1 mg/day) to healthy volunteers on a sodium restricted diet (15mmol/day). They noted that systolic blood pressure rose significantly from a mean value of 116 mm Hg to 125 mmHg and plasma volume rose from a mean of 2.8 liters to 3.6 liters. The level of increase in blood pressure was less than that seen in previous studies in which ACTH was given to subjects on a normal sodium diet, suggesting that dietary sodium restriction lessens but does not prevent the rise in blood pressure with hypercortisolemia (32). Williamson et al. demonstrated that mineralocorticoid blockade with spironolactone (400 mg/day) did not affect the increased blood pressure seen in patients given cortisol (80 and 200 mg/day), even though it completely blocked salt and water retention (33). In another study, Whitworth and colleagues showed that administration of synthetic glucocorticoids with little or no mineralocorticoid activity increased blood pressure without salt and water retention (34). Finally, the glucocorticoid antagonist mifepristone can normalize blood pressure in Cushing’s syndrome but does not bind to the mineralocorticoid receptor (35). These findings indicate that a functional mineralocorticoid excess state is not the sole pathogenic mechanism, and the glucocorticoid receptor is involved in the development of hypertension in Cushing’s syndrome.
Glucocorticoid effects on vasculature
Enhanced action of the renin angiotensin system (RAS)
In rodent models, glucocorticoids increase angiotensinogen production (36). Despite this potential increase in substrate, plasma angiotensin II levels and renin activity are usually normal or suppressed in patients with Cushing’s syndrome. However, animal studies have shown that glucocorticoids increase angiotensin II receptor type 1 concentration in brain and peripheral tissue. They also enhance angiotensin II stimulated inositol phosphate-3 production in vascular smooth muscle cells (37 - 38) and its central pressor effects (39). Patients with Cushing’s syndrome show an increased pressor response to angiotensin II, suggesting increased sensitivity to the agent. These data support the concept that the RAS may play a role in the pathophysiology of glucocorticoid-induced hypertension through upregulation of central and peripheral angiotensin II receptors.
Inhibition of vasodilators
In addition to enhancing RAS activity, glucocorticoids impair vasodilation (40). Although circulating levels of the vasodilator atrial natriuretic peptide (ANP) are increased in experimental and clinical conditions of glucocorticoid excess, in vitro studies show that glucocorticoids decrease its biological activity (41). Sala and colleagues administered physiological doses of ANP to normotensive healthy volunteers, and patients with Cushing’s disease or essential hypertension. Despite a 4-fold increase in ANP levels in all groups, the biological response, increase in plasma and urine cGMP, was much lower in patients with Cushing’s disease (40).
Glucocorticoids also decrease production of nitric oxide synthase, which is responsible for the synthesis of another vasodilator, nitric oxide (42). Glucocorticoids also inhibit production of other potent vasodilators like prostacyclin, prostaglandin E2 and kallikrein (43 – 44). This in turn may increase blood pressure by decreasing peripheral vasodilation.
Enhanced vascular reactivity to vasopressors
Glucocorticoids increase the vascular sensitivity to the effects of catecholamines. Studies in healthy volunteers demonstrate that glucocorticoids increase the sensitivity to infusions of phenylephrine, angiotensin II, and norepinephrine resulting in an increase in peripheral vascular resistance and mean arterial blood pressure (44 - 46). Although plasma concentrations of various vasopressor hormones are normal in patients with Cushing’s syndrome, an increase in beta-adrenergic receptor sensitivity has been documented (45).
Endothelin-1 (ET-1) is a potent vasoconstrictor produced by vascular smooth muscle cells and endothelial cells. Plasma levels of ET-1 are significantly elevated in patients with Cushing’s syndrome (47). However, studies looking at the role of ET-1 in glucocorticoid induced hypertension have conflicting results. On one hand, dexamethasone decreases endothelin receptors in the kidney and the nonselective endothelin antagonist bosentan has no effect on ACTH-induced hypertension in rats. On the other hand, in animal studies, chronic use of endothelin type-A receptor antagonist normalizes blood pressure in 11-β HSD inhibitor induced hypertension (48). Therefore, the contribution of the endothelin-1 pathway to glucocorticoid-induced hypertension requires further study.
Glucocorticoids also appear to down regulate the expression of the sodium-calcium exchanger in vascular smooth muscle cells, which in turn increases the cytoplasmic concentration of calcium, and leads to vasoconstriction (49). Plasma levels of erythropoietin, another vasoconstrictor, have also been shown to increase with glucocorticoids and may play a role in glucocorticoid induced hypertension (50).
Other Indirect Factors
Obstructive sleep apnea has been reported in patients with Cushing’s syndrome (51). As OSA is associated with hypertension, this represents an additional etiology. Moreover, obesity-associated hypertension may occur irrespective of cortisol levels and also may also contribute to hypertension in Cushing’s syndrome.
Management of Hypertension in Cushing’s Syndrome
As hypertension is mediated by excess cortisol levels in Cushing’s syndrome, the therapeutic goal is to find and surgically remove the cause of excess glucocorticoids. While this may lead to resolution or improvement of hypertension, in some cases complete normalization is not achieved. In patients with occult ACTH-secreting tumors, as well as in those awaiting surgery, antihypertensive agents and anti-glucocorticoid agents are useful adjunctive therapies.
Medical Management
Although the optimal therapy for Cushing’s syndrome is surgical, medical treatment of hypercortisolism is often required while awaiting surgery and also when surgery is contraindicated or a tumor cannot be found. Medical agents include compounds that modulate ACTH release (dopamine and somatostatin agonists), inhibit steroidogenesis (metyrapone, ketoconazole, and mitotane), or block glucocorticoid action at its receptor (mifepristone). Normalization of cortisol is generally associated with improved blood pressure, although metyrapone may exacerbate hypertension by increasing mineralocorticoid production.
The steroidogenesis inhibitors metyrapone and ketoconazole have a rapid onset of action. However, due to an escape phenomenon, where ACTH secretion overrides control of hypercortisolemia, these drugs are usually not effective as the sole long-term treatment for Cushing’s disease. They are usually used as adjunctive agents after surgery or radiotherapy, or in preparation for surgery. The escape phenomenon is less likely with very high doses of mitotane, presumably because of its adrenolytic effects. The choice of agent is individualized depending on the side effect profile and other factors (Table 2). One of the main concerns with all medical agents is overtreatment that can render the patient adrenally insufficient. Therefore, all patients receiving such treatment should be educated in the use of glucocorticoids in emergency.
Table 2.
Steroidogenesis inhibitors and glucocorticoid antagonist for treatment of hypercortisolism
| Name | Mechanism ofaction | Time toonset ofaction | Side-effects | Other concerns |
|---|---|---|---|---|
| Metyrapone | Inhibits CYP11B1 | Days | Hirsutism,hypertension,gastrointestinaleffects | In US, available onlyfrom the manufactureras of 2010 |
| Ketoconazole | Inhibits CYP11B1and CYP11A1 | Days | Gastrointestinal,gynecomastia, rarelyhepatic dyscrasia | Enzyme inhibition leadsto decreasedtestosterone;requires gastric acidityfor bioavailability |
| Mitotane | Adrenolytic; inhibitsCYP11B1 andCYP11A1; possiblyother actions | Months | Gastrointestinal(common); CNS(lethargy, dizziness) | Hepatic enzymeinducer; teratogenic; fatsoluble |
| Mifepristone | Reversible blockadeof glucocorticoidreceptor | Days | Risk of adrenalinsufficiency | Also antagonizesandrogen andprogesterone receptor |
Agents evaluated for inhibition of ACTH secretion include bromocriptine, cabergoline, octreotide, and the investigational somatostatin-dopamine chimeric drug SOM230 (52). Successful normalization or control of hypercortisolemia leads to better control of hypertension. However, these agents are not uniformly effective and their role in treatment of ACTH-dependent Cushing’s syndrome is not defined. Despite early promise, peroxisome proliferator activated receptor γ (PPAR γ) agonists are not effective (53 - 54).
In addition to anti-glucocorticoid agents, antihypertensive agents should be used. Patients usually require more than one agent to reach the target blood pressure levels recommended by JNC 7. Since upregulation of RAS may be involved in glucocorticoid induced hypertension, angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) are recommended. ACEIs and ARBs ameliorate hypertension in almost 50% of hypertensive patients with Cushing’s disease (55 - 57). While adrenergic blockade and calcium channel blockers may be ineffective alone, they may be useful in combination therapy (58).
Hypertension can be difficult to control with antihypertensive agents without normalization of hypercortisolemia. Fallo and colleagues conducted a retrospective study of 40 patients with hypertension in Cushing’s syndrome; 28 had received conventional antihypertensive therapy while 12 were treated with ketoconazole. Blood pressure normalized in only 4 of the 28 patients on conventional treatment. Twelve of the remaining 24 patients were placed on ketoconazole, with normalization of blood pressure in all but one patient. In the second group, 11 of the 12 patients achieved normal blood pressure with ketoconazole alone (59). Whether this reflects bias in allocation to either treatment or suboptimal use of antihypertensive medications is not known. Specific treatment of hypertension and normalization of cortisol levels is essential in Cushing’s syndrome.
Surgical treatment of Cushing’s syndrome
Surgical excision of cortisol or ACTH producing tumors remains the optimal treatment of Cushing’s syndrome. Worldwide, transsphenoidal resection of pituitary adenoma for Cushing’s disease has immediate post-operative cure rates of 78 to 97%, with best results with microadenomas and experienced neurosurgeons. Unilateral or bilateral adrenalectomy is used for primary adrenal disease, depending on its location. Localization and surgical excision of non-metastatic ectopic ACTH-secreting tumors leads to cure. Bilateral adrenalectomy can be considered when a tumor cannot be localized or other treatments have failed (10).
Even after ‘curative’ surgery for Cushing’s syndrome, not all patients become normotensive. Studies have shown that about one-third of adult patients after surgery continue to have hypertension (60 - 62). Persistent hypertension after surgery correlates with the duration, but not the severity, of preoperative hypertension. These findings are similar to rates of persistent hypertension after treatment of other forms of secondary hypertension in adults, and probably reflect irreparable damage or remodeling of the vasculature from long-standing hypertension. By contrast, children and adolescents show complete resolution of hypertension within one year after surgical cure (15). The exact reason for this is not known but it may be related to the shorter duration of hypercortisolemia and/or vascular protective factors in younger patients.
Conclusion
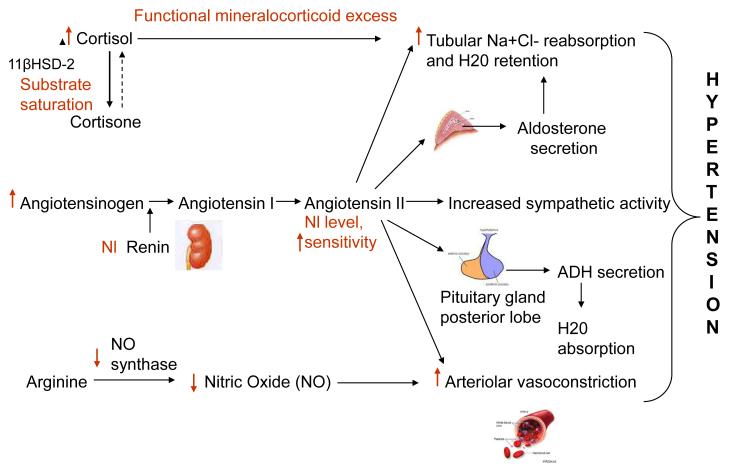
Cushing’s syndrome results from chronic pathological exposure to glucocorticoids. Hypertension is present in almost 80% of patients with Cushing’s syndrome and if left uncontrolled can lead to an increased cardiovascular risk and mortality. Although the exact pathophysiology leading to hypertension in Cushing’s syndrome is still not known, several possible mechanisms have been proposed (Figure 1). These include the presence of a functional mineralocorticoid excess state secondary to substrate saturation of the 11-β HSD 2 enzyme, upregulation of the renin angiotensin system, inhibition of vasodilators, and an enhanced vasoreactivity to vasopressors. Control of hypercortisolemia is important for blood pressure control. Normalization of hypercortisolemia via surgical resection of the source of excess glucocorticoids – adrenal pathology, pituitary adenoma or an ectopic tumor – remains the therapeutic goal. Anti-glucocorticoid drugs and antihypertensive agents should be used as adjunctive modes of treatment to normalize blood pressure.
Figure 1.
Pathogenesis of hypertension in Cushing’s Syndrome. Text and arrows in red show the effect of excess glucocorticoids on the renin-angiotensin system and other pathways involved.  = Increased,
= Increased,  = decreased, Nl = normal.
= decreased, Nl = normal.
Acknowledgments
This work was supported by the intramural program of the National Institute of Child Health and Human Development, National Institutes of Health
On part of the NIH, Dr. Nieman participates in a Cooperative Research and Development Agreement (CRADA with HRA-Pharma to evaluate mifepristone treatment of ectopic ACTH secretion. Dr. Sharma has nothing to disclose.
References
- 1.Lindholm J, Juul S, Jorgenson JO, et al. Incidence and late prognosis of Cushing’s syndrome: a population based study. J Clin Endocrinol Metab. 2001;86:117–23. doi: 10.1210/jcem.86.1.7093. [DOI] [PubMed] [Google Scholar]
- 2.Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf) 1994;40:479–84. doi: 10.1111/j.1365-2265.1994.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 3.Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605–17. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 4.Catargi B, Rigalleau V, Poussin A, et al. Occult Cushing’s syndrome in type-2 diabetes. J Clin Endocrinol Metab. 2003;88:5808–13. doi: 10.1210/jc.2003-030254. [DOI] [PubMed] [Google Scholar]
- 5.Leibowitz G, Tsur A, Chayen SD, et al. Pre-clinical Cushing’s syndrome: an unexpected frequent cause of poor glycaemic control in obese diabetic patients. Clin Endocrinol (Oxf) 1996;44:717–22. doi: 10.1046/j.1365-2265.1996.737558.x. [DOI] [PubMed] [Google Scholar]
- 6.Nugent CA, Warner HR, Dunn JT, et al. Probability theory in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1964;24:621–7. doi: 10.1210/jcem-24-7-621. [DOI] [PubMed] [Google Scholar]
- 7.Ross EJ, Linch DC. Cushing’s syndrome-killing disease: discriminatory value of signs and symptoms aiding early diagnosis. Lancet. 1982;2(8299):646–9. doi: 10.1016/s0140-6736(82)92749-0. [DOI] [PubMed] [Google Scholar]
- 8.Magiakou MA, Mastorakos G, Oldfield EH, et al. Cushing’s syndrome in children and adolescents: Presentation, diagnosis, and therapy. N Eng J Med. 1994;331:629–36. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- 9.Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieman LK, Ilias I. Evaluation and treatment of Cushing’s syndrome. Am J Med. 2005;118:134–46. doi: 10.1016/j.amjmed.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 11.Ilias I, Torpy DJ, Pacak K, et al. Cushing’s syndrome due to ectopic corticotrophin secretion: twenty years experience at National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–62. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]
- 12.Torpy DJ, Mullen N, Ilias I, et al. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion. Ann N Y Acad Sci. 2002;970:134–44. doi: 10.1111/j.1749-6632.2002.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 13.Faggiano A, Pivonello R, Spiezia S, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab. 2003;88:2527–33. doi: 10.1210/jc.2002-021558. [DOI] [PubMed] [Google Scholar]
- 14.Stewart PM, Walker BR, Holder F. II beta-hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80:3617–20. doi: 10.1210/jcem.80.12.8530609. [DOI] [PubMed] [Google Scholar]
- 15.Magiakou MA, Mastorakos G, Zachman K, et al. Blood pressure in children and adolescents with Cushing’s syndrome before and after surgical cure. J Clin Endocrinol Metab. 1997;82:1734–38. doi: 10.1210/jcem.82.6.3985. [DOI] [PubMed] [Google Scholar]
- 16.Treadwell BLJ, Sever ED, Savage O, et al. Side-effects of long-term treatment with corticosteroids and corticotrophin. Lancet. 1964;1(7343):1121–3. doi: 10.1016/s0140-6736(64)91804-5. [DOI] [PubMed] [Google Scholar]
- 17.Fraser R, Davies DL, Connell JMC. Hormones and hypertension. Clin Endocrinol. 1989;31:701–746. doi: 10.1111/j.1365-2265.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 19.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 20.Speiker C, Barenbrock M, Rahn KH, et al. Circadian blood pressure variations in endocrine disorders. Blood Pressure. 1993;2:35–9. doi: 10.3109/08037059309077524. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, Abe S, Sasaki S, et al. Altered circadian blood pressure rhythm in patients with Cushing’s syndrome. Hypertension. 1988;12:11–9. doi: 10.1161/01.hyp.12.1.11. [DOI] [PubMed] [Google Scholar]
- 22.Zacharieva S, Orbetzova M, Stoynev A, et al. Circadian blood pressure profile in patients with Cushing’s syndrome before and after treatment. J Endocrinol Invest. 2004;27:924–30. doi: 10.1007/BF03347534. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Abe S, Sasaki S, et al. Exogenous glucocorticoid eliminates or reverses circadian blood pressure variations. J Hypertens. 1989;7:113–20. [PubMed] [Google Scholar]
- 24.Magiakou MA, Smyrnaki P, Chrousos GP. Hypertension in Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab. 2006 Sep;20(3):467–82. doi: 10.1016/j.beem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Quinkler M, Stewart PM. Hypertension and the Cortisol-Cortisone shuttle. J Clin Endocrinol Metab. 2003;88(6):2384–92. doi: 10.1210/jc.2003-030138. [DOI] [PubMed] [Google Scholar]
- 26.Hatakeyama H, Inaba S. Miyamori I. 11 betahydroxysteroid dehydrogenase in cultured human vascular cells: possible role in development of hypertension. Hypertension. 1999;33:1179–1184. doi: 10.1161/01.hyp.33.5.1179. [DOI] [PubMed] [Google Scholar]
- 27.Brem A, Bina R, King T, et al. Localization of 2 11-betahydroxysteroid dehydrogenase isoforms in aortic endothelial cells. Hypertension. 1998;31:459–462. doi: 10.1161/01.hyp.31.1.459. [DOI] [PubMed] [Google Scholar]
- 28.Edwards CR, Stewart PM, Burt D, et al. Localization of 11-beta hydroxysteroid dehydrogenase – tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2(8618):986–9. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- 29.Funder JW, Pearce PT, Smith R, et al. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 30.Dotsch J, Dorr HG, Stall GK, et al. Effect of glucocorticoid excess on cortisol/cortisone ratio. Steroids. 2001;66:817–820. doi: 10.1016/s0039-128x(01)00117-9. [DOI] [PubMed] [Google Scholar]
- 31.Ulick S, Wang JZ, Blumenfeld JD, et al. Cortisol inactivation overload: a mechanism of minerlocorticoid hypertension in the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1992;74:963–967. doi: 10.1210/jcem.74.5.1569172. [DOI] [PubMed] [Google Scholar]
- 32.Connell JM, Whitworth JA, Davies DL, et al. Hemodynamic, hormonal, and renal effects of adrenocorticotrophic hormone in sodium-restricted man. J Hypertens. 1988;6(1):17–23. [PubMed] [Google Scholar]
- 33.Williamson PM, Kelly JJ, Whitworth JA. Dose-response and mineralocorticoid activity in cortisol-induced hypertension in humans. J Hypertens Suppl. 1196;14(5):S37–41. [PubMed] [Google Scholar]
- 34.Whitworth JA, Gordon D, Andrews J, et al. The hypertensive effect of synthetic glucocorticoids in man: role of sodium and volume. J Hypertens. 1989;7:537–549. doi: 10.1097/00004872-198907000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Nieman LK, Chrousos GP, Kellner C, et al. Successful treatment of Cushing’s syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metab. 1985;61:536–540. doi: 10.1210/jcem-61-3-536. [DOI] [PubMed] [Google Scholar]
- 36.Klett C, Ganten D, Hellmann W, et al. Regulation of hepatic angiotensinogen synthesis and secretion by steroid hormones. Endocrinology. 1992;130:3660–3668. doi: 10.1210/endo.130.6.1597163. [DOI] [PubMed] [Google Scholar]
- 37.Sato A, Suzuki H, Murakami M, et al. Glucocorticoid increases angiotensin II type 1 receptor and its gene expression. Hypertension. 1994;23:25–30. doi: 10.1161/01.hyp.23.1.25. [DOI] [PubMed] [Google Scholar]
- 38.Shelat SG, King JL, Flanagan-Cato LM, et al. Mineralocorticoids and glucocorticoids cooperatively increase salt intake and angiotensin II receptor binding in rat brain. Neuroendocrinology. 1999;69:339–351. doi: 10.1159/000054436. [DOI] [PubMed] [Google Scholar]
- 39.Scheuer DA, Bechtold AG. Glucocorticoids potentiate central actions of angiotensin to increase arterial blood pressure. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1719–R1726. doi: 10.1152/ajpregu.2001.280.6.R1719. [DOI] [PubMed] [Google Scholar]
- 40.Sala C, Ambrosi B, Morganti A. Blunted vascular and renal effects of exogenous atrial natriuretic peptide in patients with Cushing’s disease. J Clin Endocrinol Metab. 2001;86:1957–1961. doi: 10.1210/jcem.86.5.7477. [DOI] [PubMed] [Google Scholar]
- 41.Yasunari K, Kohno M, Murakawa K, et al. Glucocorticoids and atrial natriuretic factor receptors on vascular smooth muscle. Hypertension. 1990;16:581–586. doi: 10.1161/01.hyp.16.5.581. [DOI] [PubMed] [Google Scholar]
- 42.Kelm M. The L-arginine-nitric oxide pathway in hypertension. Curr Hypertens Rep. 2003;5:80–86. doi: 10.1007/s11906-003-0015-z. [DOI] [PubMed] [Google Scholar]
- 43.Axelrod L. Inhibition of prostacyclin production mediates permissive effect of glucocorticoids on vascular tone. Perturbations of this mechanism contribute to pathogenesis of Cushing’s syndrome and Addison’s disease. Lancet. 1983;23:904–906. doi: 10.1016/s0140-6736(83)91330-2. [DOI] [PubMed] [Google Scholar]
- 44.Handa M, Kondo K, Suzuki H, et al. Role of prostaglandins and pressure sensitivity to norepinephrine. Hypertension. 1984;6(2):236–241. [PubMed] [Google Scholar]
- 45.McKnight JA, Rooney DP, Whitehead H, et al. Blood pressure responses to phenylephrine infusions in subjects with Cushing’s syndrome. J Hum Hypertens. 1995;9:855–858. [PubMed] [Google Scholar]
- 46.Pirpiris M, Sudhir K, Yeung S, et al. Pressor responsiveness in corticosteroid-induced hypertension in humans. Hypertension. 1992;19(6):567–574. doi: 10.1161/01.hyp.19.6.567. [DOI] [PubMed] [Google Scholar]
- 47.Kirilov G, Tomova A, Dakovska L, et al. Elevated plasma endothelin as an additional cardiovascular risk factor in patients with Cushing’s syndrome. Eur J Endocrinol. 2003;149:549–553. doi: 10.1530/eje.0.1490549. [DOI] [PubMed] [Google Scholar]
- 48.Ruschitzka F, Quaschning T, Noll G, et al. Endothelin-1 type A receptor antagonism prevents vascular dysfunction and hypertension induced by 11 beta hydroxysteroid dehydrogenase inhibition: role of nitric oxide. Circulation. 2001;103(25):3129–3135. doi: 10.1161/01.cir.103.25.3129. [DOI] [PubMed] [Google Scholar]
- 49.Smith L, Smith JB. Regulation of sodium-calcium exchanger by glucocorticoids and growth factors in vascular smooth muscle. J Biol Chem. 1994;269(44):27527–31. [PubMed] [Google Scholar]
- 50.Kelly JJ, Martin A, Whitworth JA. Role of erythropoietin in cortisol-induced hypertension. J Hum Hypertens. 2000;14(3):195–198. doi: 10.1038/sj.jhh.1000959. [DOI] [PubMed] [Google Scholar]
- 51.Shipley JE, Schteingart DE, Tandon R, et al. Sleep architecture and sleep apnoea in patients with Cushing’s disease. Sleep. 1992;15:514–518. doi: 10.1093/sleep/15.6.514. [DOI] [PubMed] [Google Scholar]
- 52.Nieman LK. Medical therapy of Cushing’s disease. Pituitary. 2002;5:77–82. doi: 10.1023/a:1022308429992. [DOI] [PubMed] [Google Scholar]
- 53.Suri D, Weiss RE. Effect of pioglitazone on adrenocorticotropic hormone and cortisol secretion in Cushing’s disease. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2004-1746. [DOI] [PubMed] [Google Scholar]
- 54.Kreutzer J, Jeske I, Hofmann B, et al. No effect of the PPAR-gamma agonist rosiglitazone on ACTH or cortisol secretion in Nelson’s syndrome or Cushing’s disease in vitro and in vivo. Clin Neuropathol. 2009;28(6):430–9. [PubMed] [Google Scholar]
- 55.Zacharieva S, Orbetzova M, Natchev E, et al. Losartan in Cushing’s syndrome. Methods Find Exp Clin Pharmacol. 1998;20:163–168. doi: 10.1358/mf.1998.20.2.472459. [DOI] [PubMed] [Google Scholar]
- 56.Dalakos TG, Elias AN, Anderson GH, Jr, et al. Evidence for an angiotensinogenic mechanism of the hypertension of Cushing’s syndrome. J Clin Endocrinol Metab. 1978;46:114–118. doi: 10.1210/jcem-46-1-114. [DOI] [PubMed] [Google Scholar]
- 57.Zacharieva S, Torbova S, Orbetzova M, et al. Trandolapril in Cushing’s disease: short-term trandolapril treatment in patients with Cushing’s disease and essential hypertension. Methods Find Exp Clin Pharmacol. 1988;20:433–438. doi: 10.1358/mf.1998.20.5.485705. [DOI] [PubMed] [Google Scholar]
- 58.Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6):493–499. doi: 10.1007/s11906-004-0046-0. [DOI] [PubMed] [Google Scholar]
- 59.Fallo F, Paoletta A, Tona F, et al. Response of hypertension to conventional antihypertensive treatment and/or steroidogenesis inhibitors in Cushing’s syndrome. J Intern Med. 1993;234:595–598. doi: 10.1111/j.1365-2796.1993.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 60.Fallo F, Sonino N, Barzon L, et al. Effect of surgical treatment on hypertension in Cushing’s syndrome. Am J Hypertens. 1996;9:77–80. doi: 10.1016/0895-7061(95)00299-5. [DOI] [PubMed] [Google Scholar]
- 61.Colao A, Pivonello R, Spiezia S, et al. Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful cure. J Clin Endocrinol Metab. 1999;84:2664–2672. doi: 10.1210/jcem.84.8.5896. [DOI] [PubMed] [Google Scholar]
- 62.Mishra AK, Agarwal A, Gupta S, et al. Outcome of adrenalectomy for Cushing’s syndrome: experience from a tertiary care center. World J Surg. 2007;31(7):1425–32. doi: 10.1007/s00268-007-9067-6. [DOI] [PubMed] [Google Scholar]