A Partially Folded Structure of Amyloid-Beta(1-40) in an Aqueous Environment (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 29.
Published in final edited form as: Biochem Biophys Res Commun. 2011 Jun 25;411(2):312–316. doi: 10.1016/j.bbrc.2011.06.133
Abstract
Aggregation of the Aβ1-40 peptide is linked to the development of extracellular plaques characteristic of Alzheimer’s disease. While previous studies commonly show the Aβ1-40 is largely unstructured in solution, we show that Aβ1-40 can adopt a compact, partially folded structure. In this structure, the central hydrophobic region of the peptide forms a 310 helix from H13 to D23 and the N- and C-termini collapse against the helix due to the clustering of hydrophobic residues. Helical intermediates have been predicted to be crucial on-pathway intermediates in amyloid fibrillogenesis, and the structure presented here presents a new target for investigation of early events in Aβ1-40 fibrillogenesis.
Keywords: Structure, Amyloid-beta, Alzheimer’s, Disordered protein, Misfolding, Amyloidogenesis
INTRODUCTION
Alzheimer’s disease is a common neurodegenerative disorder characterized morphologically by the formation of extracellular clumps of aggregated proteins and symptomatically by severe cognitive decline. The cause of Alzheimer’s is unknown, however, the ubiquity of aggregated forms of the Aβ peptide in Alzheimer’s patients and the apparent cytoxicity of aggregated forms of these proteins has led to a theory, the amyloid cascade hypothesis, linking the misfolding of the Aβ peptide to the ultimate upstream cause of Alzheimer’s disease.[1] The amyloid cascade hypothesis is supported by a strong but circumstantial evidence linking aggregated forms of the Aβ peptide to neuronal cell death, either directly through calcium dysregulation,[2] oxidative stress,[3] or inflammation[4] or indirectly through causing the hyperphosphorylation and aggregation of the tau protein.[5; 6] Regardless of the exact mechanism, the cytotoxicity of Aβ has been definitively linked to its aggregation, as the monomeric form has little apparent toxicity.[7]
To stop the formation of toxic aggregated Aβ, it is necessary to first understand the molecular forces that drive aggregation. Structural models have been constructed for the final amyloid product of aggregation.[8; 9; 10] However, structural information for earlier species along the aggregation pathway remains elusive. Models for earlier species are essential, as kinetic studies have implicated a partially folded, non β-sheet, intermediate as being essential for aggregation.[11; 12] Although individual studies have probed the conformational preferences of the Aβ1-40 monomer,[13; 14; 15; 16; 17] a high-resolution structure of Aβ1-40 in aqueous solution, in the absence of organic solvents that can alter the energetics of folding, has not been available. The absence of a high-resolution structure has made understanding of the starting point of aggregation difficult. Towards this end, we have solved the high-resolution structure of Aβ1-40 at 15°C at pH 7.3 with 50 mM NaCl. Under this condition Aβ1-40 adopts a folded conformation notably different from that seen in most previous NMR experiments. [18; 19]
MATERIALS AND METHODS
Sample preparation
Aβ1-40 was purchased from Anaspec (Fremont, CA). To break up preformed aggregates in the sample, the peptide was first dissolved in 10% NH4OH and lyophilized. The lyophilized product was then dissolved to 77 μM concentration in 20 mM potassium phosphate buffer with 50 mM NaCl at pH 7.3 using a 93% H2O/7% D2O solution. The buffer was initially chilled to 4°C before adding to the peptide and the sample was kept in ice for three hours before the start of the NMR experiments.
NMR spectroscopy and structure calculations
All data were acquired on a 900 MHz Bruker NMR spectrometer equipped with a cryogenic triple-resonance pulse-field gradient probe. 2D NOESY and TOCSY spectra were acquired at 288 K in phase-sensitive mode using time-proportional phase incrementation (TPPI) for quadrature detection in the t1 dimension.[20; 21] For each spectrum, 32 transients were collected using 128 dummy scans with spectral widths of 13 KHz in both dimensions, using 2048 complex points for F2 and 512 complex points for F1. All chemical shifts were measured from two-dimensional TOCSY spectra referenced to the internal DSS signal at 0.00 ppm. The solvent signal was suppressed using pulse-field gradients. NOESY data were collected with a mixing time of 300 ms to derive 1H-1H distance constraints.
Spectra were processed using standard Bruker software (TOPSPIN, version 2.1) and analyzed with SPARKY. Data were zero filled to 2048 points in both F1 and F2 without linear prediction and then Fourier transformed with a sine-bell-squared window function shifted between π/2 and π/4. A polynomial baseline correction was applied to both sides of the residual water signal. Sequence specific back-bone resonance assignments and side-chain assignments for all residues were obtained using a combination of 2D TOCSY and NOESY experiments as described elsewhere.[22] The NOE cross-peak assignments were obtained by an iterative procedure using a combination of manual and automatic approaches. Stereo-specific assignments were not obtained due to the degeneracy of proton chemical shifts. Upper distance limit constraints for the structure calculation were obtained from the NOESY spectrum and backbone dihedral angle restraints for residues located in locally well defined helices were derived from the observed chemical shifts by using the TALOS program.
RESULTS
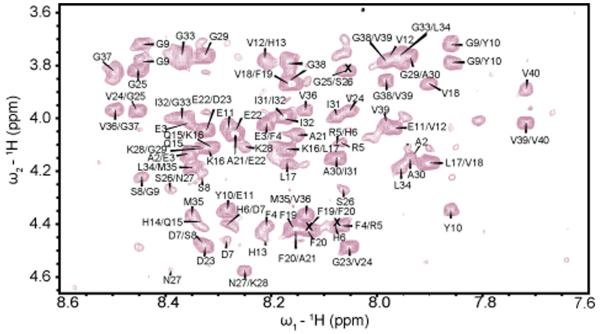
The structure of Aβ1-40 was determined using solution NMR through 2D 1H-1H TOCSY and NOESY data collected as mentioned in the Materials and Methods section. In an analysis of different experimental conditions, it a temperature of 15°C was found to be optimal as spectra acquired at higher temperatures suffered from poor signal intensity and resolution. Figure 1 shows the Hα-NH region of the NOESY spectrum. The presence of numerous well-resolved cross-peaks in the NOESY spectra along with the significant degree of spectral dispersion in the amide region is a strong indication that the peptide is at least partially folded, as a completely disordered structure would have both poor chemical shift dispersion due to the similarity of chemical shift values in the random coil state and poor NOE cross-peak intensity due to the high mobility of the structure.
Figure 1.
Hα-NH region of a 2D-NOESY spectrum (300 ms, 900 MHz) of Aβ1-40 in 20 mM potassium phosphate, 50 mM NaCl, pH 7.3 at 288 K. Only the NOEs corresponding to sequential assignment H_αi_-N_Hi+1_ are labeled.
Continuous stretches of dNN(i,i+1), _d_αN(i,i+3), and _d_αN(i,i+2) medium rangeNOE connectivities indicative of the i,i+3 hydrogen bond formation suggest the presence of either α- or 310 helices in the central region of the peptide (H13 to D23) (Fig. 2A). The negative chemical shift index values in this region of the peptide further confirms the presence of a helical structure (Fig. 2B).[23] The particular NOE pattern observed argues for the existence of a 310 rather than an α- helix (Fig. 2A), as the existence of _d_αN(i,i+2) NOEs is consistent with the tighter coiling of the 310 helix compared to the α-helix.[23] Similarly, _d_αN(i,i+4) interactions expected to occur in an α-helix were not observed in the NOESY spectrum (Fig. 2A).[23] Therefore, residues H13 to D23 most likely exist in a 310 helical conformation as seen in the structure of the peptide in Figure 3; however some conformational exchange between 310 helix and other conformations in this region cannot be ruled out.[24]
Figure 2.
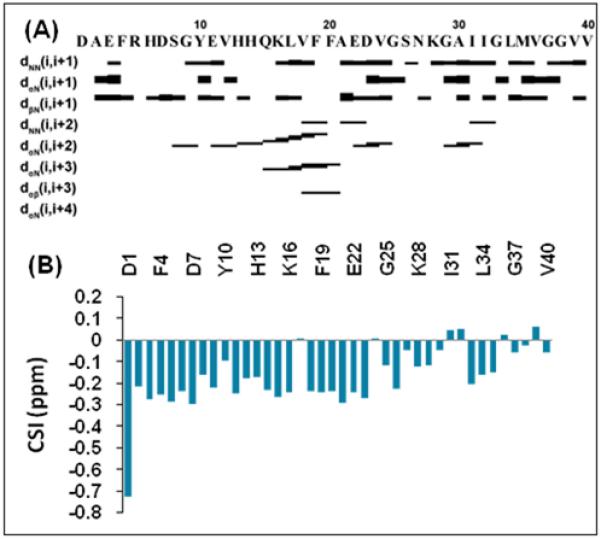
(A) NOE connectivity plot showing the formation of a 310 helix for residues H13 to D23 of Aβ1-40. The strengths of sequential NOEs are indicated by the height of the bars, graded into categories of strong, medium, or weak. (B) Deviations from Hα random coil chemical shift values showing the propensity of Aβ1-40 to form a helix.
Figure 3.
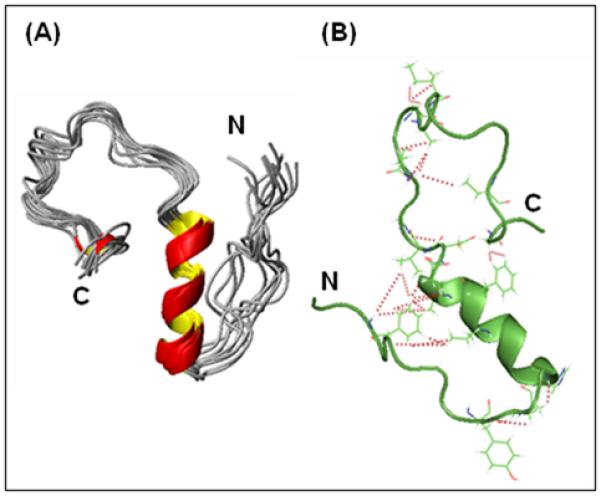
(A) High-resolution NMR structures of Aβ1-40at 15 °C at pH 7.3 in 50 mM NaCl. (B) A cartoon plot showing the long-range NOEs stabilizing the formation of the hairpin structure and the bends in the N- and C-termini (red lines).
From the NOE constraints collected it was possible to construct a 3D structure of the peptide (shown in Fig. 3A). The statistics of the structure determination of Aβ1-40 are summarized in Table S1. A total of 489 intramolecular NOE distance constraints, including 26 long-range (i,i+>4) NOEs, were determined for the structure calculations. In addition, 29 dihedral constraints were derived from chemical shift data using the TALOS program and an additional 25 conformational constraints were obtained from the NOE patterns observed in the NOESY spectrum. None of the 20 structures have any violated NOE distance or dihedral-angle constraint. Most of the residues (78.9%) were found in the most favored regions of the Ramachandran plot. The root-mean-square (r.m.s.) deviation of 20 superposition of structures in the well ordered region between 13th and 23rd residues is 0.18±0.08 Å for the backbone and 0.72±0.08 Å for all heavy atoms.
The formation of the helix in the central region of the peptide clusters hydrophobic residues together, creating hydrophobic surfaces on both sides of the helix. The generation of this surface facilitates the interaction of hydrophobic residues in the unstructured N-and C-termini with the central hydrophobic core. This interaction is reflected in several long-range NOEs that connect both termini to the central helix (see Fig. 3B for a cartoon depiction, a complete list can be found in Table S2 in the Supporting Information). In particular, several long-range NOEs were observed from F4 to most of the residues in the middle of the helical structured region of Aβ1-40 peptide (the aromatic region of the NOESY spectra is shown in Fig. 4). For example, Hβ and the aromatic ring protons of residue F4 show NOE cross-peaks with V18 Hγ as well as many medium to weak NOEs to the side-chains of A21, E22 and V24. Together these NOEs constrain the N-terminus in a hairpin conformation with the central helix. The packing of the hydrophobic phenyl ring of F4 against the hydrophobic side chains of V18 and A21in the central helix is likely to be responsible for this constraint. Hydrogen bonds from the side-chain of Q15 on the central helix to exposed carbonyls in the N-terminal coil that are observed in many of the structures also likely contribute to the packing of the N-terminus against the helix. In the C-terminus, the hairpin conformation is constrained to lie against the other side of the C-terminal helix by long-range NOEs between the aromatic ring protons of F19 to Hα of G38. Similar NOEs from the C-terminus to central hydrophobic core of Aβ1-40 have been detected in other NMR studies[13] and MD simulations.[25; 26] NOEs in the interior of the hairpin, in particular from Hδ of N27 to the side-chain of V36 further confirms a hairpin conformation. Like the N-terminus, hydrophobic clustering appears to be responsible for this interaction. In this case, it is the clustering of F19 with the hydrophobic residues at the C-terminus that appears to be responsible for the collapse of the C-terminus against the central helix. The interaction of the C-terminus with the central hydrophobic core would presumably be stronger in Aβ1-42, which possesses two additional hydrophobic residues at the C-terminus (IA), in agreement with fluorescence measurements.[27]
Figure 4.
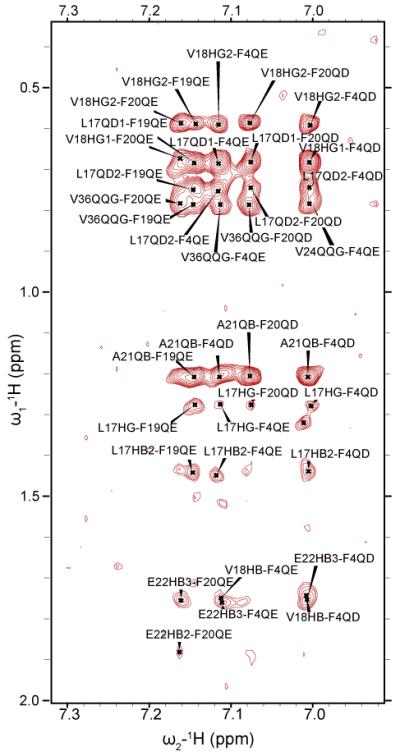
The aromatic region of a 2D-NOESY spectrum showing crosspeaks between F19 and F20 and the C-terminus and F4 and the central helix.
Although both the N- and C-termini lack stable secondary structure, they are not completely unstructured, as both contain many twists and turns caused by local interactions between side-chains after the end of helical secondary structure. The chemical shift index plot shows substantial negative deviations from random coil values from D1 to G29, as well as negative deviations in the G33-M35 region (Fig. 2B). While negative deviations in the chemical shift from the corresponding random coil values are consistent with the formation of helical structure, the absence of corresponding dαN(i,i+3) and dαβ(i,i+3) NOEs reflective of an α- or 310 helix suggests the formation of transient, unstable secondary structure, as has been previously observed for Aβ1-40 and the similarly unstructured amyloidogenic IAPP peptide.[24; 28] The presence of bends or turns in the peptide chain are further supported by several (i,i+2) NOEs. In the N-terminal region, two (i,i+2) NOEs are consistent with turn-like structures: from Y10-Hβ to V12-Hγ and from V12-Hγ to H14-Hα. At the C-terminus, (i,i+2) NOEs between D23-Hα and G25-H NOEs from G29 Hα to the side-chain, and NOEs due to side-chain to side-chain interactions between N27-NH2 to the methyl protons of A30 produce bends in the largely unstructured region near the C-terminus.
DISCUSSION
The structure of Aβ1-40 reported here differs substantially from previously reported NMR studies of Aβ1-40 and Aβ1-42 in solution. Although the exact features of the conformation of the Aβ1-40 monomer differ on the sample conditions employed, previous NMR studies are in general agreement that the monomer is primarily unstructured with a small amount of fluctuating residual structure,[18; 19] the composition of which changes as the temperature is altered.[24; 29] An exception is a solution study of the 10-35 fragment of Aβ at pH 5.7, which found that the peptide adopted a compact collapsed coil structure with well-defined loops and turns but without regular repeating secondary structure.[13] Similar compact states have been observed for other amyloidogenic proteins,[30; 31] in agreement with the theory that natively unfolded amyloidogenic proteins occupy a premolten globule state.[12] The central hydrophobic core has a tendency to adopt turn or bend-like structures from Phe20-Ser26,[18] in agreement with the finding that this region is protease resistant.[19] Outside the central hydrophobic core, the C-terminus is more extended than would be expected from a statistical random coil[15; 16; 17] and relaxation experiments indicate the N-terminus is weakly structured at low temperatures.[14] Similarly, molecular dynamics simulations predict a partially structured monomeric state of Aβ1-40.[25; 26; 32] Overall, both experiment and simulation are consistent with monomeric Aβ1-40 existing in an ensemble of rapidly converting, nearly isoenergetic conformations, the exact proportions of which depend on the experimental conditions.
Notably, previous NMR studies did not, in general, detect medium range NOEs reflective of stable secondary structure or long-range NOEs reflective of a tertiary structure.[19] By contrast, under our experimental conditions Aβ1-40 adopts a well-defined three-dimensional structure, with a stable helical structure evident in the central hydrophobic core and contacts of the N- and C-termini with the central helix. Because the potential energy landscape of Aβ1-40 is flat with small differences in energy separating individual conformers in the ensemble,[26] an apparently small perturbation in sample conditions can translate to large differences in the average structure. Variations in pH (most NMR experiments on Aβ have been performed at acidic pH),[33] temperature,[24; 29] and ionic strength[34] can all strongly effect the final conformation adopted by Aβ1-40. Of particular interest here is the ionic strength of the sample, as most of the NMR studies performed on the Aβ peptide (with some notable exceptions)[34; 35; 36] were run in the absence of salt. Salt can change the conformational preferences of proteins, as the exclusion of Cl− anions from exposed hydrophobic surfaces (Hofmeister effect) is thermodynamically unfavorable. Minimization of these surfaces can be accomplished either through compaction of the protein or by the formation of a secondary structure, which is reflected in our structure by the collapse of the hydrophobic portions of the N and C- termini against the central hydrophobic core and the formation of the central helix. Narayanan et al has showed that inclusion of salt has a complicated effect on the conformational preferences and aggregation of the Aβ1-40 peptide, shifting low molecular weight species from unstructured to more helical conformations and decreasing the amount of primarily β-strand high molecular weight species in equilibrium with the monomer to a degree reflective of the anion’s position in the Hofmeister series.[34] In particular, at 50 mM NaCl, the condition employed here, they observe a CD spectra with a high degree of helical content, similar to that observed in our spectra.[34] The partially helical structure presented here is also consistent with structures of Aβ1-40 and Aβ1-42 in organic cosolvents, which are expected to reveal underlying conformational tendencies of the peptide. In all structures with organic solvents (TFE or HFIP), the central hydrophobic core (17LVFFA21) of Aβ1-40 and Aβ1-42 forms a helix similar to that observed in our structure,[32] suggesting this region is the most favorable for helix formation in the absence of organic cosolvents. As the concentration of organic solvent is increased, the central helix extends further on the C-terminal side and an additional helix is formed near the C-terminus, suggesting the C-terminus can form loop like structures but that the propensity for such is lower than in the central hydrophobic core.[32] Overall, the structure is consistent with predictions of conformations of Aβ1-40 that are expected to be energetically close to the disordered state.[32]
An α-helical conformation has also been detected as a transient on-pathway intermediate during amyloid formation.[11; 37] The chemical shift distribution strongly argues that the majority of the NMR visible low-molecular weight species is in a partially helical conformation (Fig. 2B). However, it cannot be excluded that some of the NOEs, particularly the weak long-range NOEs, actually represent transfer NOEs from a high molecular weight, NMR invisible, Aβ1-40 oligomer, [34; 38] as the Aβ1-40 peptide is not stable long enough under the conditions employed here for a DOSY-NOESY experiment to separate contributions from individual aggregation states. While details of the conformational states are expected to vary depending on the experimental conditions, the availability of a high-resolution structure is expected to yield insights into the early conformational transitions that drive the misfolding of the Aβ1-40 peptide.
Supplementary Material
01
Research highlights.
- Amyloid beta (1-40) is partially folded at 15 °C, pH 7.3 in 50 mM NaCl.
- Amyloid beta (1-40) forms a 310 helix from H13 to D23.
- N- and C-termini are unstructured but collapse against the helix.
- Low temperature and moderate salt conformations may favor helical conformations of amyloid beta (1-40).
ACKNOWLEDGMENTS
This study was supported by research funds from NIH (DK078885 to A. R.). We thank Dr. Shivani Ahuja for help with NMR measurements, and the 900 MHz NMR facility at the Michigan State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ABBREVIATIONS: Aβ, amyloid-β peptide; DSS, 4,4-dimethyl-4-silapentane-1-sulfonic acid; NOE, Nuclear Overhauser Effect; MD, molecular dynamics; TOCSY, Total Correlation Spectroscopy; NOESY, Nuclear Overhauser Effect Spectroscopy; NMR, Nuclear Magnetic Resonance.
SUPPORTING INFORMATION AVAILABLE: Table of structural statistics and a list of long-range NOEs.
REFERENCES
- [1].Hardy J, Allsop D. Amyloid deposition as the central event in the etiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- [2].Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J. Biol. Chem. 2010;285:12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim. Biophys. Acta. 1768;2007:1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- [4].Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ott S, Henkel AW, Henkel MK, Redzic ZB, Kornhuber J, Wiltfang J. Pre-aggregated Aβ1-42 peptide increases tau aggregation and hyperphosphorylation after short-term application. Mol. Cell. Biochem. 2011;349:169–177. doi: 10.1007/s11010-010-0671-7. [DOI] [PubMed] [Google Scholar]
- [6].Fein JA, Sokolow S, Miller CA, Vinters HV, Yang FS, Cole GM, Gylys KH. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am. J. Pathol. 2008;172:1683–1692. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shankar GM, Li SM, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paravastu AK, Petkova AT, Tycko R. Polymorphic fibril formation by residues 10-40 of the Alzheimer’s beta-amyloid peptide. Biophys. J. 2006;90:4618–4629. doi: 10.1529/biophysj.105.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Petkova AT, Yau WM, Tycko R. Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Doeli H, Schubert D, Riek R. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fezoui Y, Teplow DB. Kinetic studies of amyloid beta-protein fibril assembly - differential effects of alpha-helix stabilization. J. Biol. Chem. 2002;277:36948–36954. doi: 10.1074/jbc.M204168200. [DOI] [PubMed] [Google Scholar]
- [12].Uversky VN, Fink AL. Conformational constraints for amyloid fibrillation: The importance of being unfolded. Biochim. Biophys. Acta. 2004;1698:131–53. doi: 10.1016/j.bbapap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- [13].Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, Lu Y, Felix AM, Maggio JE, Lee JP. The Alzheimer’s peptide a beta adopts a collapsed coil structure in water. J. Struct. Biol. 2000;130:130–141. doi: 10.1006/jsbi.2000.4288. [DOI] [PubMed] [Google Scholar]
- [14].Lim KH. A weakly clustered n terminus inhibits a beta(1-40) amyloidogenesis. ChemBioChem. 2006;7:1662–1666. doi: 10.1002/cbic.200600270. [DOI] [PubMed] [Google Scholar]
- [15].Lim KH, Henderson GL, Jha A, Louhivuori M. Structural, dynamic properties of key residues in a beta amyloidogenesis: Implications of an important role of nanosecond timescale dynamics. ChemBioChem. 2007;8:1251–1254. doi: 10.1002/cbic.200700194. [DOI] [PubMed] [Google Scholar]
- [16].Yan YL, Wang CY. Aβ42 is more rigid than Aβ40 at the c terminus: Implications for Aβ aggregation and toxicity. J. Mol. Biol. 2006;364:853–862. doi: 10.1016/j.jmb.2006.09.046. [DOI] [PubMed] [Google Scholar]
- [17].Yan YL, Liu JJ, McCallum SA, Yang DW, Wang CY. Methyl dynamics of the amyloid-beta peptides Aβ40 and Aβ42. Biochem. Biophys. Res. Commun. 2007;362:410–414. doi: 10.1016/j.bbrc.2007.07.198. [DOI] [PubMed] [Google Scholar]
- [18].Hou LM, Shao HY, Zhang YB, Li H, Menon NK, Neuhaus EB, Brewer JM, Byeon IJL, Ray DG, Vitek MP, Iwashita T, Makula RA, Przybyla AB, Zagorski MG. Solution NMR studies of the Aβ1-40 and Aβ(1-42) peptides establish that the Met35 oxidation state affects the mechanism of amyloid formation. J. Am. Chem. Soc. 2004;126:1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- [19].Lazo ND, Grant MA, Condron MC, Rigby AC, Teplow DB. On the nucleation of amyloid beta-protein monomer folding. Protein Sci. 2005;14:1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- [21].Sklenar V, Piotto M, Leppik R, Saudek V. Gradient-tailored water suppression for H-1-N-15 HSQC experiments optimized to retain full sensitivity. Journal of Magnetic Resonance Series A. 1993;102:241–245. [Google Scholar]
- [22].Wuthrich K, editor. NMR of proteins and nucleic acids. John Wiley and Sons; New York: 1986. [Google Scholar]
- [23].Wagner G, Neuhaus D, Worgotter E, Vasak M, Kagi JHR, Wuthrich K. Nuclear-magnetic-resonance identification of half-turn and 310-helix secondary structure in rabbit liver metallothionein-2. J. Mol. Biol. 1986;187:131–135. doi: 10.1016/0022-2836(86)90413-4. [DOI] [PubMed] [Google Scholar]
- [24].Danielsson J, Jarvet J, Damberg P, Graslund A. The Alzheimer beta-peptide shows temperature-dependent transitions between left-handed 3(10)-helix, beta-strand and random coil secondary structures. FEBS J. 2005;272:3938–3949. doi: 10.1111/j.1742-4658.2005.04812.x. [DOI] [PubMed] [Google Scholar]
- [25].Vitalis A, Caflisch A. Micelle-like architecture of the monomer ensemble of Alzheimer’s amyloid-beta peptide in aqueous solution and its implications for a beta aggregation. J. Mol. Biol. 2010;403:148–165. doi: 10.1016/j.jmb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [26].Yang MF, Teplow DB. Amyloid beta-protein monomer folding: Free-energy surfaces reveal alloform-specific differences. J. Mol. Biol. 2008;384:450–464. doi: 10.1016/j.jmb.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maji SK, Amsden JJ, Rothschild KJ, Condron MM, Teplow DB. Conformational dynamics of amyloid beta-protein assembly probed using intrinsic fluorescence. Biochemistry. 2005;44:13365–13376. doi: 10.1021/bi0508284. [DOI] [PubMed] [Google Scholar]
- [28].Yonemoto IT, Kroon GJ, Dyson HJ, Balch WE, Kelly JW. Amylin proprotein processing generates progressively more amyloidogenic peptides that initially sample the helical state. Biochemistry. 2008;47:9900–9910. doi: 10.1021/bi800828u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lim KH, Collver HH, Le YTH, Nagchowdhuri P, Kenney JM. Characterizations of distinct amyloidogenic conformations of the Aβ(1-40) and (1-42) peptides. Biochem. Biophys. Res. Commun. 2007;353:443–449. doi: 10.1016/j.bbrc.2006.12.043. [DOI] [PubMed] [Google Scholar]
- [30].Soong R, Brender JR, Macdonald PM, Ramamoorthy A. Association of highly compact type ii diabetes related islet amyloid polypeptide intermediate species at physiological temperature revealed by diffusion NMR spectroscopy. J. Am. Chem. Soc. 2009;131:7079–7085. doi: 10.1021/ja900285z. [DOI] [PubMed] [Google Scholar]
- [31].Vaiana SM, Best RB, Yau WM, Eaton WA, Hofrichter J. Evidence for a partially structured state of the amylin monomer. Biophys. J. 2009;97:2948–2957. doi: 10.1016/j.bpj.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tomaselli S, Esposito V, Vangone P, van Nuland NAJ, Bonvin A, Guerrini R, Tancredi T, Temussi PA, Picone D. The alpha-to-beta conformational transition of Alzheimer’s Aβ-(1-42) peptide in aqueous media is reversible: A step by step conformational analysis suggests the location of beta conformation seeding. ChemBioChem. 2006;7:257–267. doi: 10.1002/cbic.200500223. [DOI] [PubMed] [Google Scholar]
- [33].Barrow CJ, Yasuda A, Kenny PTM, Zagorski MG. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimers-disease - analysis of circular-dichroism spectra. J. Mol. Biol. 1992;225:1075–1093. doi: 10.1016/0022-2836(92)90106-t. [DOI] [PubMed] [Google Scholar]
- [34].Narayanan S, Reif B. Characterization of chemical exchange between soluble and aggregated states of beta-amyloid by solution-state NMR upon variation of salt conditions. Biochemistry. 2005;44:1444–52. doi: 10.1021/bi048264b. [DOI] [PubMed] [Google Scholar]
- [35].Jarvet J, Damberg P, Bodell K, Eriksson LEG, Graslund A. Reversible random coil to beta-sheet transition and the early stage of aggregation of the a beta(12-28) fragment from the Alzheimer peptide. J. Am. Chem. Soc. 2000;122:4261–4268. [Google Scholar]
- [36].Yamaguchi T, Matsuzaki K, Hoshino M. Transient formation of intermediate conformational states of amyloid-beta peptide revealed by heteronuclear magnetic resonance spectroscopy. FEBS Lett. 2011;585:1097–1102. doi: 10.1016/j.febslet.2011.03.014. [DOI] [PubMed] [Google Scholar]
- [37].Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J. Mol. Biol. 2001;312:1103–1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- [38].Fawzi NL, Ying J, Torchia DA, Clore GM. Kinetics of amyloid beta monomer-to-oligomer exchange by NMR relaxation. J. Am. Chem. Soc. 2010;132:9948–9951. doi: 10.1021/ja1048253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01