Identifying the Early Post-HIV Antibody Seroconversion Period (original) (raw)
Abstract
Background. Identifying persons with recent human immunodeficiency virus (HIV) antibody seroconversion is useful for treatment, research, and prevention, but the sensitivity and specificity of tests for this purpose are uncertain.
Methods. We used longitudinal specimens panels from 155 persons identified prior to HIV seroconversion to assess antibody-based methods for classifying persons as within 30, 60, or 90 days of seroconversion, including 2 incidence assays, a less-sensitive (LS) enzyme immunoassay (EIA), and the BED assay.
Results. Sensitivity and specificity, respectively, for identifying persons within 30 days of seroconversion were: 34%–57% and 98%–100% for 2 standard EIAs (employing a signal-to-cutoff ≤4.0; ≥1.0 defines HIV positive), 84% and 73% for the LS-EIA (≤0.2 cutoff), 88% and 72% for the BED (≤0.2 cutoff), and 43%–58% and 98% (≤3 bands) for 2 Western blot (WB) assays. By area under the receiver operator curves, the best test for identifying persons within 30 days of seroconversion was the number of bands on the Bio-Rad WB (0.90); within 60 days, the LS-EIA and BED (both 0.85); and for persons within 90 days the BED (0.86).
Conclusions. Standard EIAs, Western blots, and HIV incidence assays provide useful information for identifying persons 30 to 90 days after seroconversion.
Human immunodeficiency virus type 1 (HIV-1) antibody tests have been optimized to become positive as soon as possible after infection. Current tests typically become positive within 3–6 weeks of infection and 1–3 weeks after the onset of acute HIV symptoms [1]. While detection of early infection is a strength of these tests, it creates challenges in identifying persons with very early HIV. Individuals who might have been classified as “pre-seroconversion” primary infection cases with older antibody assays are often difficult to distinguish from chronic HIV cases based on results of current antibody tests. Approaches such as defining a specific number of positive bands on Western blot assays as evidence of recent infection have been used in some studies [2, 3]. These approaches have not been rigorously tested, however, to determine the sensitivity and specificity with which they correctly classify persons with recent HIV antibody seroconversion.
Identifying persons who are within weeks of HIV-1 antibody seroconversion is potentially useful for treatment, pathogenesis, and epidemiologic studies, as well as clinical decision-making and HIV prevention. Observational studies have suggested that persons treated within 2 weeks of antibody seroconversion may have sustained CD4+ T-cell count and viral load benefits [4]. Because events within the first months of infection predict the course of infection [5–8], standardized methods of identifying persons with recent HIV seroconversion are potentially important for conducting pathogenesis studies. Identification of very early HIV-1 infection is also useful in studying the characteristics of viral variants responsible for initiating infection, which has potential importance for vaccine design [9]. Finally, persons in the first 2.5 months of HIV infection have been reported to have a 10-fold higher risk of transmission than persons in chronic infection [10]. Methods that could identify persons who are still in early infection—even if seroconversion has already occurred—would allow clinicians to target these individuals (and their partners) for appropriate interventions to reduce the risk of HIV transmission.
We sought to determine the sensitivity and specificity of antibody testing approaches to identify whether persons who are positive on standard antibody tests for HIV-1 have very recently seroconverted. We studied persons for whom longitudinal plasma specimens were available following a well-characterized date of HIV-1 seroconversion. Several widely available clinical antibody tests, as well as tests that are currently available in research laboratories but could readily be performed in clinical laboratories, were evaluated.
METHODS
Subjects and Specimens
We assembled longitudinal specimen panels from persons in the Acute Infection and Early Disease Research Program (AIEDRP) cohort, a multicenter, North American early HIV infection research program. We selected participants in whom HIV antibody testing using standard assays was either negative or indeterminate at initial evaluation, so that dates of antibody seroconversion could be accurately estimated. An HIV-1 viral load ≥5000 copies/mL performed within 7 days after the negative or indeterminate antibody test was used to confirm HIV infection. In addition, 2 or more specimens of stored plasma that met the following criteria were required: (1) specimens were collected before or on the day that antiretroviral therapy was started; (2) specimens were collected within 180 days of the negative or indeterminate antibody test; (3) specimens were obtained ≥1 week apart from other specimens used for the analyses. Subjects in the study were enrolled in protocols approved by the Institutional Review Boards of each of the participating centers.
Measures
Specimens were tested using 2 HIV-1 viral lysate-based enzyme immunoassay (EIA)-antibody tests approved by the US Food and Drug Administration (FDA), the Vironostika HIV-1 (Organon Teknika, OTV), and the Genetics Systems rLAV (Bio-Rad). We evaluated signal-to-cutoff (SCO) ratios above the 1.0 threshold used in clinical diagnostic testing to define a sample as reactive. We performed Western blot testing using 2 assays licensed for clinical diagnosis of HIV-1 infection in the United States, one originally developed by Ortho (now manufactured by Calypte) and the other developed by Genetics Systems (now manufactured by Bio-Rad). We also tested 2 assays that have been designed for HIV incidence studies; these require greater quantities of antibody with higher binding avidity than standard EIAs used for HIV diagnosis. The less sensitive EIA (LS-EIA, also called a detuned EIA) uses a standard EIA kit but increases the plasma or serum dilution and reduces the incubation times; results are reported in standardized optical density (SOD) units [11]. The LS-EIA assay we used was based on the Organon Teknika OTV EIA kit. The second incidence assay we evaluated was the BED assay developed by the Centers for Disease Control and Prevention (CDC). This is an EIA that uses a branched peptide that includes sequences from HIV-1 subtypes B, E, and D and detects increasing proportions of anti-HIV immunoglobulin G (IgG) relative to total IgG [12]. The results of the BED assay are expressed as Normalized Optical Density (NOD) units. For all assays, specimen identity was coded and laboratories were blinded to other data on seroconversion dates.
Analysis
Analyses were based on time from the estimated date of HIV-antibody seroconversion on conventional antibody testing. For persons with an indeterminate antibody test, we determined that the median number of days between an indeterminate and first positive antibody test in our population was 14; we took the midpoint (7 days) and added this to the date of an indeterminate antibody test to estimate the seroconversion date. For persons with an initial negative antibody test/positive HIV RNA assay but no indeterminate antibody test, the seroconversion date was taken as the midpoint point between the last negative and first positive antibody tests if the interval between the tests was <28 days, and 14 days after the negative antibody test if the difference was >28 days.
As there is not a specific length of time since seroconversion that can be clearly defined as the most important duration to measure, we considered 3 time windows: within 30 days, 60 days, or 90 days of conventional antibody seroconversion. For each time window, we defined sensitivity as the proportion of specimens obtained within the specified time period with results below a specified test threshold, and specificity as the proportion of specimens obtained after the specified time period with results above the threshold. For example, in testing a cutoff of 5 or fewer bands on Western blot testing to identify persons with 30 days from seroconversion, a specimen with 4 bands was considered a true positive for early infection if it was obtained <30 days from seroconversion, and a false positive if it was obtained >30 days from seroconversion. These definitions differ substantially from those used to diagnose HIV infection; a false positive interpretation means results potentially consistent with a short period of time from infection in someone with a longer duration of infection, rather than the absence of HIV infection. Maximum likelihood estimates of sensitivity and specificity were obtained using a generalized linear model for the binomial family with a logit link function, that is, a logistic regression. Analysis was performed using R software version 2.5.0 for Windows. To account for the correlation between observations obtained on the same individual over time, empirical (sandwich) standard errors were calculated in R using the sandwich package [13, 14] and substituted for model-based standard errors and used in combination with the delta method to calculate standard errors and 95% confidence intervals for sensitivity and specificity estimates. In cases where none or all of the observations were below the specified cutoff value for the outcome, exact binomial 95% confidence intervals were calculated. Positive and negative likelihood ratios (LR) with 95% confidence intervals were also calculated for different threshold values for each assay in defining the state of recent infection at each time cutoff. We used standard formulas to calculate post-test probabilities based on pre-test probabilities and likelihood ratios [15]. To assess the accuracy of each test for the diagnosis of <30, <60, or <90 days of HIV infection, test performance was modeled by estimating post-test probabilities across a range of plausible pre-test probabilities of having recent HIV infection using Bayes theorem.
Data on symptoms of acute HIV infection were collected using a standardized questionnaire [16]. For analysis of the duration of time between onset of acute HIV symptoms and seroconversion, we excluded symptoms that were present for >45 days to remove potentially chronic symptoms.
This study was performed by the AIEDRP, which was funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the United States. NIAID staff provided input into the design of network data collection, which was used for this study, and reviewed the final manuscript without suggesting changes. Testing was performed for the Genetics Systems rLAV (Bio-Rad) EIA assay and the Western blot assays by the manufacturers at no charge. The BED assay was performed at CDC laboratories at no charge. Participating laboratories were not involved in data analysis.
RESULTS
The 155 participants included in this study were 90% male, and most were infected with HIV through sex with other men (Table 1). Because of specimen availability, the numbers of observations for certain tests varied. For both the Bio-Rad rLAV EIA and the OTV EIA, there were 351 observations on 139 individuals, for the LS-EIA assay 337 observations on 134 individuals, and for the BED-EIA 397 observations on 149 individuals. For Western blot assays, there were 343 observations on 142 individuals.
Table 1.
Participant Characteristics
| Characteristic | n = 155 |
|---|---|
| Male (%) | 90.3 |
| Race/ethnicity (%) | |
| Black | 10.9 |
| White | 87.7 |
| Asian | 1.4 |
| Age, median years (range) | 35 (15–58) |
| Route of HIV exposure (%) | |
| Injection drug use (No MSM Risk) | 4.9 |
| MSM only (No IDU) | 75.5 |
| MSM and IDU | 2.8 |
| Heterosexual | 8.4 |
| Missing all | 7.7 |
| Median days from onset of symptoms to seroconversion (interquartile range, n = 95) | 18 (12–26) |
| EIA antibody negative at initial specimen (%) | |
| rLAV (n = 155) (%) | 23 (14.9) |
| OTV (n = 154) (%) | 33 (21.4) |
| Initial HIV-1 RNA, median log10 copies/mL (interquartile range) | 5.35 (4.43, 5.87) |
| Initial CD4+ T cells, median cells/μL (interquartile range) | 513 (399, 643) |
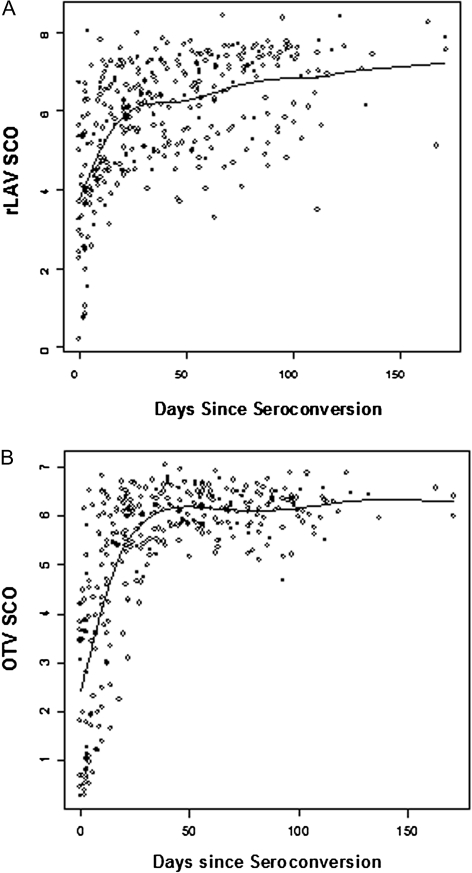
When results are reported from standard antibody testing, EIA results are usually reported as “negative” or “positive.” However, it is also possible to analyze the ratio of the signal provided by the specimen to the cutoff for a positive assay (the SCO ratio) (Figure 1 and Table 2). Ratios above the value of 1 used to determine a positive EIA antibody test provided very high specificity for classifying persons as being within 30 days of seroconversion, at or near 100% for SCO ratios up to 4. However, sensitivity for such identification with this cutoff was only 57% for the OTV EIA and 34% for the rLAV EIA.
Figure 1.
Enzyme immunoassay (EIA) results by days from seroconversion. Each point represents the signal-to-cutoff (SCO) ratios from the rLAV (A) or OTV (B) EIA assays by estimated days from seroconversion. Results >1 are considered positive for clinical diagnostic purposes. There is a rapid increase in SCO ratios following seroconversion, with results <4 being highly specific for persons <30 days from seroconversion.
Table 2.
Sensitivity and Specificity of Antibody Tests for Early Seroconversion Time Periods
| < 30 days | < 60 days | < 90 days | ||||
|---|---|---|---|---|---|---|
| Test, cutoff | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity |
| OTV EIA, SCO ≤4 | .57 (.47, 67) | 1.0 (.96, 1.0) | .38 (.33, .45) | 1.0 (.94, 1.0) | .31 (.22, .41) | 1.0 (.92, 1.0) |
| rLAV EIA, SCO ≤4 | .34 (.26, .43) | .98 (.95, .99) | .24 (.18, .31) | .98 (.94, .99) | .19 (.14, .26) | .98 (.88, 1.0) |
| LS-EIA, SOD ≤0.2 | .84 (.75, .90) | .73 (.64, .80) | .68 (.59, .75) | .82 (.72, .89) | .57 (.49, .65) | .88 (.77, .95) |
| LS-EIA, SOD ≤0.5 | .91 (.82, .95) | .45 (.36, .54) | .84 (.76, .89) | .57 (.45, .68) | .77 (.70, .84) | .75 (.60, .86) |
| BED, NOD ≤ 0.2 | .88 (.80, .92) | .72 (.63, .79) | .74 (.67, .80) | .84 (.74, .90) | .88 (.80, .92) | .72 (.63, .79) |
| BED, NOD ≤0.5 | .97 (.91, .99) | .29 (.21, .38) | .93 (.88, .97) | .54 (.39, .68) | .89 (.84, .93) | .54 (.39, .68) |
| Bio-Rad WB, ≤3 bands | .43 (.32, .54) | .98 (.96, 1.0) | .30 (.22, .39) | .98 (.94, 1.0) | .23 (.17, .31) | .96 (.86, .99) |
| Ortho WB, ≤3 bands | .58 (.46, .68) | .98 (.93, .99) | .40 (.31, .50) | .99 (.93, 1.0) | .32 (.23, .43) | 1.0 (91, 1.0) |
| Bio-Rad WB, ≤6 bands | .79 (.70, .86) | .86 (.79, .91) | .61 (.52, .70) | .94 (.85, .97) | .50 (.41, .58) | .94 (.84, .98) |
| Ortho WB, ≤6 bands | .86 (.79, .92) | .68 (.57, .77) | .74 (.65, .82) | .79 (.67, .88) | .65 (.56, .73) | .91 (.78, .97) |
Compared to the standard EIA assays, the incidence tests were more sensitive for identification of recent seroconverters, as would be expected (Table 2). For example, a cutoff of 0.2 OD on the LS-OTV had a sensitivity of 84% for identifying an individual within 30 days of seroconversion, with a specificity of 73%; the BED assay had quite similar results using a cutoff of 0.2 NOD. A higher cutoff on both tests (eg, 0.5) had the best sensitivity of any of the assays tested for classifying persons as being within 90 days of seroconversion. Both of these tests had only moderate specificity, however, for a window period of 90 days post-seroconversion, with the BED being particularly weak, a specificity of 54% using a cutoff of 0.5 NOD. A more extensive list of sensitivity, specificity, and likelihood ratios for different values of each of these tests can be found in accompanying Supplementary tables.
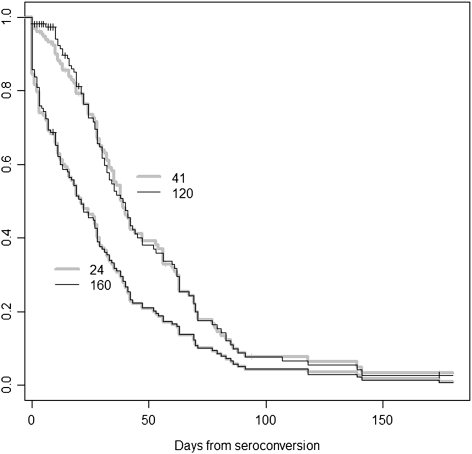
The number of positive Western blots bands was useful in classifying duration of infection. For identifying persons within 30 days of antibody seroconversion, having 3 or fewer positive bands on the Bio-Rad Western blot had a sensitivity of 43% and a specificity of 98%, whereas the same criterion on the Ortho Western blot had a sensitivity of 58% and a specificity of 98% (Table 2). Having 6 or fewer bands was still fairly specific for the early post-seroconversion period, while having substantially greater sensitivity for recent seroconversion than a cutoff of 3 or fewer bands. Although some Western blot bands turned positive rapidly, there was variability between different bands, with the median times to a positive band ranging from 21 to 64 days (Table 3). Bands such as p24 and gp160 were among the earliest bands to turn positive (Figure 2). There was some variability between equivalent bands between Western blot kits, probably due to differences in the reagents. The largest difference was in the median days to a positive p55 band, 21 days on the Bio-Rad Western blot, and 64 days on the Ortho Western blot. The key bands of p24 and gp160 (2 of the 3 bands used for determining a positive Western blot) had nearly identical median times to become positive.
Table 3.
Median Time From Estimated Date of Seroconversion to Positive Western Blot Bands
| Band | Median days | 95% CI | IQR |
|---|---|---|---|
| Bio-Rad Western blot | |||
| p18 | 41 | (35, 53) | (28,70) |
| p24 | 21 | (16, 28) | (2, 42) |
| p31 | 41 | (34, 47) | (22, 63) |
| gp41 | 39 | (34, 47) | (24, 67) |
| p55 | 21 | (15, 28) | (4, 42) |
| p65 | 47 | (39, 60) | (28, 71) |
| gp120 | 39 | (32, 47) | (24, 67) |
| gp160 | 21 | (16, 28) | (5, 42) |
| Ortho Western blot | |||
| p17 | 22 | (20, 32) | (12,39) |
| p24 | 22 | (20, 33) | (12, 40) |
| p31 | 63 | (46, 76) | (32, 82) |
| gp41 | 34 | (31, 40) | (22, 56) |
| p55 | 64 | (54, 90) | (35, 171) |
| p66 | 36 | (33, 47) | (23, 62) |
| gp120 | 31 | (23, 36) | (17, 54) |
| gp160 | 22 | (20, 32) | (12, 39) |
Figure 2.
Survival curve for conversion of Western blot bands to positive on the Bio-Rad Western blot. Patterns of time from seroconversion to development of a positive band on Western blot vary by band. On the Bio-Rad Western blot, p24 (24 on the figure) and gp 160 (160) had very similar patterns of time to positive results but usually became positive earlier than gp 41 (41) and gp 120 (120).
We used receiver-operator curves (ROC) to assess the overall performance of different tests for identifying persons within the various time periods following seroconversion (Table 4). The Bio-Rad Western blot employing the ≤3 band criteria had the greatest ROC area (0.90) for identifying persons within 30 days of seroconversion. While Western blot tests also performed well for other time periods, the LS-EIA and the BED assay performed slightly better for longer time intervals.
Table 4.
Receiver-Operator Curve Areas Under the Curve (AUC) for Accuracy of Antibody Tests in Identifying the Early Post-seroconversion Period by Various Time Periods Postseroconversion
| Test | AUC (SE) | 95% CI |
|---|---|---|
| AUC for <30 Days | ||
| Ortho SCO | 0.883 (0.021) | (0.84, 0.92) |
| Bio-Rad SCO | 0.820 (0.028) | (0.76, 0.87) |
| LS-EIA SOD | 0.865 (0.023) | (0.81, 0.90) |
| BED NOD | 0.873 (0.023) | (0.83, 0.92) |
| Bio-Rad WB bands | 0.901 (0.019) | (0.86, 0.93) |
| Ortho WB bands | 0.864 (0.028) | (0.80, 0.91) |
| AUC for <60 Days | ||
| Test | AUC (SE) | 95% CI |
| LS-EIA SOD | 0.845 (0.025) | (0.80, 0.89) |
| BED NOD | 0.845 (0.026) | (0.79, 0.90) |
| Bio-Rad WB bands | 0.833 (0.025) | (0.78, 0.88) |
| Ortho WB bands | 0.809 (0.029) | (0.75, 0.86) |
| AUC for <90 Days | ||
| Test | AUC (SE) | 95% CI |
| LS-EIA SOD | 0.838 (0.025) | (0.80, 0.89) |
| BED NOD | 0.861 (0.023) | (0.81, 0.91) |
| Bio-Rad WB bands | 0.769 (0.028) | (0.71, 0.82) |
| Ortho WB bands | 0.829 (0.032) | (0.76, 0.89) |
Positive and negative likelihood ratios (LR) with 95% confidence intervals were also calculated for various assay results (see online-only tables). These allowed us to calculate the post-test probabilities that would be associated with similar test performance in a population with different pre-test probabilities of having early HIV infection (Table 5). In many clinical and research settings, patients have known dates of prior negative HIV antibody tests. These can be used to estimate the pre-test probability of recent HIV infection. For example, an individual who currently tests HIV antibody positive and last had a negative HIV antibody test 1 year ago would have a pre-test probability of 8% of being within 30 days of antibody seroconversion based on date information alone, assuming random distributions of infection and testing over the year interval. If there were 3 or fewer bands on a Western blot, the post-test probability of being within 30 days of antibody seroconversion would be 74% (using the Bio-Rad Western blot); the post-test probability of being within 90 days of seroconversion would be essentially 100%.
Table 5.
Estimated Post-test Probability of Recent HIV Seroconversion for Newly HIV+ Individuals Testing Annually for HIV Given Specific Results on Antibody Tests
| Estimated post-test probability of recent seroconversion within specific time intervals in an annual testing population | ||||
|---|---|---|---|---|
| Assay | Test result | Infection <30 days (pre-test prob. = .08)a | Infection <60 days (pre-test prob. = .17)a | Infection <90 days (pre-test prob. = .25)a |
| LS-EIA | SOD=0.1 | 0.33 | 0.73 | 0.79 |
| 0.2 | 0.29 | 0.55 | 0.73 | |
| 0.3 | 0.22 | 0.43 | 0.62 | |
| BED | NOD=0.1 | 0.48 | 0.64 | 0.72 |
| 0.2 | 0.22 | 0.48 | 0.79 | |
| 0.3 | 0.15 | 0.32 | 0.52 | |
| Ortho WB | Bands =3 | 0.71 | 0.89 | ∼1.00b |
| 5 | 0.33 | 0.64 | 0.88 | |
| 6 | 0.20 | 0.42 | 0.71 | |
| Bio-Rad WB | Bands =3 | 0.74 | 0.79 | ∼1.00b |
| 5 | 0.47 | 0.76 | 0.88 | |
| 6 | 0.35 | 0.66 | 0.71 |
DISCUSSION
We compared several antibody-based approaches to identify persons in the early HIV post-seroconversion period. A key finding was that Western blot assays are quite accurate for identifying persons with recent HIV seroconversion. Our data suggest that simple criteria based on the total number of positive bands on the Western blot can have good-to-excellent diagnostic performance for identifying individuals with early HIV infection. Two different Western blot assays both yielded very high specificity for identifying persons within 30 days of seroconversion with the simple criterion of having 3 or fewer bands, and for persons within 90 days of seroconversion with the criterion of having 6 or fewer bands. The significance of these findings is high because Western blot tests are widely used as confirmatory tests for HIV infection. Thus, data that can help identify persons in the early post-seroconversion period is already available to many clinicians. Similar results were recently reported from Switzerland based on an immunoblot assay (INNO-LIA HIV-I/II SCORE, Innogenetics) that is not available in the United States [17].
On the 2 standard EIA assays we examined, an SCO value of <4 had high specificity for seroconversion within the past 30 days. This value, although not always reported to clinicians, can be obtained from testing laboratories. The rapid increase in reactivity on these assays, however, results in poor sensitivity. The threshold of an SCO of 4 is exceeded within days to 2 weeks of seroconversion, and nearly half of persons classified as within the first 30 days following seroconversion had already exceeded this cutoff using the OTV EIA. While results were similar between the 2 standard EIAs we tested, the OTV EIA was slightly more sensitive and more specific for the early post-seroconversion period.
In contrast to the Western blots and EIAs, the LS-EIA and BED incidence assays performed best when detecting persons within longer time windows after seroconversion. These assays had the highest sensitivity for identifying persons within 90 days of seroconversion. To achieve reasonable specificity for this time window required using cutoffs that were significantly lower than those used in incidence studies for identifying persons within longer periods (eg, 180 days) since seroconversion. Although the BED was slightly more sensitive than the LS-EIA for detecting persons within 90 days of seroconversion, it suffered from limited specificity. When the pre-test probability of recent infection is low, this can result in substantial false positive results due to misclassification of chronically infected persons as having just seroconverted. While our study was not designed to assess the specificity of these assays for classifying persons within windows beyond 90 days after seroconversion, other studies have noted limitations in the specificity of these assays in certain settings using higher cutoffs for incidence studies, including false positive results in persons with advanced HIV disease [18]. These assays are research tests that are not FDA approved and are unavailable in many settings, but they are inexpensive and can potentially be performed in laboratories equipped to perform HIV EIA antibody testing.
Besides potential applications in research settings and epidemiologic studies, estimates of duration of HIV infection based on data available from standard HIV diagnostic tests may be useful for prevention purposes. The first few months of HIV infection are a particularly infectious period [10]. In a macaque model of Simian Immunodeficiency Virus transmission, the viral load needed to transmit infection was >50 times higher when using plasma from chronically infected macaques compared to plasma from acutely infected macaques, and further experiments suggested that lack of neutralizing antibodies may have been a factor in the higher infectivity of plasma from acutely infected macaques [19]. If these findings apply to HIV infection in humans, having information to identify recently infected persons may be of particular importance in order to focus HIV prevention efforts on those most likely to transmit HIV.
HIV antibody levels can wane in advanced disease, and this can result in erroneous classifications of such individuals as recently infected using tests we assessed. For this reason, the assays we tested should typically not be applied to persons with clinical AIDS or a CD4+ T-cell count <200 cells/μL. Although we did not test persons with chronic HIV infection, there are prior studies that have addressed this issue for the assays we tested. In a study that evaluated 199 subjects with a CD4+ T-cell count <200 cells/μL, 96.5% had at least 6 of 9 Western blot bands present [20]. In a second study of persons with chronic HIV infection but without clinical AIDS (WHO stage 2 and 3), 701 of 704 patients (99.8%) had 6 or more bands on Western blot testing, and 100% had 5 or more bands [21]. These findings provide reassurance that the specificity of criteria such as having 6 or fewer bands on Western blot for identifying persons in early HIV infection should be very high when applied to persons with chronic infection. In a review of incidence assays, the median specificity from 11 estimates in longstanding infection was 98% using higher cutoffs than those used in the current study [18]. Specificity using the much lower cutoffs we employed should be considerably higher.
There are additional limitations to this study. First, by studying only specimens that were close to the time cutoffs being evaluated, it is likely that we underestimated both the specificity and sensitivity of assays for early infection in contexts in which the majority of newly diagnosed persons are infected for longer periods of time, and hence recent and longstanding infections would be easier to distinguish. Second, our results suggest that while results are generally similar across assays such as standard EIA and Western blots, there were differences between specific assays. Thus, caution must be used in extrapolating the present data to other test kits. In addition, interpretation of the Western blot entails some subjective decisions when the bands are faint. It is possible that the Western blot results we obtained from an expert research laboratory might be more carefully interpreted and hence more consistent than the results obtained from standard clinical laboratories. Third, as we did not have daily blood samples, we estimated exact seroconversion dates, and there is some imprecision in the estimates, even if only by a matter of days. Fourth, our patient population consisted primarily of men who have sex with men with sub-type B infection. It is possible that evolution of antibody responses might differ in heterosexual or injection drug use transmission, although we are not aware of data that indicate such a difference. As some of the antibody tests we used were based on sub-type B antigens, results might also differ using these assays with other HIV subtypes. Finally, one of the incidence assays we evaluated (the LS-EIA based on the OTV EIA) is no longer available. New iterations of the LS-EIA test (based on kits manufactured by Avioq) have been developed, validated, and provide similar results, although there may be some differences that could alter diagnostic performance for the applications we describe.
Despite these limitations, our results suggest that if interpreted carefully, information from standard EIA and Western blot assays, and from so-called incidence assays such as the BED and LS-EIA tests, can be useful to identify persons who are in the early post-seroconversion period of HIV infection. The approaches outlined here may be useful for clinical, research, and prevention purposes that benefit from identifying very recently HIV-infected individuals.
Supplementary Data
Supplementary Data are available at The Journal of Infectious Diseases online.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institute of Health (NIH) Acute Infection Early Disease Research Program (AI041531, AI 041535, AI 041535, AI 041535, AI 041536, AI 041536, AI 041536, AI041534, AI041534, AI 041534, AI041534, AI043638, and AI043638). Additional support for the data collected in this study came from NIH grants (R01 AI055343, P01 AI57005, AI071713, and AI74621); Centers for AIDS Research (CFAR) grants (AI027763, AI27767, and AI27757); General Clinical Research Center (GCRC) grant (5M01-RR00032-38); Rockefeller University General Clinical Research Center (M01-RR00102); the State of California’s University-wide AIDS Research Program (IS02-SD-701); and by Fonds de la recherche en santé du Québec, FRSQ, réseau SIDA et maladies infectieuses.
Supplementary Material
Supplementary Data
Acknowledgments
The authors wish to thank the patients who participated in this study and the study staffs at each of the sites who conducted the enrollment and follow-up data collection that made this study possible. We appreciate testing performed at Bio-Rad (coordinated by Christopher Benson), Ortho (coordinated by Steve Alexander), and testing at the Centers for Disease Control and Prevention (CDC) of the BED assay. Mila Lebedeva performed much of the testing done at Blood System Research Institute (LS-EIA and OTV assays), and Brandee Pappalardo helped to coordinate overall specimen testing.
References
- 1.Lindback S, Thorstensson R, Karlsson AC, et al. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. Karolinska Institute Primary HIV Infection Study Group. AIDS. 2000;14:2333–9. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz M, Vaida F, Hare CB, et al. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J Infect Dis. 2010;201:1298–302. doi: 10.1086/651664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg E, Altfeld M, Poon S, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 4.Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–33. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 5.Blattner WA, Ann Oursler K, Cleghorn F, et al. Rapid clearance of virus after acute HIV-1 infection: correlates of risk of AIDS. J Infect Dis. 2004;189:1793–801. doi: 10.1086/386306. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 7.Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J Acquir Immune Defic Syndr. 2007;45:445–8. doi: 10.1097/QAI.0b013e318074ef6e. [DOI] [PubMed] [Google Scholar]
- 8.Mellors JW, Kingsley LA, Rinaldo CR, Jr, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 11.Kothe D, Byers RH, Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33:625–34. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 12.Parekh BS, Kennedy MS, Dobbs T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18:295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 13.Zeileis A. Econometric computing with HC and HAC covariance matrix estimators. J Stat Softw. 2004;11:1–17. [Google Scholar]
- 14.Zeileis A. Object-oriented computation of sandwich Estimators. J Stat Softw. 2006;16:1–16. [Google Scholar]
- 15.Sackett DL, Sackett DL. Clinical epidemiology: a basic science for clinical medicine. 2nd ed. Boston: Little, Brown; 1991. [Google Scholar]
- 16.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 17.Schupbach J, Gebhardt MD, Tomasik Z, et al. Assessment of recent HIV-1 infection by a line immunoassay for HIV-1/2 confirmation. PLoS Med. 2007;4:e343. doi: 10.1371/journal.pmed.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy R, Gold J, Calleja JM, et al. Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect Dis. 2009;9:747–59. doi: 10.1016/S1473-3099(09)70300-7. [DOI] [PubMed] [Google Scholar]
- 19.Ma ZM, Stone M, Piatak M, Jr., et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83:3288–97. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson JB, Parsons JS, Nichols LS, Knoble N, Kennedy S, Piwowar EM. Detection of human immunodeficiency virus type 1 (HIV-1) antibody by western blotting and HIV-1 DNA by PCR in patients with AIDS. J Clin Microbiol. 1997;35:1118–21. doi: 10.1128/jcm.35.5.1118-1121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudha T, Lakshmi V, Teja VD. Western blot profile in HIV infection. Indian J Dermatol Venereol Leprol. 2006;72:357–60. doi: 10.4103/0378-6323.27752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data