Clinical Outcome of HIV-Infected Patients with Discordant Virological and Immunological Response to Antiretroviral Therapy (original) (raw)
Abstract
Background. A subgroup of human immunodeficiency virus type 1 (HIV-1)–infected patients with severe immunodeficiency show persistently low CD4+ cell counts despite sustained viral suppression. It is unclear whether this immuno-virological discordance translates into an increased risk for clinical events.
Methods. Data analysis from a large multicenter cohort incorporating 14,433 HIV-1–infected patients in Germany. Treatment-naive patients beginning antiretroviral therapy (ART) with CD4+ cell counts <200 cells/μL who achieved complete and sustained viral suppression <50 copies/mL (n = 1318) were stratified according to the duration of immuno-virological discordance (failure to achieve a CD4+ cell count ≥200 cells/μL). Groups were compared by descriptive and Poisson statistics. The time-varying discordance status was analyzed in a multivariable Cox model.
Results. During a total of 5038 person years of follow-up, 42 new AIDS events occurred. The incidence rate of new AIDS events was highest in the initial 6 months of complete viral suppression (immuno-virological discordance group, 55.06; 95% confidence interval [CI], 30.82–90.82; and immune responder group, 24.54; 95% CI, 10.59–48.35) and decreased significantly by 65% per year in patients with immuno-virological discordance (incidence risk ratio, 0.35; 95% CI, 0.14–0.92; P = .03). Immuno-virological discordance and prior AIDS diagnosis were independently associated with new AIDS events (hazard ratio, 3.10; 95% CI, 1.09–8.82; P = .03).
Conclusion. Compared with immune responders, patients with immuno-virological discordance seem to remain at increased risk for AIDS. Absolute risk is greatly reduced after the first 6 months of complete viral suppression.
Antiretroviral therapy (ART) has led to a dramatic reduction in AIDS related morbidity and mortality among human immunodeficiency virus (HIV)–infected patients [1–3]. The risk to develop AIDS clearly depends on immune reconstitution and viral suppression during therapy, and prognosis is best for patients who show both recovery of CD4+ cell counts and sustained viral suppression [4, 5]. Currently, up to 30% of patients still present late to HIV centers in Europe in advanced stages of immunodeficiency (CD4+ cell counts of <200 cells/μL) and are therefore at high risk to develop AIDS [6–10]. Results from observational studies suggest that at least 73.5% of patients initiating ART achieve complete virological suppression within 6 months [11], but 9%–45% of patients do not experience an appropriate increase in their CD4+ cell counts in the short term [12, 13]. This condition, commonly referred to as immuno-virological discordance, is associated with the CD4+ cell count nadir before initiation of ART, and the risk of progression to AIDS and death may be increased in this patient population [14–16].
However, detailed risk progression analyses are largely missing in patients with persisting immuno-virological discordance. Clinical observation suggests that the rate of new AIDS-defining events may be low in this subgroup of patients irrespective of CD4+ cell response. In contrast, some experts suggest to change ART regimen or to add immunomodifiers (eg, interleukin-2 or CCR5 antagonists) to ART regimens for those patients with immuno-virological discordance to augment CD4+ cell count increases and to prevent disease progression. In the present study, we evaluated patient characteristics associated with immuno-virological discordance and subsequently analyzed the risk of developing new AIDS-related events in patients with advanced stages of immunodeficiency who were receiving fully suppressive ART.
METHODS
Cohort
Data were extracted and analyzed from the German ClinSurv cohort (Clinical Surveillance of HIV Disease), which is an ongoing observational cohort study incorporating HIV-infected patients from 11 urban centers in Germany. All consecutive HIV-infected patients seen since 1 January 1999 are enrolled. Patients have routine follow-up visits every 3 months according to national guidelines. At the end of June 2008, 14,433 patients were included in this study. All available clinical data, as well as virological and immunological parameters and details of anti-viral therapy, are recorded in local electronic databases and transmitted biannually to the coordinating Robert Koch Institute, which is also responsible for monitoring and source data verification. The ClinSurv study has been approved by the German Federal Commissioner for Data Protection and Freedom of Information.
Data Analysis
All patients in the cohort who were treatment naive and had a CD4+ cell count of <200 cells/μL before commencing highly active ART were included in this analysis. In addition, patients were required to achieve a nondetectable viral load (<50 copies/mL) within 180 days after initiation of ART. Patients were followed-up from the first day of complete viral suppression (defined as a viral load <50 copies/mL; baseline) until either virologic failure (2 consecutive viral load measurements >50 copies/mL within 180 days or 1 viral load measurement >1000 copies/mL), a new AIDS-defining event, or 30 June 2008, whichever occurred first. ART was defined as the combination of at least 3 antiretroviral drugs (a regimen containing either a protease inhibitor [PI] or nonnucleoside reverse-transcriptase inhibitor [NNRTI] or a triple–nucleoside reverse-transcriptase inhibitor [NRTI] regimen).
Immune Response and Discordance
By definition, all patients included in the study had CD4+ cell counts of <200 cells/μL at or before initiation of ART. Immune response was defined as a documented CD4+ cell count increase to >200 cells/μL on at least one occasion, even if the CD4+ cell count decreased again to <200 cells/μL during subsequent follow-up. Immuno-virological discordance was defined as the failure to surpass the threshold of 200 CD4+ cells/μL. Patients were uniquely grouped according to duration of immuno-virological discordance (0–6, 7–12, 13–24, or >24 months of complete viral suppression). If, for example, a patient had an immune response with an increase in CD4+ cell count to >200 cells/μL at month 14 of complete viral suppression, then this patient was regarded as having immuno-virological discordance for at least 12 months but <24 months (group C in Table 1).
Table 1.
Patient Characteristics according to Duration of Immuno-virological Discordance
| Variable | Group A: immune response within 6 months (n = 837) | Group B: immuno-virological discordance for at least 6 but <12 months (n = 248) | Group C: immuno-virological discordance for at least 12, but <24 months (n = 134) | Group D: immuno-virological discordance for at least 24 months (n = 99) | P (reference-group A) |
|---|---|---|---|---|---|
| Male sex, % | 75.4 | 77.0 | 83.6 | 79.8 | .18a |
| Age, median years (IQR) | 38 (33– 46) | 38 (33.0– 45.5) | 40 (33– 49) | 42 (36– 52) | .03 b |
| CD4+ cell count nadir, median cells/μL (IQR) | 123 (62– 170) | 40 (16– 76) | 30.5 (11– 60) | 21 (10– 47) | <.001b |
| Log viral load at initiation of ART, median copies/mL (IQR) | 5.00 (4.56– 5.44) | 4.98 (4.5– 5.45) | 5.00 (4.57– 5.38) | 4.98 (4.58– 5.35) | .65a |
| Risk group | |||||
| Men who have sex with men, % | 46.8 | 41.9 | 47.0 | 40.4 | .39a |
| Injection drug user, % | 5.0 | 6.5 | 3.0 | 6.1 | .51a |
| Migrant from sub-Saharan Africa, % | 12.2 | 16.1 | 13.4 | 8.1 | .19a |
| Origin from Germany | 69.5 | 66.5 | 68.7 | 79.8 | .11a |
| AIDS diagnosis prior to starting ART, % | 37.2 | 57.4 | 62.3 | 65.4 | <.001a |
| Time from ART initiation to baseline, median days (IQR) | 106 (72–138) | 95.5 (67–126.5) | 89 (63–127) | 84 (60–116) | .004 b |
| Time from baseline to AIDS-defining event, median days (IQR) | 298 (99–493) | 43 (22–127) | 293 (222–501) | 701.5 (701–702) | .002 b |
AIDS-Defining Events
AIDS-defining events were defined according to the European AIDS surveillance case definition [17]. Cases fulfilling the criteria for AIDS were followed up by the principal investigator and validated after critical chart review with the primary treating physician in each case individually. Presumptive diagnoses that proved to be incorrect or unconfirmed diagnoses were excluded from the analysis. Opportunistic infection prophylaxis was administered according to national guidelines.
Statistics
Variables tested both in descriptive and time-dependent multivariable models included possible confounders and effect modifiers, such as age, sex, transmission risk group, ethnic origin, nadir CD4+ cell count before initiation of ART, prior AIDS-defining event, year of initiation of ART (before vs after 1 January 2001, when contemporary boosted PI–containing ART became available in Germany). Transmission risk was documented on the basis of the most likely mode of HIV transmission. Characteristics of patients with immune response and immuno-virological discordance were compared using parametric and nonparametric tests, including the χ2 test, the Student’s t test, analysis of variance, and the rank-sum test.
Incidence rates were calculated for every 6 month period in the first year and for the second, third, and fourth year and were normalized per 1000 person years. A Poisson model was fitted to calculate the incidence rate ratios (IRRs) for new AIDS events per year of complete viral suppression in immune responders and patients with immuno-virological discordance.
The Cox proportional hazards model included all of static variables mentioned above. In addition, the time-updated immuno-virological discordance (always defined by a CD4+ cell count <200 cells/μL), as well as immune response status in 2 steps (defined by CD4+ cell counts of 200–350 cells/μL and >350 cells/μL), were introduced into the model. The highest ever achieved immune response status was carried forward. Proportional hazards assumptions were confirmed using Schoenfeld's test.
All analyses were performed in Stata software, version 10 (Stata). A P value of <.05 was considered to be statistically significant, and all tests of significance were 2-sided.
RESULTS
Among a total of 14,433 patients in the entire cohort, 11,098 patients were receiving ART as of 30 June 2008. Of these individuals, 1318 (11.9%) were ART naive, started ART at a CD4+ cell count of <200 cells/μL, and achieved a viral load <50 copies/mL within 180 days after initiation of therapy and were thus eligible for this analysis. Among these patients, 96 new AIDS diagnoses were documented, of which 49 proved to be incorrect or could not be confirmed on data verification and which were thus excluded from the analysis. In addition, 5 AIDS-defining events occurred on the first day of viral suppression (baseline) and were also excluded. Reasons for incorrect diagnoses included: (1) diagnosis occurred in the past and was no longer valid; (2) diagnosis was presumptive and based on clinical assumptions only and was not confirmed by laboratory, serologic, or radiologic tests; (3) diagnosis was based on serologic test results only, without a clinical correlate. The remaining confirmed AIDS-defining events (_n_ = 42) were included in the analysis. Excluded patients were more likely to originate from Germany (_P_ = .01) but did not differ from included patients in terms of age, sex, transmission risk, prior AIDS-defining event, or nadir CD4+ cell count before initiation of ART (_P_ > .33 for all).
In total, 5038 person-years of follow-up with full suppression of viral load to levels <50 HIV RNA copies/mL were available for this analysis. A total of 129 patients (9.8%) had ≥1 or more viral blips, defined as a viral load >50 copies/mL and <1000 copies/mL followed by a viral load <50 copies/mL.
Patient Characteristics
Median patient age was 39 years (interquartile range [IQR], 33–47 years), and 77.0% of the patients were male. Men having sex with men was the most frequent mode of transmission (45.7%); 69.9% of patients originated from Germany, and 12.6% originated from sub-Sahara Africa.
A total of 599 patients (43.0%) already had an AIDS-defining diagnosis at or before initiation of ART. Nadir CD4+ cell count before initiation of ART was 80 cells/μL (IQR, 30–150 cells/μL). Viral suppression to levels below 50 copies/mL was achieved after a mean (± standard deviation [SD]) duration of 100.1 ± 42.3 days after ART was commenced.
Differences between Early Responders and Patients with Long-term Immuno-Virological Discordance
Compared with patients with immune response in the first 6 months of viral suppression (group A in Table 1), patients with long-term immuno-virological discordance for at least 24 months (group D in Table 1) were older (median age, 42 years [IQR, 36–52 years] vs 38 years [IQR, 33–46 years]; P = .03) and were more likely to have experienced an AIDS-defining event at or before initiation of ART (65.4% vs 37.2%; P < .001). Nadir CD4+ cell count before initiation of ART was lower (median CD4+ cell count, 21 cells/μL [IQR, 10–47 cells/μL] vs 123 cells/μL [IQR, 62–170 cells/μL]; P < .001) and mean time to viral load suppression (HIV RNA level <50 copies/mL) was significantly shorter (84 days [IQR, 60–116 days] vs 106 days [IQR, 72–138 days]; P = .004). Time from baseline (first day of complete viral suppression) to new AIDS-defining event was shorter in patients with early response (median duration, 298 days [IQR, 99–493 days] vs 701.5 days [IQR, 701–702 days]; P = .002). Patient characteristics according to duration of immuno-virological discordance can be seen in Table 1.
AIDS-Defining Events
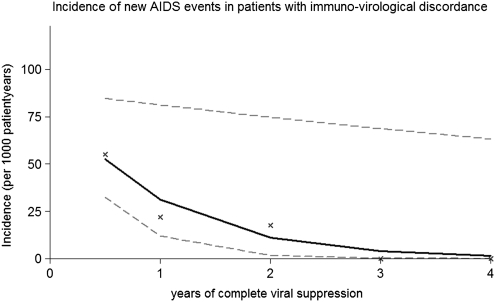
A total of 42 new AIDS-defining events occurred. Incidence for new AIDS-defining events was highest in the first year of full viral suppression in both groups. Within the first year, incidence rates were more than twice as high in the first 6 months (20.6 [95% CI, 8.9–40.7] in the immune response group and 55.1 [95% CI, 30.8–90.8] in the immuno-virological discordance group), compared with months 7–12 (8.0 [95% CI, 1.6–23.2] in the immune response group and 22.1 [95% CI, 4.6–64.5] in the immuno-virological discordance group; Table 2). Incidence rates decreased by 65% per year of complete viral suppression in patients with immuno-virological discordance (IRR, .35; 95% CI, 0.14–0.92; P = .03) and by 41% per year in patients with immune response (IRR, 0.59; 95% CI, 0.39–0.90; P = .01). The decrease in incidence rates in patients with immuno-virological discordance is depicted in Figure 1.
Table 2.
Incidence Rates of New AIDS-defining Events in Patients with Immune Response (CD4+ Cell Count ≥200 cells/μL) and Immuno-virological Discordance (Failure to Achieve CD4+ Cell Count ≥200 cells/μL)
| Immune response | Immuno-virological discordance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IR per 1000 PYFU | IR per 1000 PYFU | |||||||||
| Duration of viral suppression | PYFU | AIDS-defining events | IR | 95% CI | PYFU | AIDS-defining events | IR | 95% CI | ||
| 1 Year | 673 | 11 | 16.3 | 8.2 | 29.2 | 408.4 | 18 | 44.1 | 26.1 | 69.7 |
| 0–6 Months | 326 | 8 | 20.6 | 8.9 | 40.7 | 272.4 | 15 | 55.1 | 30.8 | 90.8 |
| 7–12 Months | 347 | 3 | 8.0 | 1.6 | 23.2 | 136.0 | 3 | 22.1 | 4.6 | 64.5 |
| 2 Years | 637 | 7 | 11.0 | 4.4 | 22.6 | 113.9 | 2 | 17.6 | 2.1 | 63.4 |
| 3 Years | 429 | 1 | 2.3 | 0.1 | 13.0 | 38.7 | 0 | 0 | 0 | 95.3 |
| 4 Yearsa | 414 | 2 | 4.8 | 0.6 | 17.4 | 22.3 | 0 | 0 | 0 | 165.5 |
Figure 1.
Incidence of new AIDS-defining events in patients with immuno-virological discordance and complete and sustained viral suppression. X represents the observed incidence for new AIDS-defining events for months 0–6, months 6–12, years 1–2, years 2–3, and years 3–4; the continuous line represents the output of a Poisson Model estimating the incidence trend with 95% confidence intervals (dashed line).
Mycobacterial disease (tuberculosis and atypical mycobacterial infections) was the most frequent diagnosis and occurred early, at a median of 28 days (IQR, 26–44 days; total of 5 events) in patients with immuno-virological discordance and at a median of 711 days (IQR, 605–834 days; total of 2 events) in patients with immune response. Esophageal candidiasis (total of 6 events) and Kaposi sarcoma (total of 5 events) were among other commonly documented AIDS-defining events.
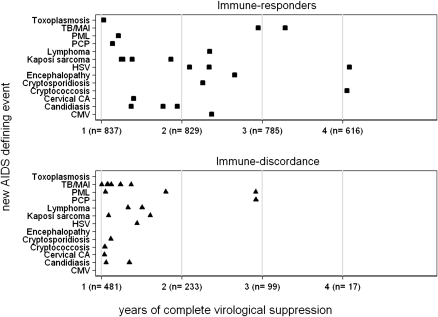
Overall, 6 (14.3%) of 42 patients had recurrence of a prior AIDS diagnosis (1 case each of recurrent cerebral toxoplasmosis, Kaposi sarcoma, chronic herpetic ulcer, and cryptococcal meningitis, and 2 cases of esophageal candidiasis). Differences in the frequencies of recurrent disease in either group were not noted (P = .22). The occurrence of AIDS-defining events is shown in Figure 2. More than one-half (54.8%) of all documented diagnoses occurred early in the first 6 months of viral suppression, and an additional 14.3% occurred in months 6–12.
Figure 2.
Occurrence of AIDS-defining events according to immune response and immuno-virological discordance. Among the immune responder group, 1 AIDS-defining event (recurrent esophageal candidiasis) occurred at month 73. Abbreviations: TB/MAI, tuberculosis/mycobacterium avium intracellulare; PML, progressive multifocal leucencephalopathy; PCP, pneumocystis pneumonia; HSV, herpes simplex virus infection;Cervical CA, cancer of the cervix; CMV, cytomegalovirus endorgan disease.
After 2 years, no additional AIDS-defining events occurred among patients with immuno-virological discordance. (Figure 2). In patients with immune response, 1 case of recurrent cryptococcal meningitis occurred at month 36, 1 case of recurrent herpetic ulcer occurred at month 36, and 1 case of recurrent esophageal candidiasis occurred at month 73 of complete viral suppression.
Cox Proportional Hazards Model
In the Cox proportional hazards model that incorporated all available follow-up data up to 3 years of complete viral suppression, receipt of an AIDS diagnosis prior to commencing ART (hazard ratio [HR], 2.58; 95% CI, 1.24–5.38; P = .01) and immuno-virological discordance (HR, 3.10; 95% CI, 1.09–8.82; P = .03, time updated) were independently associated with new AIDS-defining events (Table 3).
Table 3.
Cox Proportional Hazards Model Analyzing Risk Factors for New AIDS-defining Events in Patients with Sustained Viral Suppression
| Multivariate analysis | ||||
|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
| Male sex | 0.61 (0.26–1.46) | .27 | ||
| Age at baseline (per 10 years older) | 1.03 (1.00–1.06) | .07 | ||
| AIDS diagnosis prior to commencing ART | 2.34 (1.22–4.50) | .01 | 2.58 (1.24–5.38) | .01 |
| CD4+ cell count at baseline (per 50 cells/μL increase) | 0.91 (0.71–1.17) | .48 | 1.27 (0.93–1.74) | .13 |
| Men who have sex with mena | 1.35 (0.72–2.53) | .35 | ||
| ART initiation is before 1 January 2001 | 1.05 (0.55–2.03) | .87 | ||
| Immuno-virological discordancem, CD4+ cell count <200 cells/μLb | 2.71 (1.06–6.90) | .04 | 3.10 (1.09–8.82) | .03 |
| CD4+ cell count 200–350 cells/μLb | 1.54 (0.59–4.02) | .38 | 1.67 (0.63–4.41) | .30 |
DISCUSSION
In this analysis from a large observational cohort, we included treatment-naive HIV-infected patients who started ART with CD4+ cell counts <200 cells/μL. Risk of new AIDS events was assessed in patients who achieved a complete and sustained virological response (HIV RNA <50 copies/mL during the entire time of observation). The principal findings of this study suggest that most new AIDS events (54.8%) occur in the first 6 months after achieving complete viral suppression during ART. Incidence rates for new AIDS-defining events dramatically decreased by 65% per year in patients with immuno-virological discordance, as well as in patients with immune response (41% per year of complete viral suppression). Immuno-virological discordance (HR, 3.10; 95% CI, 1.09–8.82; P = .03) and receipt of an AIDS diagnosis prior to commencing ART (HR, 2.58; 95% CI, 1.24–5.38; P = .01) remain risk factors for new AIDS-defining events in the multivariable Cox analysis. However, absolute risk for AIDS is clearly reduced in the mid- to long-term, even among patients with immuno-virological discordance. No additional AIDS diagnoses were observed after year 2 of complete virological suppression with a minimum residual occurrence of events in immune responders.
Overall, the incidence rate of new AIDS-defining events in the whole cohort was surprisingly low. Only 42 new AIDS-defining events occurred in >5000 person-years of follow-up, underlining the clinical benefit of achieving an undetectable viral load during ART, irrespective of the individual CD4+ cell count. Moreover, in patients with immuno-virological discordance, 75% of AIDS-defining events occurred within the first 6 months after achieving sustained virological response. Mycobacterial diseases were detected frequently in this group, which may be interpreted as the occurrence of immune reconstitution inflammatory syndrome.
These data represent important and previously unreported aspects influencing clinical care of patients with HIV infection. Although normalization of CD4+ cell counts is potentially achievable in the long term [18], a number of patients do not achieve immune reconstitution despite the absence of active viral replication [19]. Current guidelines assume a constantly elevated AIDS risk and recommend prophylaxis for several opportunistic infections as long as CD4+ cell counts remain <200 cells/μL, which may contribute to additional toxicity, drug interactions, and costs [20]. So far, strategies to boost CD4+ cell counts in the light of antiretroviral treatment by co-administration of interleukin 2 have failed to show a clinical benefit [21]. Certain antiretroviral compounds (eg, zidovudine and didanosine) may impair CD4+ cell recovery [22], and boosted PI– or maraviroc-containing regimens may lead to more pronounced CD4+ cell gains [23, 24], compared with NNRTI-based combinations, but this has not been proven to translate into clinical benefits as long as viral suppression is achieved [25]. Thus, switching a virologically successful regimen just to increase the peripheral CD4+ cell count may not be beneficial or justified on the basis of current evidence. Unfortunately, we could not analyze the contribution of different antiviral compounds (eg, zidovudine, stavudine, and didanosine) on immuno-virological discordance from our database.
Results from the SMART study suggest that the latest CD4+ cell count can only predict opportunistic disease in patients who are not receiving therapy [26]. In concert with these data, our results indicate that CD4+ cell counts are of limited use as long-term surrogates for absolute AIDS risk as long as complete virological suppression is sustained. Moreover, current risk assessments according to CD4+ cell count status and recommendations for the prophylaxis of opportunistic disease are derived from studies before the ART era or in populations with uncontrolled viral replication. Overall the quality of immunological recovery seems to be better reflected by continuous control of viremia rather than by the absolute CD4+ cell count increase alone. This may be best explained by a restoration of functional immune responses against opportunistic microbes once the prevailing HIV antigens are absent during ART, rather than just increasing absolute peripheral T cell numbers. This notion is further supported by the fact that we did not observe any significant difference (P = .30) in clinical progression in patients who had a CD4+ cell count increase to >350 cells/μL, compared with the group that achieved CD4+ cell counts of 200–350/μL during therapy (Table 3).
Our analysis is limited by several factors. First, our results can only be applied to treatment-naive patient populations with advanced immune deficiency (CD4+ cell counts <200 cells/μL) at initiation of ART who achieve complete and sustained viral suppression. Second, we cannot exclude that not all opportunistic infection prophylaxis was always taken as recommended by local guidelines. Even though very few opportunistic diseases that are preventable by opportunistic infection prophylaxis occurred in patients with immuno-virological discordance, we cannot exclude that this observation is attributed to a higher use of prophylactic medication. Thus, our data do not support discontinuation of opportunistic infection prophylaxis in this group in general. However, in a previous study, the discontinuation of Pneumocystis carinii pneumonia prophylaxis with trimethoprim-sulfamethoxazole was found to be feasible even in patients with a CD4+ cell count <200 cells/μL if viral replication was sufficiently suppressed [27].
Third, the majority of our patients had rapid immune responses, with CD4+ cell counts >200 cells/μL in the first 6 months of viral suppression, and only 17 patients had long-term immuno-virological discordance beyond 2 years. For patients with long-term immuno-virological discordance, incidence rates for AIDS events are affected by large confidence intervals. However, these patients contributed 13% of total follow-up data for immuno-virological discordance, and reassuringly, not a single AIDS event was documented. Analysis of long-term immuno-virological discordance in even larger cohort collaborations could provide additional information here. Finally, we could not address the question of whether patients with immuno-virological discordance are at higher risk to develop non–AIDS-defining diseases, because these were not documented in the database from the beginning of the analysis.
In conclusion, these data from a large multicenter cohort show that the risk to develop new AIDS-defining events is substantially reduced after the first 6 months of complete viral suppression, irrespective of the numeric indicators of immune reconstitution. In patients with immuno-virological discordance, incidence rates decreased by 65% per year of complete viral suppression. Although the relative risk to develop AIDS may be higher for these patients, compared with the risk for patients with immune response, the limited reduction of absolute risk in the mid- to long-term does not support strategies to boost CD4+ cell counts, such as switching an NNRTI to a boosted PI or adding CCR5 antagonists or other immunomodifiers (eg, interleukin 2). This should further encourage physicians to define complete viral suppression as the primary goal for their patients who are receiving ART, including those patients who start therapy in advanced stages of immune deficiency.
Participating Centers in the ClinSurv Cohort
Berlin: PD Dr. K. Arastéh, S. Kowol: Auguste-Viktoria-Klinikum; Dr. F. Bergmann, M. Warnke: Charité Campus Virchow; Bochum: Prof. Dr. N. Brockmeyer, N. Mühlbächer: Ruhr Universität Bochum; Bonn: Prof. Dr. J. Rockstroh, Dr. J. Wasmuth, S. Hass: Universitätsklinikum Bonn; Düsseldorf: Dr.S.Reuter,L.Rollmann: Universitätsklinik Düsseldorf; Essen: Dr. St. Esser, P. Schenk-Westkamp: Universitätsklinik Essen; Hamburg: PD Dr. A. Plettenberg, Dr. T. Lorenzen, I. Walther: ifi Institut; Prof. Dr. Stellbrink, PD Dr. Hoffmann, H. Goey, n: IPM Studycenter, Hamburg; PD. Dr. van Lunzen, Dr. Zoufaly, K.Wassmus; Universitätsklinikum Eppendorf; Hannover: Prof. Dr. M. Stoll, S. Gerschmann: Medizinische Hochschule Hannover; Kiel: Prof. Dr. H. Horst: Universitätsklinik Kiel; Köln: Prof. Dr. G. Fätkenheuer, T. Kümmerle, C. Hertenstein: Universitätsklinik Köln; München: Prof. Dr. Bogner, B. Sonntag: Universitätsklinikum München; Regensburg: Prof. Dr. B. Salzberger: Universitätsklinik Regensburg; Rostock: Dr. C. Fritzsche; Universitätsklinik Rostock
Cohort manager: A. Kuehne, Robert Koch Institute, Berlin, Germany
Acknowledgments
Financial support. The ClinSurv study is primarily funded by the Robert Koch-Institute and was partly funded by a grant from the German Federal Ministry of Education and Research (2003–2005), and the German Federal Ministry of Health (1999).
References
- 1.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hughes MD, Daniels MJ, Fischl MA, Kim S, Schooley RT. CD4+ cell count as a surrogate endpoint in HIV clinical trials: a meta-analysis of studies of the AIDS Clinical Trials Group. AIDS. 1998;12:1823–32. doi: 10.1097/00002030-199814000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Battegay M, Fluckiger U, Hirschel B, Furrer H. Late presentation of HIV-infected individuals. Antivir Ther. 2007;12:841–51. [PubMed] [Google Scholar]
- 7.Borghi V, Girardi E, Bellelli S, et al. Late presenters in an HIV surveillance system in Italy during the period 1992-2006. J Acquir Immune Defic Syndr. 2008;49:282–6. doi: 10.1097/QAI.0b013e318186eabc. [DOI] [PubMed] [Google Scholar]
- 8.Wolbers M, Bucher HC, Furrer H, et al. Delayed diagnosis of HIV infection late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Med. 2008;9:397–405. doi: 10.1111/j.1468-1293.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 9.Chadborn TR, Delpech VC, Sabin CA, Sinka K, Evans BG. The late diagnosis consequent short-term mortality of HIV-infected heterosexuals (England and Wales, 2000-2004) AIDS. 2006;20:2371–9. doi: 10.1097/QAD.0b013e32801138f7. [DOI] [PubMed] [Google Scholar]
- 10.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4+ cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 11.Street E, Curtis H, Sabin CA, Monteiro EF, Johnson MA. British HIV Association (BHIVA) national cohort outcomes audit of patients commencing antiretrovirals from naive. HIV Med. 2009;10:337–42. doi: 10.1111/j.1468-1293.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 12.Tan R, Westfall AO, Willig JH, et al. Clinical outcome of HIV-infected antiretroviral-naive patients with discordant immunologic virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:553–8. doi: 10.1097/QAI.0b013e31816856c5. [DOI] [PubMed] [Google Scholar]
- 13.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–61. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renaud M, Katlama C, Mallet A, et al. Determinants of paradoxical CD4+ cell reconstitution after protease inhibitor-containing antiretroviral regimen. AIDS. 1999;13:669–76. doi: 10.1097/00002030-199904160-00007. [DOI] [PubMed] [Google Scholar]
- 15.Group HSMC. Human immunodeficiency virus type 1 RNA level and CD4+ count as prognostic markers and surrogate end points: a meta-analysis. HIV Surrogate Marker Collaborative Group. AIDS Res Hum Retroviruses. 2000;16:1123–33. doi: 10.1089/088922200414965. [DOI] [PubMed] [Google Scholar]
- 16.d'Arminio Monforte A, Testori V, Adorni F, et al. CD4+ cell counts at the third month of HAART may predict clinical failure. AIDS. 1999;13:1669–76. doi: 10.1097/00002030-199909100-00010. [DOI] [PubMed] [Google Scholar]
- 17.AIDS. European Centre for the Epidemiological Monitoring of AIDS. 1993 revision of the European AIDS surveillance case definition. AIDS Surveillance in Europe. Quarterly Report. 1993:23–8. [Google Scholar]
- 18.Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4+ counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–13. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4+ T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. quiz CE1-4. [PubMed] [Google Scholar]
- 21.Levy Y. In: Program and abstracts of the 16th CROI, Montreal, Canada, 8–11 February 2009. Effect of interleukin-2 on clinical outcomes in patients with CD4+ cell count 50 to 299/mm3: Primary results of the SILCAAT study [abstract 90bLB] [Google Scholar]
- 22.Huttner AC, Kaufmann GR, Battegay M, Weber R, Opravil M. Treatment initiation with zidovudine-containing potent antiretroviral therapy impairs CD4+ cell count recovery but not clinical efficacy. AIDS. 2007;21:939–46. doi: 10.1097/QAD.0b013e3280f00fd6. [DOI] [PubMed] [Google Scholar]
- 23.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359:1442–55. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann C, Jung N, Hofmann A, et al. Nucleoside-free boosted double PI regimen: significant CD4+ T-cell recovery in patients with poor immunologic response despite virologic suppression. Curr HIV Res. 2008;6:555–9. doi: 10.2174/157016208786501526. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–55. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 27.D'Egidio GE, Kravcik S, Cooper CL, Cameron DW, Fergusson DA, Angel JB. Pneumocystis jiroveci pneumonia prophylaxis is not required with a CD4+ T-cell count <200 cells/microl when viral replication is suppressed. AIDS. 2007;21:1711–5. doi: 10.1097/QAD.0b013e32826fb6fc. [DOI] [PubMed] [Google Scholar]