Inflammatory response following a short-term course of chiropractic treatment in subjects with and without chronic low back pain (original) (raw)
Abstract
Objective
Inflammatory markers interleukin-6 (IL-6) and C-reactive protein (CRP) have not been evaluated in response to a short course of lumbar spinal manipulation. The purpose of this study is to observe the responses of inflammatory markers (IL-6 and CRP) after a series of 9 chiropractic spinal manipulations.
Methods
Twenty-one participants were assigned to a treatment or a control group. Only the treatment group received 9 chiropractic interventions. Pre- and postintervention measures were recorded for blood samples for detection of proinflammatory cytokines IL-6 and CRP.
Results
Mediators of inflammation (IL-6 and high-sensitivity CRP) were modified by the intervention received in the treatment group, and the effect size demonstrated a tendency toward the control group values.
Conclusion
A total of 9 chiropractic lower back manipulations caused the mediators of inflammation to present a normalization response in individuals suffering from chronic low back pain.
Key indexing terms: Manipulation, Spinal, Diagnosis, Interleukins, C-reactive protein
Introduction
Many different outcome measures as a means of monitoring the treatment course of chiropractic adjustments have been investigated.1-8 Outcome measures, however, such as inflammation markers (interleukin-6 [IL-6] and C-reactive protein [CRP]) have never been compared with each other during the same course of treatment. Thus, the scientific relationship that may exist between manipulation and the inflammation markers is at the moment unknown.
One aspect that may be present in lower back pain is the inflammatory process typically accompanied by various expressions of plasma cytokines. In fact, different disease states exhibit unique cytokine profiles.9 Inflammation is often associated with the like of several proinflammatory cytokines that include IL-6 and CRP. Interleukin-6 is the main mediator of the acute phase inflammatory response.10 In humans, IL-6 causes a dramatic increase in hepatocyte-derived CRP synthesis.11 However, in nonacute phase inflammatory status, Meir-Ewert et al12 have shown no diurnal effects of CRP plasma concentrations over a 24-hour period. Nonetheless, several authors13-16 have found that CRP is a sensitive marker of inflammation.13-15
Recently, it was reported6-8 that a single chiropractic treatment does indeed affect the level of circulating cytokines (tumor necrosis factor–α and interleukin-1) but not that of substance P and interleukin-2. The lumbar spine was considered from the T12 to L5 level for the chiropractic intervention; and as proposed by Maigne,17,18 “The most frequent manifestation of this thoracolumbar junction syndrome is low back pain, which is exactly like low back pain of lumbosacral or sacroiliac origin.” Thus, spinal interventions were performed using a manually assisted mechanical force lumbar spine protocol,19 whereas paraspinal computed tomography was taken in the prone position as previously described.7,20-22 The purpose of this study is to observe the responses inflammatory markers (IL-6 and CRP) after a series of 9 chiropractic spinal manipulations.
Methods
Participants
For sample size determination, we considered alterations in physiologic responses,23 and these were established in 9 participants. Thus, we elected to have 10 subjects per group.
Anthropometric characteristics of the participants are shown in Table 1. The research protocol for the evaluation and adjustment was approved by the Université du Québec à Montréal ethics committee. Written informed consent was obtained from all participants. This study was registered with clinicaltrials.gov, registration number NCT00739570. All participants received a physical examination, had spinal radiographs, completed the Oswestry questionnaire, and had blood samples drawn.
Table 1.
Anthropometric characteristics of the subjects
| Variables | Control Group | Treatment Group |
|---|---|---|
| Weight (kg) | 74.9 ± 16.9 | 80.3 ± 16.5 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 |
| BMI | 25.3 ± 3.6 | 28.0 ± 3.7 |
| Age (y) | 47.5 ± 16.2 | 45.6 ± 8.9 |
The decision to have a healthy control group that would not receive any intervention was to evaluate each variable and compare both groups to be able to compare the pre- and postintervention measurements of the control group vs the variation of the treatment group.
Control group
A total of 10 participants were recruited, 4 women and 6 men, at the beginning of June 2008 from a chiropractic clinic located at 7655 Newman Blvd, LaSalle, Quebec. The inclusion criterion was that all participants were pain free and would not receive any other treatment during the 2-week span of the research project. All participants were evaluated for all the same outcome measures as the treatment group.
Treatment group
All treatment group participants were recruited via an announcement in the newspaper Le Messager de LaSalle during the period from July 6 to 20, 2008. Forty-five subjects responded to the telephone number at the university and left a message indicating their interest in the project. The acceptance criterion was a chronic low back condition of at least 3 months in duration. Eleven participants that met the criteria were selected, whereas the others were thanked for their interest. The 11 retained participants were 4 women and 7 men.
One participant (female) did not come for the final evaluation. Our treatment group was thus composed of 10 participants: 3 women and 7 men. Those administering the chiropractic intervention were not blinded to the group assignment, but those administering the different tests were blinded to the group assignment. The final evaluation comprised the same tests as the initial examination.
Outcome measures
Analysis of inflammatory markers
Blood samples were collected by a trained licensed nurse from the province of Quebec. For every subject, the IL-6 blood sample was collected in a properly coded lavender tube (4.0 mL, BD Vacutainer, K2 EDTA, lot 7344830; BD, Franklin Lakes, NJ). Similarly, CRP blood samples were collected in properly coded yellow tubes for every subject (5.0 mL, BD Vacutainer, SST, lot 8032322; BD). All samples remained at room temperature for 15 minutes before being centrifuged for 15 minutes at 1000_g_. Afterward, they were immediately decanted; and the plasma or serum (IL-6 and CRP analysis, respectively) was transferred via disposable transfer pipettes (Category number 13-711-7; Fisher Scientific, Pittsburgh, PA) into clearly coded Eppendorf tubes (Sarstedt, Nümbrecth, Germany). The Eppendorf tubes were then closed and stored on dry ice until they were delivered to Hôpital St-Luc's clinical laboratory in Montréal at the end of the day. All sample collecting needles were disposed of after single use in a biohazard container (BD, Sharps collector, reference number 305648, lot 7103001; BD Medical, Oakville, Ontario) provided by the Centre Local de Services de Santé, an organism from the Quebec Ministry of Health. The IL-6 and CRP quantification was performed under the supervision of Dr Line Labrecque, PhD (biochemistry). The results were provided by electronic means and were identified only by the patient's code. The laboratory procedure for the CRP and IL-6 follows; it was respected by the hospital laboratory personnel for the evaluation of both markers.
CRP analysis protocol
(Techne Corporation, 614 McKinley Place NE, Minneapolis, MN. R&D Systems, Quantikine, Human IL-6 Immunoassay, Catalog Number D6050, http://www.rndsystems.com/pdf/d6050.pdf).
Sample preparation
Serum and plasma samples require a 100-fold dilution. A suggested 100-fold dilution is 10 _μ_L sample + 990 _μ_L Calibrator Diluent RD5P (1×). Use polypropylene test tubes.
Reagent preparation
Bring all reagents to room temperature before use.
Wash Buffer—If crystals have formed in the concentrate, warm to room temperature and mix gently until the crystals have completely dissolved. Dilute 20 mL of Wash Buffer Concentrate into deionized or distilled water to prepare 500 mL of Wash Buffer.
Substrate Solution—Color Reagents A and B should be mixed together in equal volumes within 15 minutes of use. Protect from light. Two hundred microliters of the resultant mixture is required per well. Calibrator Diluent RD5P (1×)—Dilute 20 mL of Calibrator Diluent RD5P Concentrate into 80 mL of deionized or distilled water to prepare 100 mL of Calibrator Diluent RD5P (1×). CRP Standard—Use polypropylene tubes.
Pipette 200 _μ_L of Calibrator Diluent RD5P (1×) into each of 6 tubes. Add 200 _μ_L of the standard to the 25-ng/mL tube and continue the 2-fold dilution series (below). Mix each tube thoroughly before the next transfer. The 50-ng/mL standard serves as the high standard. The Calibrator Diluent RD5P (1×) serves as the zero standard (0 ng/mL).
Assay procedure
Bring all reagents and samples to room temperature before use. It is recommended that all samples, controls, and standards be assayed in duplicate.
- Prepare all reagents, working standards, and samples as directed in the previous sections.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 _μ_L of Assay Diluent RD1F to each well. May contain a precipitate. Mix well before and during use.
- Add 50 _μ_L of standard, control, or sample* per well. Cover with the adhesive strip provided. Incubate for 2 hours at room temperature.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes. Wash by filling each well with Wash Buffer (400 _μ_L) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.
- Add 200 _μ_L of CRP Conjugate to each well. Cover with a new adhesive strip. Incubate for 2 hours at room temperature.
- Repeat the aspiration/wash as in step 5.
- Add 200 _μ_L of Substrate Solution to each well. Incubate for 30 minutes at room temperature on the bench top. Protect from light.
- Add 50 _μ_L of Stop Solution to each well. The color in the wells should change from blue to yellow. If the color in the wells is green or the color change does not appear uniform, gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well within 30 minutes, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 or 570 nm. If wavelength correction is not available, subtract readings at 540 or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
*Serum and plasma samples require dilution. See “Sample preparation.”
Calculation of results
Average the duplicate readings for each standard, control, and sample; and subtract the average zero standard optical density. Create a standard curve by reducing the data using computer software capable of generating a 4-parameter logistic curve fit. As an alternative, construct a standard curve by plotting the mean absorbance for each standard on the y-axis against the concentration on the x-axis and draw a best fit curve through the points on the graph. The data may be linearized by plotting the log of the CRP concentrations vs the log of the OD, and the best fit line can be determined by regression analysis. This procedure will produce an adequate but less precise fit of the data. If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.
Sensitivity
Forty assays were evaluated, and the minimum detectable dose (MDD) of CRP ranged from 0.005 to 0.022 ng/mL. The mean MDD was 0.010 ng/mL. The MDD was determined by adding 2 SDs to the mean optical density value of 20 zero standard replicates and calculating the corresponding concentration.
Calibration
This immunoassay is calibrated against a highly purified NS0-expressed recombinant human CRP produced at R&D Systems. The recombinant protein is directly calibrated to the National Institute for Biological Standards and Control (NIBSC)/World Health Organization (WHO) First International Standard 85/506.
Specificity
This assay recognizes recombinant and natural human CRP. The factors listed below were prepared at 500 ng/mL in Calibrator Diluent RD5P (1×) and assayed for cross-reactivity. Preparations of the following factors at 500 ng/mL in a midrange recombinant human CRP control were assayed for _μ_interference. No significant cross-reactivity or microinterference was observed.
IL-6 analysis protocol
(Techne Corporation, 614 McKinley Place NE, Minneapolis, MN. R&D Systems, Quantikine Human C-Reactive Protein Immunoassay, Catalog Number DCRP00, http://www.rndsystems.com/pdf/d6050.pdf).
Reagent preparation
Bring all reagents to room temperature before use.
Wash Buffer—If crystals have formed in the concentrate, warm to room temperature and mix gently until the crystals have completely dissolved. Dilute 20 mL of Wash Buffer Concentrate into deionized or distilled water to prepare 500 mL of Wash Buffer.
Substrate Solution—Color Reagents A and B should be mixed together in equal volumes within 15 minutes of use. Protect from light. Two hundred microliters of the resultant mixture is required per well. IL-6 Standard—Reconstitute the IL-6 Standard with 5.0 mL of Calibrator Diluent RD5T (for cell culture supernatant samples) or Calibrator Diluent RD6F (for serum/plasma samples). This reconstitution produces a stock solution of 300 pg/mL. Allow the standard to sit for a minimum of 15 minutes with gentle agitation before making dilutions. Pipette 667 _μ_L of the appropriate Calibrator Diluent into the 100-pg/mL tube and 500 _μ_L of diluent into each remaining tube. Use the stock solution to produce a dilution series (below). Mix each tube thoroughly before the next transfer. The undiluted standard serves as the high standard (300 pg/mL). The appropriate Calibrator Diluent serves as the zero standard (0 pg/mL).
Assay procedure
Bring all reagents and samples to room temperature before use. It is recommended that all samples, standards, and controls be assayed in duplicate.
- Prepare all reagents and working standards as directed in the previous sections.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 _μ_L of Assay Diluent RD1W to each well.
- Add 100 _μ_L of standard, sample, or control per well. Cover with the adhesive strip provided. Incubate for 2 hours at room temperature. A plate layout is provided to record standards and samples assayed.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes. Wash by filling each well with Wash Buffer (400 _μ_L) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.
- Add 200 _μ_L of IL-6 Conjugate to each well. Cover with a new adhesive strip. Incubate for 2 hours at room temperature.
- Repeat the aspiration/wash as in step 5.
- Add 200 _μ_L of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Protect from light.
- Add 50 _μ_L of Stop Solution to each well. The color in the wells should change from blue to yellow. If the color in the wells is green or the color change does not appear uniform, gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well within 30 minutes, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 or 570 nm. If wavelength correction is not available, subtract readings at 540 or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Calculation of results
Average the duplicate readings for each standard, control, and sample; and subtract the average zero standard optical density. Create a standard curve by reducing the data using computer software capable of generating a 4-parameter logistic curve fit. As an alternative, construct a standard curve by plotting the mean absorbance for each standard on the y-axis against the concentration on the x-axis and draw a best fit curve through the points on the graph. The data may be linearized by plotting the log of the IL-6 concentrations vs the log of the OD, and the best fit line can be determined by regression analysis. This procedure will produce an adequate but less precise fit of the data. If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.
Sensitivity
The minimum detectable dose of IL-6 is typically less than 0.70 pg/mL.
The minimum detectable dose was determined by adding 2 SDs to the mean optical density value of 20 zero standard replicates and calculating the corresponding concentration.
Calibration
This immunoassay is calibrated against highly purified _Escherichia coli_–expressed recombinant human IL-6 produced at R&D Systems. The NIBSC/WHO International Standard for IL-6 (89/548) (40), which was intended as a potency standard, was evaluated in this kit. The NIBSC/WHO standard is a CHO cell–derived recombinant human IL-6. The dose-response curve of the International Standard (89/548) parallels the Quantikine standard curve. To convert sample values obtained with the Quantikine IL-6 kit to equivalent NIBSC 89/548 units, use the following equation: NIBSC (89/548) equivalent value (IU/mL) = 0.131 × Quantikine IL-6 value (pg/mL).
Specificity
This assay recognizes both natural and recombinant human IL-6. The factors listed below were prepared at 50 ng/mL in Calibrator Diluent RD5T and at 100 ng/mL in Calibrator Diluent RD6F, and assayed for cross-reactivity. Preparations of the following factors at 50 ng/mL in a midrange rhIL-6 control prepared in Calibrator Diluent RD5T and 100 ng/mL in a midrange rhIL-6 control prepared in Calibrator Diluent RD6F were assayed for interference. No significant cross-reactivity or interference was observed.
Experimental procedures
Control and treatment group interventions
The participants of the control group received no treatment, only the evaluation of the outcome measures at the pre– and post–2-week intervention interval and the Activator Methods (AM) evaluation to determine their pelvic-deficient side (PD). A PD is explained as follows: “Traditionally, the short leg has been designated the Pelvic Deficient, or PD leg. It is referred to as the reactive leg because of its tendency to appear shorter or longer during different testing procedures. The PD leg is visually observed during the initial leg check following placement of the patient in the prone position on the adjusting table.”19
In the treatment group, the participants received the above-mentioned chiropractic evaluation using the AM basic scan protocol for the lumbar spine. They received a chiropractic adjustment with the AM instrument, which was an Activator IV, signature series. It was set at number 4 (176 N) for all patients19; only the lumbar area from T12 to L5 was treated according to the PD side. The treating clinician held an advanced proficiency rating in the AM.24 The duration of the treatment schedule was 2 weeks.25 All participants in the treatment group had outcome measures taken at the pre– and post–2-week intervention interval.
Experimental protocol
When the participants arrived for a recording session, they were asked to dress with a cotton gown that had an open slit in the back while keeping only their underclothing. They then proceeded to the room were blood samples were taken. Following the collection of blood samples, the patient went to the evaluation room. The participants then proceeded to lie prone on an AM table. For the control group, this was the end of the recording session; and the participants were instructed to get dressed and make an appointment for the next evaluation in 2 weeks. The participants in the treatment group proceeded to make 9 appointments to receive AM treatments for the next 2 weeks.25 Subsequently, when the treatment group subjects would arrive for a treatment session, they were shown to the treatment room and were treated according to the AM basic scan for the lumbar spine.19 After the treatment, they would confirm their reservation for the following day. The participants were treated from Monday, July 28, to August 1 and then from Monday, August 4, to Thursday, August 7. Friday, August 8, was the last visit and consisted of the complete reevaluation where the initial evaluation procedure was repeated; but there was no treatment administered. One participant did not come for the reevaluation. On the day of the reevaluations, one participant did not want to have blood drawn that day. These were the only 2 protocol deviations, and there were no adverse events for all participants throughout the entire length of the experiment. The final count for participants in the treatment group was n = 9. On the last day of recording, all the participants were thanked for their participation and received a $30.00 payment for their travel expenses.
Statistical analysis
Descriptive statistics (mean ± SD unless stated otherwise) were computed for all independent and dependent variables for all conditions. Standardized effect size (Cohen d) calculation was performed to estimate the power of group differences (control vs treatment) where d ≤ 0.2, d > 0.2 and ≤ 0.5, and d > 0.5 and ≤ 0.8 are considered, respectively, small, medium, and large effect sizes.26,27
Results
There were no significant differences for the anthropometric characteristics of the subjects between both groups (Table 1).
Markers of inflammation
Inflammation mediators responses for both treatment and control groups, pre- and postintervention, are illustrated in Fig 1. There is a large standardized effect size difference for both CRP and IL-6 between the treatment and control groups preintervention (CRP: 2.50 ± 0.79 vs 1.05 ± 0.34 g/dL and IL-6: 3.97 ± 0.44 vs 3.12 ± 0.00 ng/L, respectively). As well, the IL-6 difference between the treatment and control groups preintervention is different (P = .06). A medium effect size difference for postintervention is noticeable between groups (treatment and control groups) for both high-sensitivity CRP and IL-6 (CRP: 1.94 ± 0.49 vs 1.38 ± 0.51 g/dL and IL-6: 2.98 ± 0.34 vs 3.24 ± 0.12 ng/L, respectively).
Fig 1.
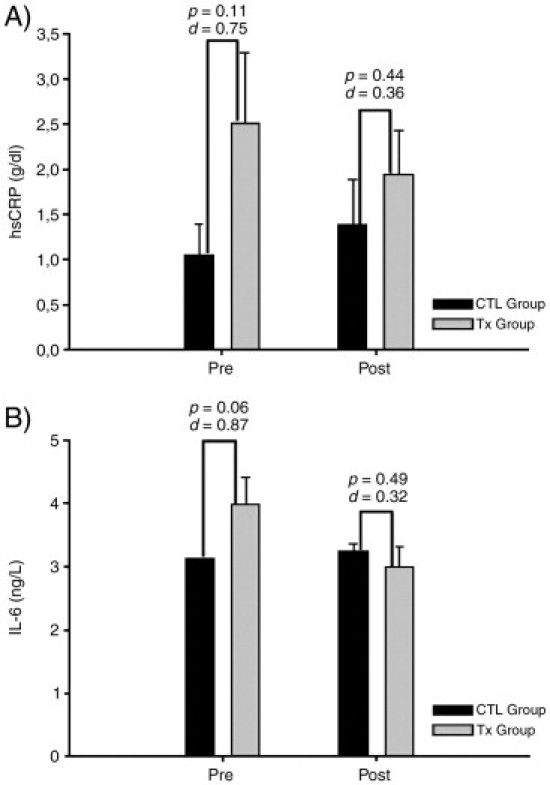
Inflammatory mediator response for both treatment and control groups. Error bars represent SEM for the sake of clarity. Dark bars represent control. Gray bars represent treatment. Numbers above bars represent the significance level (P, α error) and Cohen effect size factor (d coefficient, see “Statistical analysis” in “Methods”) based on between-subject differences (control vs treatment). Tx, Treatment; CTL, control; Pre, before treatment period; Post, after treatment period.
Discussion
Inflammatory mediators
Mediators of inflammation were shown to respond to a treatment course of 9 interventions (Fig 1). What is noticeable is that both CRP and IL-6 were observed to trend toward the control group values. The effect size differences measured indicate that both groups were highly different at preintervention (large standardized effect size), but at postintervention, the standardized effect size difference was smaller (medium) for both CRP and IL-6. Interleukin-6 has been shown to be an acute phase proinflammatory cytokine.28 Interleukin-6 is responsible for the synthesis of CRP from the liver.29 Thus, to observe in the treatment group a postintervention reduction in CRP is a normal physiologic reaction because IL-6 was also reduced, suggesting that 9 interventions are capable of attenuating the inflammatory response.
The patient subjective improvement demonstrated in the current report is similar to the results obtained by Quon et al30 upon treating patients with side posture manipulation. They reported that “the patients improved considerably during only two weeks of treatment.”30 We can nonetheless speculate on the relationship with the different inflammatory markers measured in this current report. As indicated for the treatment group participants, the changes for both cytokines tended toward reaching the control group values. Thus, it is plausible to consider that the inflammation processes were being reversed but that complete healing was not achieved following 2 weeks of treatment.
Limitations
The major limitation in this study was group size. Nonetheless, differences in the results were reported as effect size (Cohen d statistic).26,27 Future investigations warrant larger cohorts for this type of research on cytokines. Another limitation is that the control group included asymptomatic participants, whereas the treatment group included chronic pain patients, which may have impacted the results.
Another limitation is the choice of cytokines used for analysis or perhaps the treatment group population. The cytokine IL-6 is an acute phase proinflammatory mediator.31 The treatment cohort in this study was chronic lower back pain individuals (for at least 3 months). Nonetheless, modulations of IL-6 and CRP trending toward the control group were observed. On the other hand, a larger cohort would have perhaps been preferable with our choice of inflammatory markers retained for this study. These comments and limitations are certainly matters for future investigations.
Conclusion
Inflammatory markers appear promising, but are cumbersome to control in a clinical setting, especially mediators of inflammation. These could be useful when treatment course progression is not clearly defined. Surely, more research is needed to better understand the relationship between inflammation and manipulation. A longer treatment period would possibly help to gain better knowledge on the relation between inflammatory processes and manipulation.
Funding sources and potential conflicts of interest
Funding was received from the Fondation Chiropractique du Québec in relation to this study. No conflicts of interest were reported for this study.
Footnotes
☆
Disclaimer: Nothing of value related to this research was received from a commercial entity.
References
- 1.Gregory P., Hayek R., Hayek A.M. Correlating motion palpation with functional x-rays findings in patients with low back pain. Australas Chiropr Osteopathy. 1998;7:15–19. [PMC free article] [PubMed] [Google Scholar]
- 2.French S.D., Green S., Forbes A. Reliability of chiropractic methods commonly used to detect manipulable lesions in patients with chronic low-back pain. J Manipulative Physiol Ther. 2000;23:231–238. doi: 10.1067/mmt.2000.106101. [DOI] [PubMed] [Google Scholar]
- 3.Fryer G., Morris T., Gibbons P. Paraspinal muscles and intervertebral dysfunction: part two. J Manipulative Physiol Ther. 2004;27:348–357. doi: 10.1016/j.jmpt.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Owens E.F., Hart J.F., Donofrio J.J., Haralambous J., Mierzejewski E. Paraspinal skin temperature patterns: an interexaminer and intraexaminer reliability study. J Manipulative Physiol Ther. 2004;27:155–159. doi: 10.1016/j.jmpt.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 5.DeVocht J.W., Pickar J.G., Wilder D.G. Spinal manipulation alters electromyographic activity of paraspinal muscles: a descriptive study. J Manipulative Physiol Ther. 2005;28:465–471. doi: 10.1016/j.jmpt.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Teodorczyk-Injeyan J.A., Injeyan H.S., Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. 2006;29:14–21. doi: 10.1016/j.jmpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Roy R.A., Boucher J.P., Comtois A.S. Effects of a manually assisted mechanical force on cutaneous temperature. J Manipulative Physiol Ther. 2008;31:230–236. doi: 10.1016/j.jmpt.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Teodorczyk-Injeyan J.A., Injeyan H.S., McGregor M., Harris G.M., Ruegg R. Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment. Chiropr Osteopat. 2008;16:5. doi: 10.1186/1746-1340-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heney D., Banks R.E., Whicher J.T., Evans S.W. Cytokine measurements in disease. In: Mackiewicz A., Kushner I., Baumann H., editors. Acute phase proteins: molecular biology, biochemistry and clinical applications. CRC Press; Boca Raton: 1993. pp. 604–620. [Google Scholar]
- 10.Bartalena L., Brogioni S., Grasso L., Martino E. Increased serum interleukin-6 concentration in patients with subacute thyroiditis: relationship with concomitant changes in serum T4-binding globulin concentration. J Endicronol Invest. 1993;16:213–218. doi: 10.1007/BF03344951. [DOI] [PubMed] [Google Scholar]
- 11.Thorn C.F., Lu Z.Y., Whitehead A.S. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor–alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol. 2004;59:152–158. doi: 10.1111/j.0300-9475.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 12.Meier-Ewert H.K., Ridker P.M., Rifai N., Price N., Dinges D.F., Mullington J.M. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47:426–430. [PubMed] [Google Scholar]
- 13.Jain V.C., Misra S.S. C-reactive protein test: a clinical evaluation of its value in rheumatic fever and rheumatic heart disease. Indian J Pediatr. 1967;34:237. doi: 10.1007/BF02756911. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J., Whincup P., Walker M., Lennon L., Thomson A., Appleby P. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rifai N., Ridker P.M. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem. 2001;47:403–411. [PubMed] [Google Scholar]
- 16.Macy E.M., Hayes T.E., Tracy R.P. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 17.Maigne R. Low back pain of thoracolumbar origin. Arch Phys Med Rehabil. 1980;61:389–395. [PubMed] [Google Scholar]
- 18.Maigne R. Sémiologie des dérangements intervertébraux mineurs. Ann Med Phys. 1972;15:277–289. [Google Scholar]
- 19.Fuhr A.W., Fischer R.S. The Activator Method. 2nd ed. Mosby Elsevier; St-Louis: 2009. pp. 141–161. [Google Scholar]
- 20.Roy R.A., Boucher J.P., Comtois A.S. Digitized infrared segmental thermometry: time requirements for stable recordings. J Manipulative Physiol Ther. 2006;29:468.e1–468.e10. doi: 10.1016/j.jmpt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Roy R.A., Boucher J.P., Comtois A.S. Heart rate variability modulation in pain-free patients vs patients in pain. J Manipulative Physiol Ther. 2009;32:277–286. doi: 10.1016/j.jmpt.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Roy R.A., Boucher J.P., Comtois A.S. Paraspinal lumbar cutaneous temperature modification following a single lumbar spinal manipulation. J Manipulative Physiol Ther. 2010;33:308–314. doi: 10.1016/j.jmpt.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Colloca C.J., Keller T.S., Gunzburg R. Biomechanical and neurophysiological responses to spinal manipulation in patients with lumbar radiculopathy. J Manipulative Physiol Ther. 2004;27:1–15. doi: 10.1016/j.jmpt.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Fuhr A.W., Pavia G.R. Department of records. Activator Methods International, Ltd; Phoenix: 2008. Activator Methods chiropractic technique. [Google Scholar]
- 25.Barbuto L.M. Industrial back pain and recovery time. J Can Chiropr Assoc. 1984;28:205–208. [Google Scholar]
- 26.Cohen J. Academic Press; New York: 1969. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 27.Cohen J. 2nd ed. Lawrence Earlbaum Associates; Hillsdale (N.J.): 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 28.von Haehling S., Schefold J.C., Lainscak M., Doehner W., Anker S.D. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail Clin. 2009;5(4):549–560. doi: 10.1016/j.hfc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Ho K.M., Lipman J. An update on C-reactive protein for intensivists. Anaesth Intensive care. 2009;37(2):234–241. doi: 10.1177/0310057X0903700217. [DOI] [PubMed] [Google Scholar]
- 30.Quon J.A., Cassidy J.D., O'Connor S.M., Kirkaldy-Willis W.H. Lumbar intervertebral disc herniation: treatment by rotational manipulation. J Manipulative Physiol Ther. 1989;12:220–227. [PubMed] [Google Scholar]
- 31.Feghali C.A., Wright T.M. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]