Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome (original) (raw)
Abstract
Objective
The development of non-Hodgkin's lymphoma (NHL) confers a high risk of mortality in primary Sjögren's syndrome (pSS) patients, but the sensitivity and specificity of proposed lymphoma predictors are insufficient for practical use. The performance of lymphoid organisation in the form of germinal centre (GC)-like lesions was evaluated in labial salivary gland biopsies taken at pSS diagnosis as a potential lymphoma-predicting biomarker.
Methods
Labial salivary gland tissue biopsies available from two Swedish pSS research cohorts (n=175) were re-evaluated by light microscopy in a blind study in order to identify GC-like structures as a sign of ectopic lymphoid tissue formation and organisation. A linkage study was performed with the Swedish Cancer Registry for lymphoma identification. The risk of developing NHL in GC-positive patients in comparison with GC-negative patients was evaluated using Kaplan–Meier statistics and log-rank test. Associations between GC-like structures and clinical and/or laboratory disease markers were also determined using χ2 or Fisher's exact tests.
Results
At diagnosis, 25% of pSS patients had GC-like structures in their salivary glands. Seven of the 175 patients studied (14% GC+ and 0.8% GC−) developed NHL during 1855 patient-years at risk, with a median onset of 7 years following the initial diagnostic salivary gland biopsy. Six of the seven patients had GC-like structures at diagnosis; the remaining patient was GC negative at the time of diagnosis (p=0.001).
Conclusions
The detection of GC-like structures by light microscopy in pSS diagnostic salivary biopsies is proposed as a highly predictive and easy-to-obtain marker for NHL development. This allows for risk stratification of patients and the possibility to initiate preventive B-cell-directed therapy.
Primary Sjögren's syndrome (pSS) is characterised by dryness of the mucous membranes throughout the body, and may affect various other internal organs by inflammation or vasculitis to varying degrees. In most cases, pSS presents with a milder course of disease progression than other systemic rheumatic diseases, for example, systemic lupus erythematosus.1 The increased risk of non-Hodgkin's lymphoma (NHL) in pSS was first described by Kassan et al2 in 1978, who reported a 44-fold increased risk of NHL in pSS patients compared with that of the general population. Subsequent studies have determined the risk to be between six3 and 204 times greater than within the general population, with 5–10% of all pSS patients expected to develop this potentially life-threatening complication. In fact, up to 18% of patients have been reported to develop NHL after a long follow-up,5 with one study reporting that premature mortality in pSS patients is exclusively caused by the development of NHL.6
Despite considerable efforts to identify risk factors or biomarkers for the development of NHL, neither which patients will develop NHL nor the expected time of the onset of lymphoma can be identified efficiently. Some predictors have been repeatedly documented from large patient cohorts, namely hypocomplementaemia, persistent or recurrent salivary gland swelling, and cutaneous vasculitis, palpable purpura and low C47–9 with HR of up to 9.5.9 The combination of low C4 and palpable purpura at the time of initial presentation were previously proposed as markers of type I (high-risk) pSS.7 A clear explanation as to why these factors should facilitate lymphoma development has not been elucidated. An association between ectopic germinal centre (GC) formation and the development of lymphoma in pSS was originally proposed as early as 1999,10 but a prospective study to determine the HR and predictive value of this phenomenon for lymphoma risk has not been performed.
In the present study, we aimed to determine whether GC formation in a lower labial salivary gland biopsy taken at the time of pSS diagnosis predicts the subsequent development of lymphoma at a later stage of the disease. Indeed, six out of seven pSS patients in our cohort who developed lymphoma had GC-like structures detectable by light microscopy at diagnosis; a median of 7 years (range 2–12 years) before clinical lymphoma presentation. This finding may allow the clinician to identify the target population for repeated NHL screening and possibly the selection of candidates for preventive B-cell-directed biological treatment in pSS by utilising a simple routine diagnostic procedure.11
Patients and methods
Patients and clinical information
One hundred and seventy-five pSS patients were selected for the study out of 241 consecutive pSS patients from two Swedish centres with pSS research cohorts (Uppsala and Malmö University Hospitals) participating in a Nordic collaboration study on lymphoma and genetics. The study cohort comprised 161 (92%) female patients and 14 (8%) male patients, with an average age at diagnosis of 51.3 years (±13.3). All included patients fulfilled the American European consensus criteria (AECC)11 for pSS and were regularly followed, with registration of relevant clinical parameters such as salivary gland swelling, skin vasculitis, internal organ involvement and lymphadenopathy being chronicled. Likewise, laboratory variables were repeatedly studied, including autoantibody status, blood status, immunoglobulin levels, complement function and T-cell subsets (with complement and T-cell subsets studied only in the Malmö cohort). With up to 25 years of individual follow-ups at two separate units, the methods and reference ranges for the assessment of complement activity, autoantibody status, blood cells and cell subsets varied. The local reference levels at the time of analysis were thus used as cut-offs for determining seropositivity or abnormal levels. However, for serum autoantibodies against Ro-60, Ro-52 (SSA) and La (SSB), a separate analysis was performed using a bead-based multiplexed immune assay (xMap technology; Luminex, Austin, Texas, USA). Antinuclear antibody (ANA), anti-Ro/SSA, anti-La/SSB and rheumatoid factor (RF) autoantibodies were determined to be positive in 81%, 64%, 39% and 55% of the patients, respectively. In addition, all patients were screened for organ involvement according to the recently established assessment instrument for disease activity in pSS (European League Against Rheumatism Sjögren's syndrome disease activity index (ESSDAI)).12
Salivary gland tissue re-evaluation and GC detection
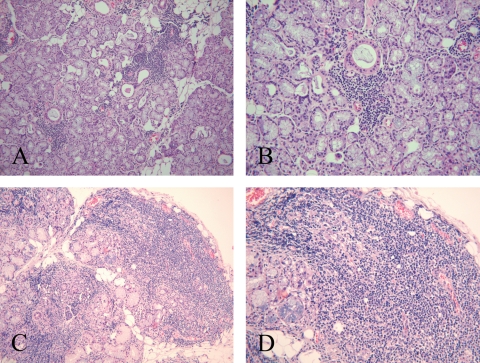
One hundred and seventy-five H&E stained paraffin-embedded minor salivary gland tissue biopsies taken at the time of pSS diagnosis were available from the 241 consecutive patients; the remaining patients either had no biopsy performed, or the biopsy material was lost or damaged and were thus excluded from the study. Tissue sections were previously evaluated for the presence of focal and periductal lymphocytic infiltration13 according to the requirements of the AECC11 (figure 1A,B) with the focus score denoting the number of inflammatory cell foci containing at least 50 mononuclear cells per 4 mm2. All available salivary gland tissue sections (n=175) were re-evaluated by one of the investigators (MVJ) at the Broegelmann Research Laboratory in Bergen, Norway. The re-assessment was performed by light microscopy without previous knowledge of clinical data or the diagnosis from the initial biopsy evaluation. Beyond the assessment of focal sialadenitis, the presence of ectopic GC-like structures was investigated. A biopsy was considered GC positive (GC+) in cases in which a well-circumscribed chronic inflammatory cell infiltrate consisting of at least 50 mononuclear cells presented with features indicative of lymphoid organisation, such as a densely packed dark zone and a light zone within otherwise normal salivary gland epithelium (figure 1C,D). Lymphoid organisation is not observed in conventional focal infiltrates (figure 1A,B) and therefore these sections were classified as GC negative (GC−). Focal infiltrates and GC-like structures may occur within the same minor salivary gland,14 and in such cases the section was characterised as GC+ (figure 1C,D).
Figure 1.
Otherwise normal salivary gland tissue with periductal focal mononuclear cell infiltrates (focal sialadenitis) but no germinal centre (GC)-like structures (GC− biopsy) (A and B) and salivary gland tissue with focal sialadenitis and GC-like structures (GC+ biopsy) (C and D).
Presence of NHL
Registry linkage was performed on the entire study cohort (n=241) with the Swedish Cancer Registry15 to 2007 using the unique national identification number.16 In patients in whom NHL was diagnosed, paraffin-embedded blocks were retrieved and consequently re-evaluated and lymphoma classified according to the WHO classification17 by a senior pathologist (GW).
Statistics
The risk of developing NHL in GC+ in comparison with GC− patients was ascertained using the Kaplan–Meier statistics/log-rank test. Clinical manifestations of GC+ and GC− patients were compared by χ2 statistics or Fisher's exact test. Multivariate analyses could not be performed due to the limited number of lymphomas.
Follow-up and observation time
The observation time for the present study was defined as the time from salivary gland biopsy to lymphoma diagnosis, death, or end of clinical follow-up, whichever occurred first.
Results
Salivary gland tissue re-evaluation and GC status establishment
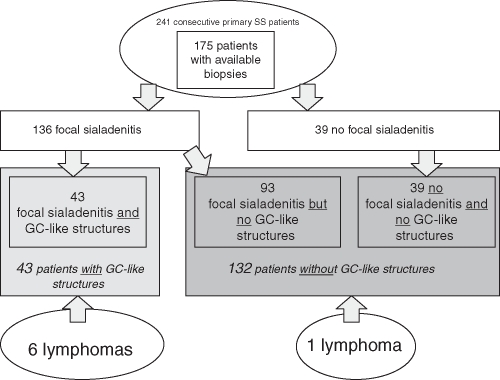
Of the 175 salivary gland tissue biopsies examined, focal sialadenitis (figure 1) with a focus score of 1 or greater was detected in 136 (78%) patients. The remaining 39 patients were categorised as having a ‘negative’ biopsy,11 13 and fulfilled pSS classification criteria due to the presence of autoantibodies against Ro/SSA and/or La/SSB. Among the 136 patients with focal sialadenitis, 43 (32%) showed GC-like structures in the diagnostic salivary gland biopsy. GC-like structures may only be found in the presence of focal lymphocyte accumulation and consequently only when autoimmune sialadenitis is present. When considering the whole cohort, 25% of the 175 patients had GC-like structures (figure 2).
Figure 2.
Patient distribution after re-evaluation of the diagnostic salivary gland biopsy and distribution of lymphoma events.
Follow-up times
The average follow-up time of all patients was 10.6 years (range 1 month to 25 years), with a mean of 11.6 years in GC+ patients and 10.3 years in GC− patients, p=0.23. The median time between the diagnosis of pSS and performing a salivary gland biopsy was 2 weeks (IQR of 0–19 weeks; total range of −227 to 624 weeks), whereas the time between salivary gland biopsy and NHL diagnosis was on average 7 years 2 months (range 2 years 4 months to 12 years 7 months).
Risk of lymphoma development in GC+ and GC− patients
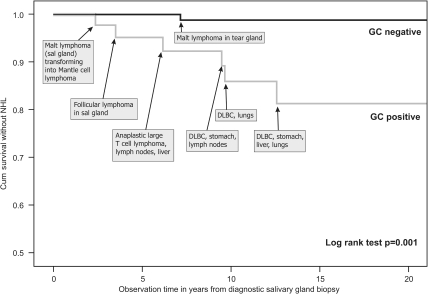
Registry linkage confirmed that seven of the 175 pSS patients had developed NHL. Six of seven patients with lymphoma had GC-like structures in their salivary glands at pSS diagnosis, performed a median of 7 years before the occurrence of lymphoma. Therefore, among the 43 patients with GC-like structures, six patients (14%) developed lymphomas in contrast to one patient (0.8%) among the 135 GC− patients (p=0.001; table 1). The difference in lymphoma-free survival between GC+ and GC− patients is illustrated by the Kaplan–Meier curve showing a log-rank test of p=0.001 (figure 3). The first NHL in the study population was diagnosed 2 years and 4 months after the salivary gland biopsy. The longest interval between GC+ salivary gland biopsy and the appearance of lymphoma was 12 years and 7 months. The positive predictive value for GC positivity was 16%, whereas the negative predictive value was 99%. For further patient and lymphoma details, please refer to supplementary table S1 (available online only).
Table 1.
Association of proposed risk factors with lymphoma occurrence in the pSS study cohort
| Lymphoma | No lymphoma | p Value* | |
|---|---|---|---|
| GC positivity† | 6 (86%) | 37 (22%) | 0.001 |
| CD4 T lymphocytopaenia†‡ | 4 (80%) | 15 (14%) | 0.003 |
| C3, lowest quartile† | 3 (50%) | 36 (24%) | 0.17 |
| C4, lowest quartile† | 4 (67%) | 29 (25%) | 0.043 |
| Cryoglobulinaemia† | 2 (33%) | 13 (16%) | 0.261 |
Figure 3.
Kaplan–Meier curve showing lymphoma-free survival in germinal centre (GC)+ and GC− patients with individual lymphoma information added. DLBC, diffuse large B cell lymphoma; NHL, non-Hodgkin's lymphoma.
Previously established risk factors were also significantly associated with lymphoma development in this cohort (table 1). However, due to the retrospective nature of the study, with incomplete assessments of the other risk markers and together with the small number of lymphoma events, a direct comparison of risk estimates cannot be reliably performed.
Differences between patients with and without GC-like structures in their salivary gland biopsy
The differential occurrence of non-exocrine disease manifestations in GC+ and GC− patients was investigated using domains from the disease activity instrument ESSDAI12 to determine the variables for comparison. In GC+ patients lymphadenopathy was present in 31%, but was observed only in 12% of GC− patients (p=0.006); haematological manifestations, mainly leucopenia and hypergammaglobulinaemia were also overrepresented among patients with GC-like structures. Furthermore, systemic disease affecting a higher number of organ systems was observed more frequently in GC+ patients in comparison with the GC− group (table 2). Initial C4 levels were significantly lower in GC+ patients, whereas C3 levels, the presence of cryoglobulins and CD4 T-cell counts did not differ significantly between the two groups. As previously shown,18 autoantibodies such as anti-Ro/SSA, anti-La/SSB, ANA and RF were more frequently detected among GC+ than GC− patients.
Table 2.
Risk factors and clinical variables in GC+ and GC− patients
| Risk factors/clinical variables* | GC+ (n/% of available or mean (SD)) | GC− (n/% of available or mean (SD)) | p Value† |
|---|---|---|---|
| Lymphoma | 6/14% | 1/0.8% | 0.001 |
| Lymphadenopathy | 13/31% | 15/12% | 0.006 |
| Leucopaenia (<4000/mm3) | 13/37% | 17/17% | 0.019 |
| CD4 T lymphocytopaenia‡ | 8/26% | 11/14% | 0.16 |
| Cryoglobulinaema | 5/18% | 10/16% | 1.00 |
| Systemic disease§ | 30/74% | 57/51% | 0.007 |
| Total n involved organ systems¶ | 2.56 (1.62) | 1.58 (1.37) | <0.001 |
| Anti-Ro/SSA antibodies | 24/77% | 48/54% | 0.022 |
| Anti-La/SSB antibodies | 19/61% | 32/36% | 0.014 |
| ANA | 38/91% | 103/79% | 0.10 |
| RF | 27/68% | 63/51% | 0.07 |
| IgG (g/l)** | 18.3 (6.46) | 15.0 (5.17) | 0.003 |
| C4 (g/l)** | 0.22 (0.087) | 0.26 (0.083) | 0.015 |
| C3 (g/l)** | 0.98 (0.26) | 1.05 (0.25) | 0.17 |
Discussion
The 20-fold increased risk of NHL with premature mortality as a consequence is one of the main problems in the clinical management of pSS. The results of the present study propose a new, potent and easy-to-use tool for risk assessment of developing NHL and possibly treatment tailoring. The labial salivary gland biopsy is performed as part of the diagnostic process, constituting one of two items within the AECC criteria set required for classification.11 We show here that six of the seven pSS patients with NHL had GC-like structures in the initial minor salivary gland biopsy.
GC-like structures were apparent in 25% of the patients in the study cohort, with NHL expected to occur in approximately 10% of all pSS patients during their lifetime. While GC are recognised as important loci for the maturation of B cells and the generation of B-cell lymphomas,19 20 GC positivity alone seems not sufficient as a sole contributor and with additional factors required for the evolvement of NHL. Furthermore, we only examined GC-like structures in minor salivary glands, yet the lymphomas in our patient cohort presented in other organs in five out of seven cases (see figure 3 and supplementary table S1, available online only). Based on our results, one might speculate that the formation of GC-like structures is a widespread or multi-local phenomenon in pSS patients prone to lymphoproliferation. Systemic findings of pSS-promoting B-cell proliferation such as upregulated interferon alpha (IFNα) signature21 22 and increased B cell activating factor of the TNF family of ligands (BAFF) levels14 23 may exert their effects in multiple sites with ongoing antigenic stimulation. The reduced levels of memory B cells in pSS peripheral blood,24 25 possibly due to migration into inflamed tissue,26 also supports a process expanding beyond the salivary glands. Whether our lymphoma patients expressed antecedent GC-like structures in the organs where the lymphoma evolved, or if lymphoma infiltration of the salivary glands was present at the time point of lymphoma detection in these other organs, is not known. Gasparotto et al27 published a single case of pSS in which salivary gland swelling and parotid lymphoproliferation was followed by pulmonary lymphoma, showing partly the same B-cell clone in both sites. This is suggestive of a contribution of the local environment in the final transition from non-neoplastic to malignant B-cell proliferation.
In our patient material, the single GC− lymphoma patient diagnosed with mucosa-associated lymphoid tissue lymphoma of a tear gland was ascertained to have borderline small foci in the minor salivary gland biopsy. In addition to other recognised risk factors for the development of lymphoma, the patient also lacked autoantibodies against Ro/SSA and La/SSB.
Treatment options beyond supportive, symptomatic and fluid replacement measures in pSS are scarce. However, expensive B-cell-directed therapies have been proposed as a potential preventive measure for the development of lymphoma. Established biomarkers and guidelines for identifying which patients to treat are urgently needed. We here propose the presence of GC-like structures as a possible marker to identify patients at a higher risk of developing NHL.
Our findings revealed only a limited association of GC formation with other well-established markers of lymphoma development. Nonetheless, low C4 levels, leucopenia and hypergammaglobulinaemia were all associated with lymphoid organisation. A trend was observed for C3 and CD4 T-cell levels to be lower in GC+ patients; however, this did not reach statistical significance, possibly due to lack of power or maybe due to the independent expression of these predictors in the pathogenic process. In addition, peripheral blood cells and their epitopes, cytokines and chemokines do not necessarily mirror the situation in the gland or at other sites of the autoimmune process. The peripheral situation may either be the cause or the consequence of the tissue process, or may simply be unrelated.28 29 In a previous study, serum levels of interleukin (IL) 17, IL-1RA, IL-15, macrophage inflammatory protein 1a, eotaxin, IFNα and IL-4 were reported to be significantly increased in GC+ patients in comparison to GC− patients.30 Unfortunately, these parameters were not available for the patients in this study due to the historic longitudinal case–control design with a long-term follow-up of up to 25 years from the index biopsy.
The strength of our study lies in the prospective collection of unselected patients, data and tissue material collected over more than 25 years, the registry linkage study design, using the well-recognised Swedish health registries for lymphoma detection and verification,15 as well as the completely independent and blinded re-evaluation of the biopsies. Moreover, the proposed risk marker is virtually available as a routine analysis in every pSS patient in whom a representative minor salivary gland biopsy has been obtained.
The main drawback of the present study is the imprecise risk estimation due to the small number of lymphoma cases in this cohort. Nevertheless, the association between lymphoid organisation and lymphoma appears convincing, with the negative predictive value of the proposed marker being 99% allowing for the reliable negative selection of a large number of patients and sparing them from unnecessary screening and subjective anxiety.
It is important to keep in mind that the development of lymphomas in pSS is not confined only to serologically positive patients. Two of our seven patients developing lymphomas were indeed negative for anti-Ro and anti-La. In one patient there was no information as to whether the patient was originally serologically positive. The other patient was always seronegative for all autoantibodies and was described only borderline positive in the biopsy, putting this patient into the borderline–SS category. Her lymphoma may represent the background population risk of lymphoma.
In conclusion, we present a new marker of lymphoma development in pSS patients present in 86% of the patients presenting with NHL later on. This marker is detectable already at diagnosis in labial salivary gland biopsies by routine light microscopy of H&E sections. Although this predictor does not allow the precise determination of the time of onset of lymphoma, it certainly aids stratification to identify high-risk patients who should be closely monitored by lymphoma screening, close follow-up and possibly advanced interventions for the treatment of both the frequently associated systemic disease manifestations and ultimately for the prevention of lymphoma.
We propose that an assessment of lymphoid organisation in labial salivary glands is routinely performed by the pathologist along with the histopathological focus score evaluation as further material or staining is not required. We also encourage rheumatologists to include the minor salivary gland biopsy in the routine work-up during the diagnosis of pSS, even if it formally would not be necessary in Ro/SSA and/or La/SSB-seropositive cases. The value of ectopic GC-like structures can thus be evaluated prospectively.
Acknowledgments
The authors would like to thank the study nurses Käth Nilsson and Rezvan Kiani for collecting patient materials. Casey Smith is acknowledged for linguistic revision of the manuscript.
Footnotes
Funding This study was supported by the Swedish Rheumatism Association (ET), the Strategic Research Program at Helse Bergen, the Western Norway Regional Health Authority and the Broegelmann Foundation (RJ, MVJ, KB), Anna-Greta Crafoord Foundation (ET), a postdoctoral grant from Astrid Karlsson Foundation (GN), Malmö University Hospital Cancer Research Foundation (ET).
Competing interests None.
Ethics approval This study was conducted with the approval of the local ethics committees at the universities of Lund, Uppsala and Bergen.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Jonsson R, Bolstad AI, Brokstad KA, et al. Sjögren's syndrome – a plethora of clinical and immunological phenotypes with a complex genetic background. Ann NY Acad Sci 2007;1108:433–47 [DOI] [PubMed] [Google Scholar]
- 2.Kassan SS, Thomas TL, Moutsopoulos HM, et al. Increased risk of lymphoma in sicca syndrome. Ann Intern Med 1978;89:888–92 [DOI] [PubMed] [Google Scholar]
- 3.Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst 2006;98:51–60 [DOI] [PubMed] [Google Scholar]
- 4.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med 2005;165:2337–44 [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe N, Inanc M, Speight P, et al. Predictors of lymphoma development in primary Sjögren's syndrome. Semin Arthritis Rheum 1998;28:80–7 [DOI] [PubMed] [Google Scholar]
- 6.Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary Sjögren's syndrome: a prospective cohort study. Arthritis Rheum 2004;50:1262–9 [DOI] [PubMed] [Google Scholar]
- 7.Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren's syndrome. Arthritis Rheum 2002;46:741–7 [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Casals M, Brito-Zerón P, Yagüe J, et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2005;44:89–94 [DOI] [PubMed] [Google Scholar]
- 9.Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjögren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006;65:796–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voulgarelis M, Dafni UG, Isenberg DA, et al. Malignant lymphoma in primary Sjögren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren's Syndrome. Arthritis Rheum 1999;42:1765–72 [DOI] [PubMed] [Google Scholar]
- 11.Vitali C, Bombardieri S, Jonsson R, et al. ; European Study Group on Classification Criteria for Sjögren's Syndrome Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002;61:554–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seror R, Ravaud P, Bowman SJ, et al. ; EULAR Sjögren's Task Force EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis 2010;69:1103–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol 1968;21:656–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsson MV, Szodoray P, Jellestad S, et al. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjögren's syndrome. J Clin Immunol 2005;25:189–201 [DOI] [PubMed] [Google Scholar]
- 15.The National Board of Health and Welfare. Cancer Incidence in Sweden 2009. Stockholm: The National Board of Health and Welfare, 2011. http://www.socialstyrelsen.se/statistik/statistikefteramne/cancer (accessed 13 April 2011)
- 16.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swerdlow SH, Campo E, Harris NL, et al. eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 4th edn Lyon: IARC Press, 2008 [Google Scholar]
- 18.Jonsson MV, Skarstein K, Jonsson R, et al. Serological implications of germinal center-like structures in primary Sjögren's syndrome. J Rheumatol 2007;34:2044–9 [PubMed] [Google Scholar]
- 19.Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer 2005;5:251–62 [DOI] [PubMed] [Google Scholar]
- 20.Illes A, Varoczy L, Papp G, et al. Aspects of B-cell non-Hodgkin's lymphoma development: a transition from immune-reactivity to malignancy. Scand J Immunol 2009;69:387–400 [DOI] [PubMed] [Google Scholar]
- 21.Nordmark G, Alm GV, Rönnblom L. Mechanisms of disease: primary Sjögren's syndrome and the type I interferon system. Nat Clin Pract Rheumatol 2006;2:262–9 [DOI] [PubMed] [Google Scholar]
- 22.Gottenberg JE, Cagnard N, Lucchesi C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc Natl Acad Sci U S A 2006;103:2770–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szodoray P, Alex P, Jonsson MV, et al. Distinct profiles of Sjögren's syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin Immunol 2005;117:168–76 [DOI] [PubMed] [Google Scholar]
- 24.Bohnhorst JØ, Bjørgan MB, Thoen JE, et al. Abnormal B cell differentiation in primary Sjögren's syndrome results in a depressed percentage of circulating memory B cells and elevated levels of soluble CD27 that correlate with serum IgG concentration. Clin Immunol 2002;103:79–88 [DOI] [PubMed] [Google Scholar]
- 25.Hansen A, Gosemann M, Pruss A, et al. Abnormalities in peripheral B cell memory of patients with primary Sjögren's syndrome. Arthritis Rheum 2004;50:1897–908 [DOI] [PubMed] [Google Scholar]
- 26.Hansen A, Lipsky PE, Dörner T. Immunopathogenesis of primary Sjögren's syndrome: implications for disease management and therapy. Curr Opin Rheumatol 2005;17:558–65 [DOI] [PubMed] [Google Scholar]
- 27.Gasparotto D, De Vita S, De Re V, et al. Extrasalivary lymphoma development in Sjögren's syndrome: clonal evolution from parotid gland lymphoproliferation and role of local triggering. Arthritis Rheum 2003;48:3181–6 [DOI] [PubMed] [Google Scholar]
- 28.Salomonsson S, Larsson P, Tengnér P, et al. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand J Immunol 2002;55:336–42 [DOI] [PubMed] [Google Scholar]
- 29.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum 2003;48:3187–201 [DOI] [PubMed] [Google Scholar]
- 30.Reksten TR, Jonsson MV, Szyszko EA, et al. Cytokine and autoantibody profiling related to histopathological features in primary Sjogren's syndrome. Rheumatology (Oxford) 2009;48:1102–6 [DOI] [PubMed] [Google Scholar]