Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study (original) (raw)
Abstract
Objective To examine short and long term time trends in mortality among patients with early onset (age 0-14 years) and late onset (15-29 years) type 1 diabetes and causes of deaths over time.
Design Population based nationwide cohort study.
Setting Finland.
Participants All Finnish patients diagnosed as having type 1 diabetes below age 30 years between 1970 and 1999 (n=17 306).
Main outcome measures Crude mortality, standardised mortality ratios, time trends, and cumulative mortality.
Results A total of 1338 deaths occurred during 370 733 person years of follow-up, giving an all cause mortality rate of 361/100 000 person years. The standardised mortality ratio was 3.6 in the early onset cohort and 2.8 in the late onset cohort. Women had higher standardised mortality ratios than did men in both cohorts (5.5 v 3.0 in the early onset cohort; 3.6 v 2.6 in the late onset cohort). The standardised mortality ratio at 20 years’ duration of diabetes in the early onset cohort decreased from 3.5 in the patients diagnosed in 1970-4 to 1.9 in those diagnosed in 1985-9. In contrast, the standardised mortality ratio in the late onset cohort increased from 1.4 in those diagnosed in 1970-4 to 2.9 in those diagnosed in 1985-9. Mortality due to chronic complications of diabetes decreased with time in the early onset cohort but not in the late onset cohort. Mortality due to alcohol related and drug related causes increased in the late onset cohort and accounted for 39% of the deaths during the first 20 years of diabetes. Accordingly, mortality due to acute diabetic complications increased significantly in the late onset cohort.
Conclusion Survival of people with early onset type 1 diabetes has improved over time, whereas survival of people with late onset type 1 diabetes has deteriorated since the 1980s. Alcohol has become an important cause of death in patients with type 1 diabetes, and the proportion of deaths caused by acute complications of diabetes has increased in patients with late onset type 1 diabetes.
Introduction
Despite great advances in diabetes care, type 1 diabetes is still associated with considerable premature mortality resulting from both acute and chronic complications of diabetes. Most of the excess late mortality after a disease duration of 20 years has been related to renal complications and cardiovascular disease,1 2 3 and early mortality has mainly been related to acute complications such as diabetic ketoacidosis.4
The age at onset of diabetes may influence the risk of late complications of diabetes. In particular, patients with onset of diabetes after age 15 have been observed to have a lower risk of diabetic nephropathy and end stage renal disease than do patients diagnosed during adolescence.5 6 7 Better long term survival could thus be expected in people diagnosed as having diabetes after adolescence. However, most of the studies of mortality have been done in patients with onset of diabetes in childhood or the age at onset has not been the focus of the analyses.
The aim of this study was therefore to investigate short term and long term time trends in mortality in patients diagnosed as having early onset (age 0-14 years) and late onset (15-29 years) type 1 diabetes in Finland, the country with the world’s highest incidence of type 1 diabetes.8 9 We also studied the causes of death to identify reasons for potential changes in mortality during the first 20 years’ duration of diabetes, as well as the effect of age at onset and duration of disease on mortality.
Methods
We identified 17 306 people diagnosed as having type 1 diabetes below 30 years of age between 1970 and 1999 from the drug reimbursement register. The diagnosis of type 1 diabetes has been verified in most (74%) of the patients in previous studies.1 2 9 10 11 All Finnish citizens with certain chronic diseases such as type 1 diabetes are entitled to full reimbursement of drug costs. Because insulin has been free of charge for patients with diabetes in Finland since 1964, all patients with permanent entitlement to free insulin are entered in the drug reimbursement register. To become eligible for the special reimbursement for the specified diseases, a patient must send an application with a certificate from a physician giving the details of the diagnosis and the treatment for the disease.
The classification of the type of diabetes in the rest of the patients with diabetes (26%) followed the same procedure as was used in a previously published study.11 Briefly, we linked the data on the patients with permanent entitlement for insulin to the hospital discharge register maintained by the National Institute for Health and Welfare. The hospital discharge register has covered all hospital discharges nationwide since 1972 and includes up to four discharge diagnoses (international classification of diseases (ICD) codes) of patients who have been admitted to a hospital. We excluded cases in the hospital discharge register with a code indicating possible secondary diabetes before the diagnosis of diabetes. Such codes were for certain genetic syndromes, diseases of the exocrine pancreas, or endocrinopathies. The codes in the hospital discharge register had to be 2500B-2508B (ICD-9) or E10.0-E10.9 or O24.0 (ICD-10) to define the patient as having type 1 diabetes.
Data on vital status and causes of deaths came from the Finnish cause of death register. We used the data from the hospital discharge register and also reviewed the death certificates to verify causes of death and contributing conditions.
Statistical methods
Follow-up started from diagnosis of diabetes and ended at death or at the end of 2007. We stratified patients into six groups by year of diagnosis: 1970-4, 1975-9, 1980-4, 1985-9, 1990-4, and 1995-9. We calculated both crude and standardised mortality ratios for the entire follow-up period, as well as at 20 years’ duration of diabetes. We calculated standardised mortality ratios for all patients but also separately for the early onset and late onset cohorts. We calculated the expected number of deaths by multiplying the number of person years at risk by 1 year age specific, calendar year specific, and sex specific mortality rates in the Finnish background population drawn from the human mortality database (www.mortality.org/). We split the data into time at risk for each sex, 1 year age, and calendar year group.
We evaluated the non-linear effect of age at onset and duration of diabetes by fitting generalised additive models. We did time trend evaluation of the standardised mortality ratios by using Poisson regression modelling with the Proc Genmod and incorporated the non-linear terms identified in the generalised additive modelling in the parametric models.
We calculated cause specific standardised mortality ratios covering the first 20 years’ duration of diabetes for deaths due to ischaemic heart disease (ICD codes 410-414 or I20-I25), cerebrovascular disease (430-438 or I60-I69 or G45), and suicide (E95 or X60-X84) on the basis of the underlying cause of death for the entire follow-up period. We evaluated standardised mortality ratios for alcohol related deaths (291, 303, 3575A, 4255A, 5353A, 5710–5713, 5770D–F, 5771C–D, E24.4, F10, F19, G31.2, G62.1, G72.1, I42.6, K29.2, K70, K85.2, K86.0, Q86.0, X45, X46, X65, X66, Y15, Y16) for the years between 1998 and 2007, as the corresponding data in the background population were available only for these years. For the calculation of mortality rates for alcohol related events, we used deaths with a reference to alcohol as an underlying or contributory cause of death. We used SAS version 9.2 for all analyses.
Results
All cause mortality
The study included 17 306 Finnish patients diagnosed as having type 1 diabetes below the age of 30 years in 1970-99, of whom 10 492 were in the early onset cohort and 6814 were in the late onset cohort. The mean length of follow-up was 21.4 years, and the maximum was 37.9 years.
A total of 1338 deaths occurred during 370 733 person years of follow-up, giving an all cause mortality rate of 361 (95% confidence interval 342 to 382) per 100 000 person years. Five hundred and forty-one of the patients who died were from the early onset cohort, and 797 were from the late onset cohort. The crude mortality rate was higher in the late onset cohort than in the early onset cohort: 531 per 100 000 person years versus 245 per 100 000 person years. However, excess mortality was lower in the late onset cohort when compared with the mortality in the background population. The standardised mortality ratio was 3.6 (95% confidence interval 3.3 to 3.9) in the early onset cohort and 2.8 (2.6 to 3.0) in the late onset cohort. The corresponding standardised mortality ratios during the first 20 years’ duration of diabetes were 3.1 (2.7 to 3.4) and 2.3 (2.1 to 2.6).
The crude mortality was higher in men in both the early onset cohort (245 per 100 000 person years) and the late onset cohort (638 per 100 000 person years). For women, it was 197 per 100 000 person years in the early onset cohort and 353 per 100 000 person years in the late onset cohort. Although the crude mortality was higher in men, women had higher standardised mortality ratios: 5.5 (4.8 to 6.3) in the early onset cohort and 3.6 (3.3 to 3.9) in the late onset cohort, compared with 3.0 (2.7 to 3.4) and 2.6 (2.4 to 2.8) for men.
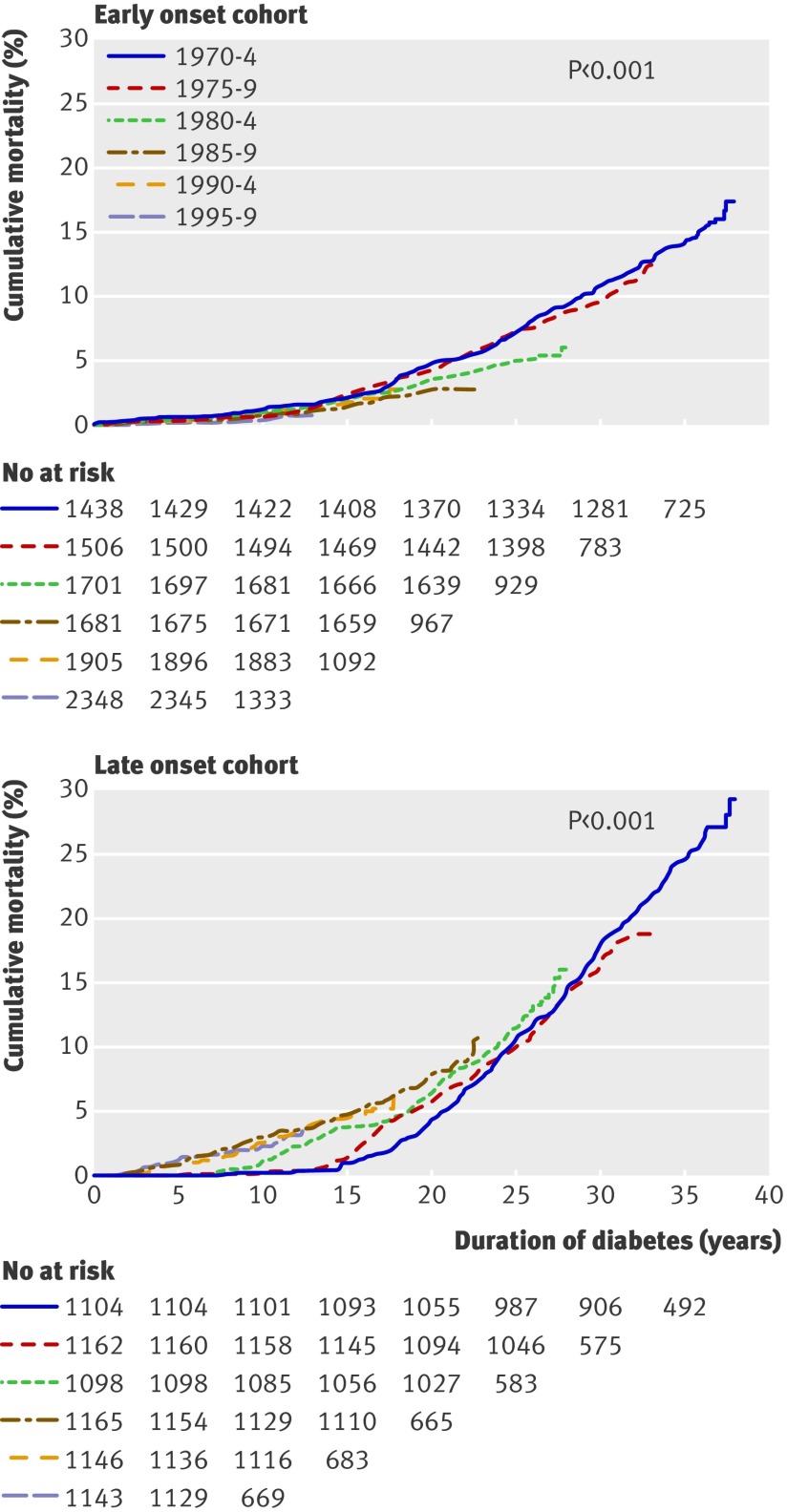
Kaplan-Meier analysis indicated that the overall cumulative mortality at 35 years’ duration of diabetes was 17.9% (95% confidence interval 17.0% to 18.8%). In the early onset cohort, the 20 year cumulative mortality in the groups diagnosed in 1970-4, 1975-9, 1980-4, and 1985-9 was 4.7% (3.7% to 5.8%), 4.3% (3.3% to 5.2%), 3.6% (2.8% to 4.5%), and 2.7% (1.9% to 3.4%) (fig 1, top). However, the follow-up time of the last group had not yet reached 20 years.
Fig 1 Cumulative mortality in patients in Finland with type 1 diabetes diagnosed between 1970 and 1999: early onset cohort (0-14 years) (top); late onset cohort (15-29 years) (bottom)
In contrast, in the late onset cohort, the cumulative mortality increased with time of diagnosis such that patients diagnosed after 1980 had a significantly higher short term mortality than did those diagnosed between 1970 and 1979 (fig 1, bottom). The 20 year cumulative mortality in the groups diagnosed in 1970-4, 1975-9, 1980-4, and 1985-9 was 4.4% (3.3% to 5.6%), 5.9% (4.6% to 7.1%), 6.5% (5.1% to 7.8%), and 7.9% (6.4% to 9.4%).
In the early onset cohort, the standardised mortality ratio decreased by 4.3% (2.8% to 5.8%) per year of diagnosis, after adjustment for duration and age at onset of diabetes. The standardised mortality ratio decreased from 4.3 (3.7 to 4.9) to 1.7 (0.9 to 2.9) (table 1). In the late onset cohort, the increase was 3.7% (2.4% to 5.0%) and the standardised mortality ratio ranged between 2.5 and 3.0. During the first 20 years’ duration in the early onset cohort, the standardised mortality ratio decreased from 3.5 to 1.9, and in the late onset cohort it increased from 1.4 to 2.9 (table 2).
Table 1.
All cause standardised mortality ratios (SMRs) in early onset type 1 diabetes cohort (0-14 years) and late onset type 1 diabetes cohort (15-29 years) diagnosed between 1970 and 1999 and followed from 1970 to 2007
| Diagnosis cohort | Follow-up range (period) | Follow-up range (years) | Age range (years) | Mortality/100 000 | Observed No of deaths | Person years | Expected No of deaths | SMR (95% CI) | Risk ratio* | P value for risk ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Early onset | ||||||||||
| All | 1970-2007 | 0-37 | 0-51 | 245 | 541 | 220 862 | 148.8 | 3.6 (3.3 to 3.9) | ||
| Men | 1970-2007 | 0-37 | 0-51 | 286 | 341 | 119 080 | 112.8 | 3.0 (2.7 to 3.4) | 1.00 | – |
| Women | 1970-2007 | 0-37 | 0-51 | 197 | 200 | 101 781 | 36.0 | 5.5 (4.8 to 6.3) | 1.33 | 0.002 |
| Subgroup†: | ||||||||||
| 1970-4 | 1970-2007 | 0-37 | 0-51 | 441 | 214 | 48 544 | 50.3 | 4.3 (3.7 to 4.9) | 1.00 | – |
| 1975-9 | 1975-2007 | 0-32 | 0-46 | 348 | 154 | 44 287 | 35.6 | 4.3 (3.7 to 5.1) | 0.90 | NS |
| 1980-4 | 1980-2007 | 0-27 | 0-41 | 200 | 82 | 40 940 | 26.4 | 3.1 (2.5 to 3.8) | 0.59 | <0.001 |
| 1985-9 | 1985-2007 | 0-22 | 0-36 | 128 | 43 | 33 580 | 17.8 | 2.4 (1.7 to 3.2) | 0.52 | <0.001 |
| 1990-4 | 1990-2007 | 0-17 | 0-31 | 124 | 36 | 29 103 | 11.5 | 3.1 (2.2 to 4.3) | 0.93 | NS |
| 1995-9 | 1995-2007 | 0-12 | 0-26 | 49 | 12 | 24 407 | 7.1 | 1.7 (0.9 to 2.9) | 0.17 | <0.001 |
| Late onset | ||||||||||
| All | 1970-2007 | 0-37 | 15-66 | 531 | 796 | 149 904 | 282.6 | 2.8 (2.6 to 3.0) | ||
| Men | 1970-2007 | 0-37 | 15-66 | 638 | 597 | 93 559 | 229.6 | 2.6 (2.4 to 2.8) | – | |
| Women | 1970-2007 | 0-37 | 15-66 | 353 | 199 | 56 345 | 53.1 | 3.7 (3.2 to 4.3) | 1.39 | <0.001 |
| Subgroup†: | ||||||||||
| 1970-4 | 1970-2007 | 0-37 | 15-66 | 764 | 279 | 36 511 | 94.7 | 2.9 (2.5 to 3.2) | 1.00 | – |
| 1975-9 | 1975-2007 | 0-32 | 15-61 | 585 | 198 | 33 840 | 78.7 | 2.5 (2.0 to 2.9) | 0.90 | NS |
| 1980-4 | 1980-2007 | 0-27 | 15-56 | 506 | 137 | 27 051 | 47.6 | 2.9 (2.4 to 3.4) | 1.17 | NS |
| 1985-9 | 1985-2007 | 0-22 | 15-51 | 414 | 96 | 23 172 | 32.5 | 3.0 (2.4 to 3.6) | 1.48 | 0.003 |
| 1990-4 | 1990-2007 | 0-17 | 15-46 | 320 | 56 | 17 492 | 18.5 | 3.0 (2.3 to 3.9) | 2.00 | <0.001 |
| 1995-9 | 1995-2007 | 0-12 | 15-41 | 253 | 30 | 11 839 | 10.7 | 2.8 (1.9 to 3.9) | 2.59 | <0.001 |
Table 2.
All cause standardised mortality ratios (SMRs) at 20 years’ duration of diabetes in early onset type 1 diabetes cohort (0-14 years) and late onset type 1 diabetes cohort (15-29 years) diagnosed between 1970 and 1989
| Diagnosis subgroup | Follow-up range (period) | Follow-up range (years) | Age range (years) | Mortality/100 000 | Observed No of deaths | Person years | Expected No of deaths | SMR (95% CI) | Risk ratio* | P value for risk ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Early onset† | ||||||||||
| 1970-4 | 1970-94 | 0-20 | 0-34 | 240 | 68 | 28 346 | 19.6 | 3.5 (2.7 to 4.4) | 1.00 | – |
| 1975-9 | 1975-99 | 0-20 | 0-34 | 216 | 64 | 29 696 | 18.9 | 3.4 (2.6 to 4.3) | 0.81 | NS |
| 1980-4 | 1979-2004 | 0-20 | 0-34 | 176 | 57 | 32 373 | 18.7 | 3.0 (2.3 to 3.9) | 0.64 | 0.02 |
| 1985-9 | 1985-07 | 0-20 | 0-34 | 99 | 19 | 19 219 | 10.1 | 1.9 (1.2 to 2.9) | 0.36 | <0.003 |
| Late onset† | ||||||||||
| 1970-4 | 1970-94 | 0-20 | 15-49 | 223 | 49 | 21 924 | 35.1 | 1.4 (1.0 to 1.8) | 1.00 | – |
| 1975-9 | 1975-99 | 0-20 | 15-49 | 296 | 68 | 22 961 | 37.4 | 1.8 (1.4 to 2.3) | 1.32 | NS |
| 1980-4 | 1979-2004 | 0-20 | 15-49 | 330 | 71 | 21 546 | 31.8 | 2.2 (1.8 to 2.8) | 1.68 | 0.006 |
| 1985-9 | 1985-2007 | 0-20 | 15-49 | 408 | 57 | 13 966 | 19.8 | 2.9 (2.2 to 3.7) | 2.23 | <0.001 |
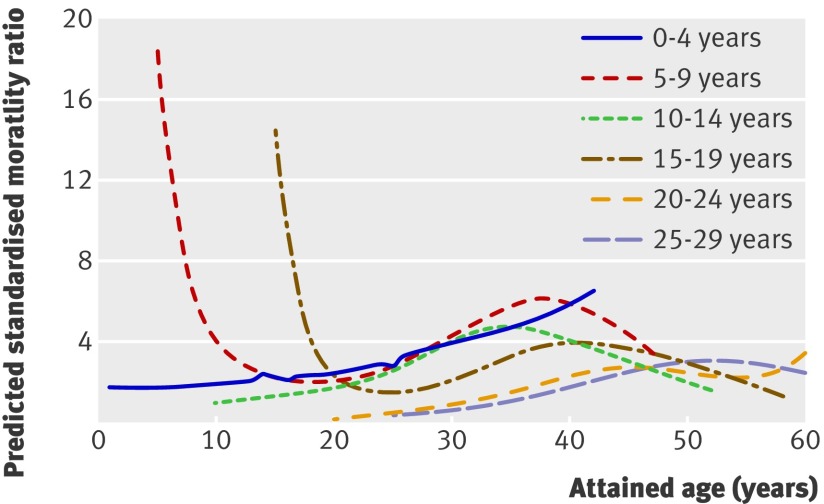
Figure 2 shows the standardised mortality ratios by attained age according to 5 year groups of age at onset. Interestingly, the younger the age at onset of diabetes the longer the standardised mortality ratios took to reach a peak. In general, the peak in the standardised mortality ratio occurred when the duration of diabetes was between 20 and 30 years. The only exception was for age at onset under 5 years, with a linear increase until 40 years of age despite more than 35 years’ duration. Mortality at or shortly after diagnosis in the groups with age at onset of 5-9 and 15-19 years showed extreme standardised mortality ratios before the age of 20 years. However, this might simply be a result of very low mortality in the background population at that young age. When the age at onset was 20 years or more, the standardised mortality ratio was much lower than that in the young age at onset groups. The average standardised mortality ratios by age at onset of diabetes were 3.1 (2.3 to 4.0), 4.0 (3.4 to 4.6), 3.6 (3.2 to 4.1), 3.2 (2.9 to 3.7), 2.4 (2.1 to 2.8), and 2.7 (2.5 to 3.0) for the groups with age at onset of 0-4, 5-9, 10-14, 15-19, 20-24, and 25-29 years.
Fig 2 Predicted standardised mortality ratios according to 5 year age at onset groups
Cause specific mortality
Mortality due to chronic complications of diabetes declined from 10.0 (5.8 to 13.1) per 10 000 person years in patients who were diagnosed as having diabetes in 1970-4 to 2.2 (0.9 to 4.5) per 10 000 person years in those diagnosed in 1985-9 (P for trend <0.001) in the early onset cohort (table 3). In the late onset cohort, the mortality did not decrease with year of diagnosis; the corresponding rates were 13.0 (8.6 to 18.7) per 10 000 person years and 10.5 (6.7 to 15.7) per 10 000 person years. Mortality due to acute complications of diabetes (non-alcohol related) showed a non-significant tendency to increase in the early onset cohort from 2.1 (0.8 to 4.6) per 10 000 person years to 4.1 (1.9 to 6.5) per 10 000 person years, and acute diabetic complications accounted for 29% of the deaths in those diagnosed in 1985-9 (table 4). In the late onset cohort, the increase was significant (P=0.03) and the corresponding mortality rates were 0.5 (0.01 to 2.6) per 10 000 person years and 5.0 (2.5 to 9.0) per 10 000 person-years (table 3).
Table 3.
Cause specific mortality per 10 000 person years in early onset type 1 diabetes cohort (0-14 years) and late onset type 1 diabetes cohort (15-29 years) diagnosed between 1970 and 1989
| Cause of death | Incidence/10 000 person years (95% CI) | P value for trend | |||
|---|---|---|---|---|---|
| 1970-4 | 1975-9 | 1980-4 | 1985-9 | ||
| Early onset cohort | |||||
| All acute diabetic complications | 3.6 (1.7 to 6.6 ) | 4.8 (2.6 to 8.0 ) | 3.7 (1.9 to 6.5 ) | 5.0 (2.9 to 8.1) | NS |
| Non-alcohol related acute diabetic complications | 2.1 (0.8 to 4.6) | 3.1 (1.4 to 5.8) | 3.1 (1.5 to 5.7) | 4.1 (1.9 to 6.5) | NS |
| Any chronic diabetic complication | 10.0 (5.8 to 13.1) | 6.8 (4.2 to 10.5) | 4.3 (2.4 to 7.3) | 2.2 (0.9 to 4.5) | <0.001 |
| Suicides | 3.6 (1.7 to 6.6) | 4.1 (2.1 to 7.1) | 3.1 (1.5 to 5.7) | 1.3 (0.3 to 3.2) | NS |
| Alcohol/drug related deaths | 7.1 (4.4 to 11.0) | 6.1 (3.6 to 9.7) | 4.3 (2.4 to 7.3) | 3.4 (1.7 to 6.1) | 0.06 |
| All deaths | 24.2 (18.8 to 30.7) | 21.8 (16.8 to 27.8) | 17.7 (13.4 to 22.9) | 12.8 (9.2 to 17.3) | 0.003 |
| Late onset cohort | |||||
| All acute diabetic complications | 1.9 (0.5 to 4.7) | 5.33 (2.8 to 9.3) | 5.6 (2.9 to 9.8) | 6.4 (3.5 to 10.7) | 0.04 |
| Non-alcohol related acute diabetic complications | 0.5 (0.01 to 2.6) | 1.3 (0.3 to 3.9) | 2.3 (0.8 to 5.5) | 5.0 (2.5 to 9.0) | 0.03 |
| Any chronic diabetic complication | 13.0 (8.6 to 18.7) | 12.9 (8.6 to 18.5) | 15.4 (10.6 to 21.7) | 10.5 (6.7 to 15.7) | NS |
| Suicide | 0.9 (0.1 to 3.3) | 1.3 (0.3 to 3.9) | 1.9 (0.5 to 4.8) | 5.0 (1.0 to 6.0) | 0.008 |
| Alcohol/drug related deaths | 6.0 (3.2 to 10.3) | 6.7 (3.7 to 11.0) | 9.8 (6.1 to 15.0) | 15.5 (10.7 to 21.7) | <0.001 |
| All deaths | 22.7 (16.8 to 30.0) | 29.8 (23.1 to 37.8) | 32.7 (25.5 to 41.3) | 40.1 (32.2 to 49.5) | <0.001 |
Table 4.
Classification of cause specific deaths in early onset type 1 diabetes cohort (0-14 years) and late onset type 1 diabetes cohort (15-29 years) diagnosed between 1970 and 1989
| Cause of death | No (%) | |||
|---|---|---|---|---|
| 1970-4 | 1975-9 | 1980-4 | 1985-9 | |
| Early onset cohort | (n=68) | (n=64) | (n=57) | (n=41) |
| Acute diabetic complications (DKA, hypoglycaemia, hyperosmolar coma): | ||||
| Alcohol/drug related | 4 (6) | 5 (8) | 2 (4) | 4 (10) |
| Other | 6 (9) | 9 (14) | 10 (18) | 12 (29) |
| Chronic diabetic complications | 7 (10) | 4 (6) | 3 (5) | 1 (2) |
| Cardiovascular: | ||||
| Acute myocardial infarction | 7 (10) | 5 (8) | 3 (5) | 3 (7) |
| Cerebrovascular disease | 0 | 0 | 0 | 0 |
| Other | 4 (6) | 3 (5) | 2 (4) | 1 (2) |
| Infection (septicaemia, pneumonia, myocarditis, other infections) | 7 (10) | 8 (13) | 6 (11) | 2 (5) |
| Any chronic diabetic complication | 25 ( 37) | 20 (31) | 14 (25) | 7 (17) |
| Neoplasm | 4 (6) | 4 (6) | 3 (5) | 0 |
| Sudden death | 3 (4) | 2 (3) | 0 | 2 (5) |
| Suicide: | ||||
| Alcohol/drug related | 7 (10) | 5 (8) | 6 (11) | 3 (7) |
| Other | 3 (4) | 7 (11) | 4 (7) | 1 (2) |
| Accidents (not intoxications): | ||||
| Alcohol/drug related | 4 (6) | 6 (9) | 5 (9) | 1 (2) |
| Other | 5 (7) | 2 (3) | 10 (18) | 6 (15) |
| Other alcohol/drug related (alcoholic cirrhosis of liver, alcohol/drug intoxications) | 5 (7) | 2 (3) | 1 (2) | 2 (5) |
| Other cause | 2 (3) | 2 (3) | 2 (4) | 3 (7) |
| All alcohol/drug related deaths | 20 (29) | 18 (28) | 14 (25) | 11 (27) |
| Late onset cohort | (n=48) | (n=67) | (n=70) | (n=88) |
| Acute diabetic complications (DKA, hypoglycaemia, hyperosmolar coma): | ||||
| Alcohol/drug related | 3 (6) | 9 (13) | 7 (10) | 3 (3) |
| Other | 1 (2) | 3 (4) | 5 (7) | 11 (13) |
| Chronic diabetic complications | 4 (8) | 5 (7) | 5 (7) | 4 (5) |
| Cardiovascular | ||||
| Acute myocardial infarction | 9 (19) | 14 (21) | 7 (10) | 11 (13) |
| Cerebrovascular disease | 5 (10) | 0 | 2 (3) | 1 (1) |
| Other | 0 | 0 | 6 (9) | 3 (3) |
| Infection (septicaemia, pneumonia, myocarditis, other infections) | 10 (21) | 10 (15) | 13 (19) | 4 (5) |
| Any chronic diabetic complication | 28 (58) | 29 (43) | 33 (47) | 23 (26) |
| Neoplasm | 3 (6) | 5 (7) | 3 (4) | 5 (6) |
| Sudden death | 2 (4) | 1 (1) | 1 (1) | 5 (6) |
| Suicide: | ||||
| Alcohol/drug related | 2 (4) | 2 (3) | 4 (6) | 9 (10) |
| Other | 0 | 1 (1) | 0 | 2 (2) |
| Accidents (not intoxications): | ||||
| Alcohol/drug related | 1 (2) | 1 (1) | 2 (3) | 1 (1) |
| Other | 0 | 6 (9) | 4 (6) | 3 (3) |
| Other alcohol/drug related (alcoholic cirrhosis of liver, alcohol/drug intoxications) | 7 (15) | 6 (9) | 10 (14) | 22 (25) |
| Other cause | 1 (2) | 4 (6) | 1 (1) | 4 (5) |
| All alcohol/drug related deaths | 13 (27) | 15 (22) | 21 (30) | 34 (39) |
Notably, the proportion of alcohol and drug related deaths was unexpectedly high during the first 20 years in the early onset cohort; one out of four deaths was associated with alcohol or drugs. In the late onset cohort, a conspicuous increase in such deaths occurred in those diagnosed after 1985 (table 4). The mortality rate increased 2.6 times from 6.0 (3.2 to 10.3) per 10 000 person years in patients diagnosed in 1970-4 to 15.5 (10.7 to 21.7) per 10 000 person years in those diagnosed in 1985-9 (P<0.001), and alcohol or drugs accounted for 39% of the deaths.
The standardised mortality ratio for alcohol related deaths was 1.5 (1.1 to 1.9) in the early onset cohort and 1.5 (1.2 to 2.7) in the late onset cohort. The standardised mortality ratio for ischaemic heart disease was 17.4 (12.6 to 22.1) in the early onset cohort. Excess mortality due to ischaemic heart disease was almost four times higher in women in the early onset cohort and three times higher in the late onset cohort compared with men. The standardised mortality ratio for cerebrovascular disease was 5.1 (2.3 to 7.9) in the early onset cohort and 2.8 (2.3 to 2.8) in the late onset cohort. Women had a 2.4 times higher standardised mortality ratio for cerebrovascular disease in the early onset cohort and a 1.5 times higher standardised mortality ratio in the late onset cohort compared with men.
Altogether, 110 suicides occurred during follow-up. Half of the suicides occurred during the first 20 years, accounting for 10-20% of the total deaths in the early onset cohort compared with less than 10% in the late onset cohort. Mortality due to suicide was increased only in women with early onset diabetes, with a standardised mortality ratio of 3.3 (1.9 to 4.7). We found an increasing trend in mortality due to suicide in the late onset cohort (table 3).
Discussion
This study provides a contemporary picture of mortality in early onset as well as late onset type 1 diabetes between 1970 and 2007 in Finland, the country with the highest incidence of type 1 diabetes in the world.8 9 An encouraging finding is that survival in the early onset cohort has improved in those patients most recently diagnosed as having diabetes. This was mainly because of a decrease in mortality due to chronic complications of diabetes. This encouraging finding in the early onset cohort is, however, overshadowed by the unfavourable findings in the late onset cohort, in which we saw an increasing trend in both short term and long term standardised mortality ratios. However, the cumulative mortality in the late onset cohort was very low in the 1970s during the first 15 years’ duration of diabetes (between 1% and 1.5%).
Comparison with other studies
Many studies have reported a decrease in mortality in type 1 diabetes, particularly due to a decrease in late diabetic complications and cardiovascular disease.12 13 Some other studies have reported no changes in mortality.14 15 16 This is partly a result of differences between populations but also a result of discrepancies in the age at onset and duration of diabetes as well as differences in time period between the studies. Many of the studies of mortality have compared patients diagnosed after and before the 1970s,17 18 but little evidence exists regarding changes in mortality during recent decades after many breakthroughs in the management of diabetes or results in young adults.19
The prognosis of patients with type 1 diabetes with regard to diabetic complications has improved during recent decades owing to great advances in diabetes care.12 20 21 22 Consequently, the incidence of end stage renal disease has also decreased during the past four decades in Finland.5 One could thus expect improvements in survival regardless of the age at onset of diabetes. This was not the case in this study, however, as improvement in survival occurred only in the patients with early onset diabetes. Given that all patients with type 1 diabetes in principle have access to the improvements in diabetes care, the observed discrepancies between the early onset and late onset cohorts may be due to differences in management practices and psychosocial aspects and temporal changes in these.
Changes in diabetes management in Finland
The Finnish healthcare system has guaranteed equal healthcare to everyone for a long time. With regard to diabetes, Finland has had a nationally organised diabetes care system and insulin has been available free of charge since 1965. Healthcare has, however, limited means to reduce premature mortality if the system is based on responding to acute physical problems rather than preventive strategies and holistic care. According to the report of the National Development Programme for the Prevention and Care of Diabetes in Finland, geographical disparities in the availability, organisation, and quality of diabetes care have increased in Finland.23 This has particularly happened since Finland experienced a deep economic recession in the early 1990s, which caused considerable reductions in healthcare spending and cuts in preventive health services in the primary healthcare centres,24 where care for patients with adult onset diabetes is predominantly arranged. The implemented cuts may have caused healthcare to deteriorate in particular among patients with late onset type 1 diabetes. Furthermore, many healthcare centres have given up the diabetes nurse system.23 This may have resulted in irregular attendance at diabetes clinics, a lack of longlasting patient-doctor-nurse relationships, and poor adherence to treatment regimens in the patients with onset of diabetes at young adult ages.
Alcohol and drug related deaths
In the late onset cohort, the increase in alcohol and drug related deaths as well as acute complications of diabetes outweighed some reduction in chronic complications of diabetes. Alcohol and drug related deaths accounted for 39% of deaths at 20 years’ duration in patients diagnosed between 1985 and 1989. Alcohol and drug related deaths were also high in the early onset group, varying between 25% and 30%. The higher proportion in the late onset cohort is, however, mostly explained by the older age, as alcohol related deaths increase with increasing age in Finland. This is confirmed by comparing the standardised mortality ratios; although the absolute numbers of alcohol and drug related deaths were higher in the late onset cohort, the standardised mortality ratio was similar in both cohorts indicating no differences in excess mortality compared with the background population.
The high proportion of, and increase in, alcohol related deaths among patients with type 1 diabetes reflects that of the background population. Since 2005, alcohol induced diseases have been the most common cause of death in men and women of working age (15-64 years) in Finland, exceeding the number of deaths from cardiovascular disease (Statistics Finland: www.stat.fi).
In accordance with the results of this study, alcohol and drug misuse also played an important role together with mental dysfunction or suicide as the cause of deaths in Swedish patients with diabetes diagnosed between the ages of 15 and 34 years.25 Likewise, drug misuse was an important cause of death in patients with type 1 diabetes diagnosed in young adulthood in the Yorkshire study.26
However, the high numbers of alcohol and drug related deaths in Finland are not straightforwardly comparable with those in the other countries. The high rate found in Finland might be due to high reliability of causes of death, as the medical death certificate is issued only after autopsy, when this is done, with all its accessory examinations completed.27 The autopsy rate is very high among patients with type 1 diabetes—about 80% in this study. Postmortem toxicology allows the diagnosis of alcohol or drug intoxication more accurately, so alcohol or drugs as a cause of death may be found more frequently in Finland than in other countries.
Acute diabetic complications
The increase in the proportion of acute complications of diabetes—hypoglycaemia, ketoacidosis, or diabetic coma—in the late onset cohort over time in this study is of concern. Diabetic ketoacidosis has been reported to be the leading cause early mortality, before the peak in chronic complications of diabetes.4 14 28 The EURODIAB mortality study reported that as many as 35% of the deaths in children diagnosed after 1989 were due to acute complications.4 Although acute diabetic complications could be largely preventable, they are still an important cause of death. Alcohol consumption is a well known risk factor for severe hypoglycaemia in type 1 diabetes,29 and an increase in both alcohol and drug related deaths and deaths due to acute diabetic complications may be related.
Depression and suicides
People with type 1 diabetes have significantly higher rates of depression than do those without diabetes.30 We frequently found a diagnosis of depression in the medical history of patients who died as a result of alcohol or drug misuse in this study. Heavy drinking is often associated with clinically significant depression.31 Furthermore, depression is highly correlated with suicidal ideation. Patients with type 1 diabetes have been found to be at higher risk of suicide than people without diabetes in some, but not all, studies.32 33 34 35 Although more men than women committed suicide, the standardised mortality ratio was significant only in women with early onset diabetes. In accordance with this, women had a higher risk of suicides in studies from Slovenia and the United Kingdom.36 37 Intoxication with drugs was the most commonly used method of suicide in women, whereas hanging and shooting were the predominant methods in men. Easily available diabetes related methods may make women more vulnerable to suicide.
Effect of age at onset and duration of diabetes on mortality
Not only the magnitude but also the peak in the standardised mortality ratio varied according to age at onset of diabetes, reflecting the effect of corresponding duration of diabetes. The patterns seen in the peaks of standardised mortality ratios and the effect of duration of diabetes resemble the model of incidence of diabetic nephropathy and the variability of risk by age at onset of diabetes. On average, the incidence of diabetic nephropathy first increases linearly with duration of diabetes, starting at five to 10 years’ duration, but after 20-25 years from diagnosis the incident cases start to decline.38 39 However, this pattern is modified by the age at onset of diabetes. In the youngest age at onset group, the standardised mortality ratio did not reach a peak, probably because the duration of diabetes was too short. This is in accordance with the population based studies on end stage renal disease that have shown greatly reduced or delayed risk of end stage renal disease in patients with onset of diabetes before the age of 5 years.5 40 This prolonged peak in the standardised mortality ratio is encouraging, as the age at onset of diabetes has decreased and children with onset before the age of 5 are becoming more common.9 41 42
Strengths and limitations of study
The main strength of this study is that it is population based with a large sample size, long follow-up time, and the ability to compare differences in mortality not only by different time of diagnosis but also between early onset and late onset type 1 diabetes. Mortality studies in type 1 diabetes should be population based and first identify patients with type 1 diabetes, as in many countries the information on death certificates does not allow identification of all patients with diabetes.43
The main limitation is the lack of precise information about type of diabetes for all patients. The classification of diabetes in young adults is challenging, and misclassification may occur. However, we used several data sources to exclude patients with secondary or type 2 diabetes. Furthermore, the vast majority of people with diabetes below the age of 30 have type 1 diabetes. Thus, we believe that misclassification of patients is not a problem.
Obtaining precise estimates of cause specific mortality rates is also challenging, because the underlying cause of death is not always unequivocal. Patients with diabetes and several late complications, mainly diabetic nephropathy, are at high risk of cardiovascular disease. Furthermore, acute respiratory infections, including pneumonia, tend to cluster in patients at high risk of coronary heart disease and may even play a role in triggering acute coronary syndromes.44 Patients with diabetic nephropathy often die from heart failure, although the immediate cause of death is pneumonia. If all these conditions were present, the order in the classification in this study was infection, cardiovascular disease, and diabetic nephropathy. The deaths due to heart failure and infection may thus mask diabetic nephropathy, and infection in turn may mask undetected cardiovascular death. Therefore, we also grouped these causes of death together and denoted them “any chronic diabetic complications.”
Conclusions and policy implications
In conclusion, we found improvement in the survival of patients with early onset type 1 diabetes followed from 1970 to 2007. Improved survival in the early onset cohort was explained by a decrease in chronic complications of diabetes during the first 20 years of diabetes. However, an alarming finding was that both short term and long term trends showed an increase in the standardised mortality ratios in patients diagnosed at the age of 15 to 29 years. The increase in mortality was due to an increase in alcohol and drug related mortality and acute complications of diabetes. This highlights the importance of permanent and long lasting patient-doctor-nurse relationships, close supervision, and guidance on the short term and long term effects of alcohol in young people with type 1 diabetes, especially in our alcohol permissive cultures.
What is already known on this topic
- Type 1 diabetes is still associated with premature mortality due to both acute and chronic complications of diabetes
- Few studies have compared trends in mortality between early onset and late onset type 1 diabetes
What this study adds
- Survival of patients with early onset type 1 diabetes (age 0-14 years) has improved with time owing to a decrease in chronic diabetic complications
- Survival of patients with late onset type 1 diabetes (age 15-29 years) has deteriorated since the 1980s
- Alcohol has become an important cause of death in patients with type 1 diabetes, and the proportion of acute diabetic complications as a cause of death has increased in patients with late onset type 1 diabetes
Contributors: VH was responsible for study design, statistical analyses, and writing of the manuscript. CF participated in regrouping causes of death, interpretation of the results, and critical revision of the manuscript. P-HG participated in regrouping causes of death, interpretation of the results, and the writing and critical revision of the article. VH is the guarantor.
Funding: This research was supported by grants from the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, and Liv och Hälsa Foundation. The sponsors of the study had no role in the design and conduct of the study or in the collection, management, analysis, and interpretation of the data. The authors are independent from the funders.
Competing interests: All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: this research was supported by grants from the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, and Liv och Hälsa Foundation; the authors have no relationship with companies that might have an interest in the submitted work in the previous three years; and they have no non-financial interests that may be relevant to the submitted work.
Ethical approval: This study was approved by the ethical committee of the National Institute for Health and Welfare, Finland.
Data sharing: No additional data available.
Cite this as: BMJ 2011;343:d5364
References
- 1.Diabetes Epidemiology Research International Mortality Study Group. International evaluation of cause-specific mortality and IDDM. Diabetes Care 1991;14:55-60. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Epidemiology Research International Mortality Study Group. Major cross-country differences in risk of dying for people with IDDM. Diabetes Care 1991;14:49-54. [DOI] [PubMed] [Google Scholar]
- 3.Groop PH, Thomas MC, Moran JL, Wadén J, Thorn LM, Mäkinen VP, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, Svensson J, et al. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia 2007;50:2439-42. [DOI] [PubMed] [Google Scholar]
- 5.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782-7. [DOI] [PubMed] [Google Scholar]
- 6.Harjutsalo V, Katoh S, Sarti C, Tajima N, Tuomilehto J. Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes 2004;53:2449-54. [DOI] [PubMed] [Google Scholar]
- 7.Harjutsalo V, Maric C, Forsblom C, Thorn L, Wadén J, Groop PH. Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia 2011;54:1992-9. [DOI] [PubMed] [Google Scholar]
- 8.DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857-66. [DOI] [PubMed] [Google Scholar]
- 9.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777-82. [DOI] [PubMed] [Google Scholar]
- 10.Lammi N, Blomstedt PA, Moltchanova E, Eriksson JG, Tuomilehto J, Karvonen M. Marked temporal increase in the incidence of type 1 and type 2 diabetes among young adults in Finland. Diabetologia 2008;51:897-9. [DOI] [PubMed] [Google Scholar]
- 11.Lammi N, Taskinen O, Moltchanova E, Notkola IL, Eriksson JG, Tuomilehto J, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia 2007;50:1393-400. [DOI] [PubMed] [Google Scholar]
- 12.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463-9. [DOI] [PubMed] [Google Scholar]
- 13.Allemann S, Saner C, Zwahlen M, Christ ER, Diem P, Stettler C. Long-term cardiovascular and non-cardiovascular mortality in women and men with type 1 and type 2 diabetes mellitus: a 30-year follow-up in Switzerland. Swiss Med Wkly 2009;139:576-83. [DOI] [PubMed] [Google Scholar]
- 14.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298-305. [DOI] [PubMed] [Google Scholar]
- 15.Burnet DL, Cooper AJ, Drum ML, Lipton RB. Risk factors for mortality in a diverse cohort of patients with childhood-onset diabetes in Chicago. Diabetes Care 2007;30:2559-63. [DOI] [PubMed] [Google Scholar]
- 16.Waernbaum I, Blohmé G, Östman J, Sundkvist G, Eriksson JW, Arnqvist HJ, et al. Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 2006;49:653-9. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ. Mortality trends in type 1 diabetes: the Allegheny County (Pennsylvania) registry 1965-1999. Diabetes Care 2001;24:823-7. [DOI] [PubMed] [Google Scholar]
- 18.Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, Matsushima M, et al. Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care 2003;26:2037-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno G, Cerutti F, Merletti F, Novelli G, Panero F, Zucco C, et al. Short-term mortality risk in children and young adults with type 1 diabetes: the population-based registry of the province of Turin, Italy. Nutr Metab Cardiovasc Dis 2009;19:340-4. [DOI] [PubMed] [Google Scholar]
- 20.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003;26:1258-64. [DOI] [PubMed] [Google Scholar]
- 21.Astrup AS, Tarnow L, Rossing P, Pietraszek L, Riis Hansen P, Parving HH. Improved prognosis in type 1 diabetic patients with nephropathy: a prospective follow-up study. Kidney Int 2005;68:1250-7. [DOI] [PubMed] [Google Scholar]
- 22.Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type 1 diabetes—the Linkoping Diabetes Complications Study. Diabetologia 2004;47:1266-72. [DOI] [PubMed] [Google Scholar]
- 23.Finnish Diabetes Association DC. Development programme for the prevention and care of diabetes in Finland 2000-2010. Finnish Diabetes Association, 2001.
- 24.Forssas E, Keskimäki I, Reunanen A, Koskinen S. Widening socioeconomic mortality disparity among diabetic people in Finland. Eur J Public Health 2003;13:38-43. [DOI] [PubMed] [Google Scholar]
- 25.Wibell L, Nyström L, Östman J, Arnqvist H, Blohmé G, Lithner F, et al. Increased mortality in diabetes during the first 10 years of the disease: a population-based study (DISS) in Swedish adults 15-34 years old at diagnosis. J Intern Med 2001;249:263-70. [DOI] [PubMed] [Google Scholar]
- 26.Feltbower RG, McKinney PA, Parslow RC, Stephenson CR, Bodansky HJ. Type 1 diabetes in Yorkshire, UK: time trends in 0-14 and 15-29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003;20:437-41. [DOI] [PubMed] [Google Scholar]
- 27.Lahti RA. From findings to statistics: an assessment of Finnish medical cause-of-death information in relation to underlying-cause coding. Helsinki University Printing House, 2005.
- 28.Laing SP, Jones ME, Swerdlow AJ, Burden AC, Gatling W. Psychosocial and socioeconomic risk factors for premature death in young people with type 1 diabetes. Diabetes Care 2005;28:1618-23. [DOI] [PubMed] [Google Scholar]
- 29.Richardson T, Weiss M, Thomas P, Kerr D. Day after the night before: influence of evening alcohol on risk of hypoglycemia in patients with type 1 diabetes. Diabetes Care 2005;28:1801-2. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069-78. [DOI] [PubMed] [Google Scholar]
- 31.Paljärvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmäki L, Mäkelä P. Binge drinking and depressive symptoms: a 5-year population-based cohort study. Addiction 2009;104:1168-78. [DOI] [PubMed] [Google Scholar]
- 32.Dahlquist G, Kallen B. Mortality in childhood-onset type 1 diabetes: a population-based study. Diabetes Care 2005;28:2384-7. [DOI] [PubMed] [Google Scholar]
- 33.Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HA. Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabetes Care 2003;26:1052-7. [DOI] [PubMed] [Google Scholar]
- 34.Fuller-Thomson E, Sawyer JL. Lifetime prevalence of suicidal ideation in a representative sample of Canadians with type 1 diabetes. Diabetes Res Clin Pract 2009;83:e9-11. [DOI] [PubMed] [Google Scholar]
- 35.Goldston DB, Kovacs M, Ho VY, Parrone PL, Stiffler L. Suicidal ideation and suicide attempts among youth with insulin-dependent diabetes mellitus. J Am Acad Child Adolesc Psychiatry 1994;33:240-6. [DOI] [PubMed] [Google Scholar]
- 36.Radobuljac MD, Bratina NU, Battelino T, Tomori M. Lifetime prevalence of suicidal and self-injurious behaviors in a representative cohort of Slovenian adolescents with type 1 diabetes. Pediatr Diabetes 2009;10:424-31. [DOI] [PubMed] [Google Scholar]
- 37.Roberts SE, Goldacre MJ, Neil HA. Mortality in young people admitted to hospital for diabetes: database study. BMJ 2004;328:741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 1983;25:496-501. [DOI] [PubMed] [Google Scholar]
- 39.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785-94. [DOI] [PubMed] [Google Scholar]
- 40.Möllsten A, Svensson M, Waernbaum I, Berhan Y, Schön S, Nyström L, et al. Cumulative risk, age at onset and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population based cohort study. Diabetes 2010;59:1803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuomilehto J, Karvonen M, Pitkäniemi J, Virtala E, Kohtamäki K, Toivanen L, et al. Record-high incidence of type I (insulin-dependent) diabetes mellitus in Finnish children. Diabetologia 1999;42:655-60. [DOI] [PubMed] [Google Scholar]
- 42.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027-33. [DOI] [PubMed] [Google Scholar]
- 43.Mühlhauser I, Sawicki PT, Blank M, Overmann H, Richter B, Berger M. Reliability of causes of death in persons with type I diabetes. Diabetologia 2002;45:1490-7. [DOI] [PubMed] [Google Scholar]
- 44.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010;10:83-92. [DOI] [PubMed] [Google Scholar]