Outcomes Among Black Patients With Stage II and III Colon Cancer Receiving Chemotherapy: An Analysis of ACCENT Adjuvant Trials (original) (raw)
Abstract
Background
Among patients with resected colon cancer, black patients have worse survival than whites. We investigated whether disparities in survival and related endpoints would persist when patients were treated with identical therapies in controlled clinical trials.
Methods
We assessed 14 611 patients (1218 black and 13 393 white) who received standardized adjuvant treatment in 12 randomized controlled clinical trials conducted in North America for resected stage II and stage III colon cancer between 1977 and 2002. Individual patient data on covariates and outcomes were extracted from the Adjuvant Colon Cancer ENdpoinTs (ACCENT) database. The endpoints examined in this meta-analysis were overall survival (time to death), recurrence-free survival (time to recurrence or death), and recurrence-free interval (time to recurrence). Cox models were stratified by study and controlled for sex, stage, age, and treatment to determine the effect of race. Kaplan–Meier estimates were adjusted for similar covariates to control for confounding. All statistical tests were two-sided.
Results
Black patients were younger than whites (median age, 58 vs 61 years, respectively; P < .001) and more likely to be female (55% vs 45%, respectively; P < .001). Overall survival was worse in black patients than whites (hazard ratio [HR] of death = 1.22, 95% confidence interval [CI] = 1.11 to 1.34, P < .001). Five-year overall survival rates for blacks and whites were 68.2% and 72.8%, respectively. When subsets defined by sex, stage, and age were analyzed, overall survival was consistently worse in black patients. Recurrence-free survival was worse in black patients than whites (HR of recurrence or death = 1.14, 95% CI = 1.04 to 1.24, P = .0045). Three-year recurrence-free survival rates in blacks and whites were 68.4% and 72.1%, respectively. In contrast, recurrence-free interval was similar in black and white patients (HR of recurrence = 1.08, 95% CI = 0.97 to 1.19, P = .15). Three-year recurrence-free interval rates in blacks and whites were 71.3% and 74.2%, respectively.
Conclusions
Black patients with resected stage II and stage III colon cancer who were treated with the same therapy as white patients experienced worse overall and recurrence-free survival, but similar recurrence-free interval, compared with white patients. The differences in survival may be mostly because of factors unrelated to the patients’ adjuvant colon cancer treatment.
CONTEXT AND CAVEATS
Prior knowledge
During 1999–2005, the 5-year relative survival rates for black and white colorectal cancer patients were 57% and 68%, respectively, in the United States. The disparity in survival between black and white patients remains substantial, and the reasons for disparity are still not well understood.
Study design
Data were obtained from the Adjuvant Colon Cancer ENdpoinTs (ACCENT) collaborative group database to analyze time-to-event endpoints for black and white patients participating in 12 randomized controlled adjuvant phase III trials of resected stage II and III colon cancer. The endpoints were overall survival (time to death), recurrence-free survival (time to recurrence or death), and recurrence-free interval (time to recurrence). Statistical models were stratified by study and controlled for sex, stage, age, and treatment.
Contribution
Black patients showed a worse 5-year overall survival rate compared with white patients. Recurrence-free survival was also worse in black patients, but the recurrence-free interval was similar in black and white patients.
Implication
The disparity in overall survival and recurrence-free survival between black and white patients is not explained by differential response to adjuvant treatment.
Limitation
ACCENT database has no information on toxicity, comorbid conditions, and treatment for recurrent disease, thereby limiting the analysis on disparities in outcomes associated with race.
From the Editors
In the United States, there were an estimated 146 970 new cases of colorectal cancer diagnosed, resulting in 49 920 deaths or a death-to-diagnosis ratio of 34 to 100 in 2006 (1). Of those diagnosed, approximately 15 000 colorectal cancer cases were expected to occur in individuals of African ancestry (African American or black patients) and are expected to result in nearly 7000 deaths or a 47 to 100 death-to-diagnosis ratio over the patients’ lifetimes (1). Although survival outcomes in the US colorectal cancer population have improved in recent years, the disparity in survival between blacks and whites has not declined and remains substantial. The 5-year relative survival rates in the United States for black and white colorectal cancer patients during 1999–2005 were 57% and 68%, respectively (1). Higher death rates from colorectal cancer account for 25% of the disparity in cancer death rates between black and white women and 11% of the disparity between black and white men (1). Despite the identification of several putative causes for worse outcomes in blacks, which include more advanced cancer stage at diagnosis, lack of access to medical care, suboptimal treatment, and lower socioeconomic status, the reasons for this disparity remain poorly understood (2).
In addition to the above-mentioned reasons for lower survival rates in blacks, it has been proposed that colorectal cancer in black patients is less responsive to treatment because of a number of genetic factors related to both tumor characteristics in this population and to ethnicity-based differences in pharmacogenetics that lead to disparate activity and toxicities of chemotherapy agents (3). However, race, in and of itself, may not be important in determining the prognosis of patients diagnosed with colorectal cancer. It is possible that by adjusting for comorbid conditions and socioeconomic factors associated with race, the poor survival outcome observed for black patients may be better understood and then either moderated or eliminated. In this study, we examined survival among black and white patients participating in adjuvant phase III cooperative group trials of resected colon cancer with data from the Adjuvant Colon Cancer ENdpoinT (ACCENT) collaborative group database (4–15). The cooperative group trial design defines the extent of disease at study entry and provides for uniform therapy, thus controlling for two major confounding factors affecting prognosis and allowing for other determinants of prognosis to be investigated.
The mandate of the National Institutes of Health Revitalization Act of 1993 required that all National Institutes of Health–sponsored clinical trials (including cancer cooperative group trials) include minorities and women in sufficient numbers to allow valid subset analysis to ascertain differences in a treatment’s effect among women and minority participants (16). Our present analysis is an attempt to fulfill the intent of that mandate in a rational manner.
Methods
Patients and Data
We analyzed data from 14 611 individual patients on 12 phase III randomized controlled clinical trials conducted in North America from 1977 to 2002 for adjuvant colon cancer (4–15). These trials provided standardized adjuvant treatment following resection of the primary tumor for these patients. Subsequent treatment in the event of recurrent disease was outside the scope of these studies and was not controlled by the study protocols; neither were data collected on salvage therapy. Our data come from the ACCENT collaborative group database. ACCENT is an international collaboration of colon cancer researchers who have pooled outcome data to better answer difficult questions related to our research. We included all trials from the database that had the following data available: treatment group, age, stage of disease (II or III), sex, time and censoring variables for our three endpoints (overall survival [time to death], recurrence-free survival [time to recurrence or death], and recurrence-free interval [time to recurrence]), and race (black or white). The analysis was restricted to patients with stage II and III disease and patients with race self-reported as black or white. A shortcoming of this database for the purpose of the present analysis is that it does not include data on toxicity or comorbid conditions.
Trials Included
Table 1 provides a summary of the ACCENT adjuvant trials included in this analysis. All included studies were approved by institutional review committees in accordance with the Helsinki Declaration. Written informed consent was obtained from all included participants. Dignam et al. (17) published a similar analysis of disparities in outcome by race on 6632 patients from five National Surgical Adjuvant Breast and Bowel Project (NSABP) studies in 1999; patients were a subset of the current study. A similar study based on participants from Eastern Cooperative Oncology Group trials was published in 2002, with largely similar findings (18).
Table 1.
ACCENT studies included in the analysis*
| Study† (reference) | No. | Race, % | Treatment type, % | ||||
|---|---|---|---|---|---|---|---|
| White | Black | Surgery alone | 5-FU or MOF | 5-FU + LV or LEV | 5-FU + LV + oxaliplatin or irinotecan | ||
| CALGB 89803 (4) | 1176 | 92.9 | 7.1 | 49.4 | 50.6 | ||
| INT 0035 (5) | 849 | 93.2 | 6.8 | 51.1 | 48.9 | ||
| NCCTG 894651 (6) | 814 | 97.1 | 3.0 | 100.0 | |||
| NCCTG 914653 (7) | 607 | 96.9 | 3.1 | 100.0 | |||
| S9415 (8) | 906 | 90.9 | 9.1 | 100.0 | |||
| NSABP C-01 (9) | 965 | 81.9 | 18.1 | 68.2 | 31.8 | ||
| NSABP C-02 (10) | 646 | 90.6 | 9.4 | 49.9 | 50.1 | ||
| NSABP C-03 (11) | 988 | 91.2 | 8.8 | 50.2 | 49.8 | ||
| NSABP C-04 (12) | 1979 | 91.8 | 8.2 | 100.0 | |||
| NSABP C-05 (13) | 1999 | 91.5 | 8.5 | 100.0 | |||
| NSABP C-06 (14) | 1457 | 91.4 | 8.6 | 100.0 | |||
| NSABP C-07 (15) | 2225 | 92.4 | 7.6 | 50.6 | 49.4 | ||
| Total‡ | 14611 | 91.7 | 8.3 | 9.7 | 7.7 | 71.0 | 11.6 |
Statistical Analysis
Endpoints evaluated included overall survival (determined from time of study entry until death from any cause), recurrence-free survival (determined from the time of study entry until disease recurrence or death from any cause), and recurrence-free interval (determined from the time of study entry until disease recurrence but censoring for death before recurrence). The meta-analysis of data from the included trials was accomplished by multivariable Cox proportional hazard models (19) stratified by study. The Cox models were used to determine the hazard ratio (HR) and associated 95% confidence interval (CI) for race and to test for statistical significance after controlling for potential confounding because of sex, stage, age, or treatment type. The HR for race is calculated as the hazard of the event of interest (death for overall survival, recurrence or death for recurrence-free interval, and recurrence for recurrence-free interval) in black patients divided by the hazard of the event of interest in white patients so that values less than one favor blacks and values greater than one favor whites. Tests for interaction of the effect of race with individual prognostic covariates in the model were accomplished by sequentially adding race–covariate interaction terms to the model above and testing for statistical significance of the interaction in the Cox model. The lone exception to this pattern of interaction testing was for treatment, where to limit complexity, the test was restricted to the three studies comparing surgery alone to chemotherapy [INT 0035 (5), NSABP C-01 (9), and NSABP C-02 (10)]. Possible heterogeneity of the effect of race across studies was tested by evaluating the race–study interaction terms in a Cox model where the interaction terms and terms for individual studies were added to the model instead of stratifying for study. The proportional hazards assumption for the variable of interest for statistical inference in each model was tested by the method of Grambsch and Therneau (20). Kaplan–Meier estimates were adjusted for study, sex, stage, age, and type of treatment using the Xie and Liu method (21) to control for possible confounding from these prognostic factors. All P values are two-sided and values less than .05 are considered statistically significant.
Results
Among the 14 611 patients included in this analysis, 1218 (8.3%) described themselves as black. The distribution of treatment and patient and/or tumor characteristics by race for all patients is shown in Table 2. Black patients were younger than white patients (median age, 58 [range 17–85 years] vs 61 years [range 15–90 years], respectively, P < .01) and more likely to be female (55% vs 45%, P < .01). There was a borderline statistically significant difference between blacks and whites by stage of disease (35% stage II in blacks vs 33% in whites, P = .069). There were racial differences in the distribution of treatments received (P < .01) because of different rates of black patient participation among the trials studied, but treatments were balanced by race within individual studies. To control for these observed differences in the distribution of covariates by race, subsequent analyses adjusted for differences in sex, stage, age, and treatment type.
Table 2.
Patient and tumor characteristics of patients included in the analysis*
| Category | White patients, % (N = 13 393) | Black patients, % (N = 1218) | P† |
|---|---|---|---|
| Cancer stage‡ | |||
| II | 32.6 | 35.1 | .069 |
| III | 67.4 | 64.9 | |
| Sex | |||
| Women | 45.0 | 54.7 | <.01 |
| Men | 55.0 | 45.3 | |
| Age, y | |||
| <40 | 5.5 | 7.6 | <.01 |
| 40–49 | 12.6 | 15.4 | |
| 50–59 | 26.9 | 32.6 | |
| 60–69 | 37.4 | 32.3 | |
| ≥70 | 17.6 | 12.2 | |
| Treatment | |||
| Surgery alone | 9.3 | 14.4 | <.01 |
| 5-FU or MOF | 7.4 | 10.8 | |
| 5-FU + LV or LEV | 71.7 | 64.0 | |
| 5-FU + LV + oxaliplatin/irinotecan | 11.7 | 10.8 |
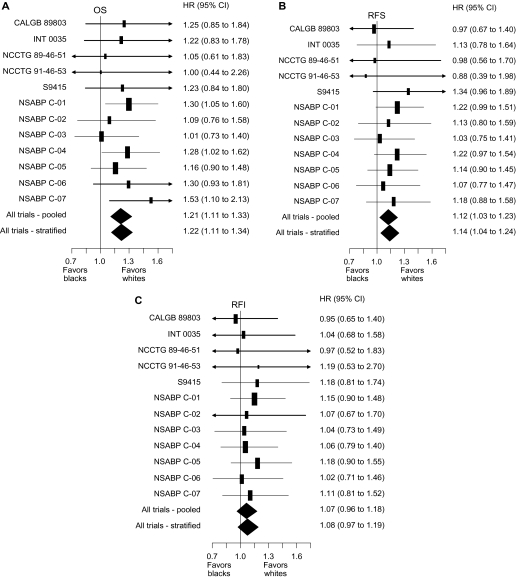
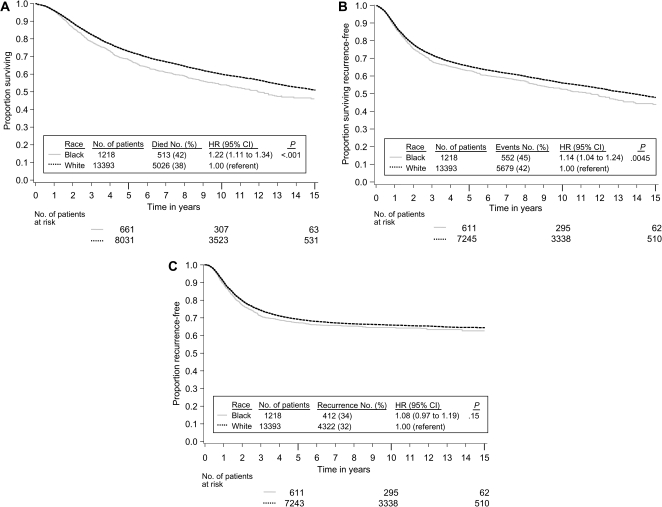
We began analysis by verifying that the effect of race on our three endpoints did not vary by treatment, by prognostic covariates, or by included study. There was no evidence that the effect of treatment differed by race in the three studies [INT 0035 (5), NSABP C-01 (9), and NSABP C-02 (10)] that compared surgery alone to surgery plus 5-fluorouracil (5-FU)–based chemotherapy (race–treatment interaction: for overall survival, _P_interaction = .76; for recurrence-free survival, _P_interaction = .85; and for recurrence-free interval, _P_interaction = .51). Across all studies (4–15), there was no strong evidence that the effect of race varied by stage of disease (race–stage interaction: for overall survival, _P_interaction = .97; for recurrence-free survival, _P_interaction = .80; and for recurrence-free interval, _P_interaction = .37); by sex (race–sex interaction: for overall survival, _P_interaction = .49; for recurrence-free survival, _P_interaction = .49; and for recurrence-free interval, _P_interaction = .15), or by age (race–age interaction: for overall survival, _P_interaction = .38; for recurrence-free survival, _P_interaction = 0.40; and for recurrence-free interval, _P_interaction = .18). The hazard ratios (black divided by white) for overall survival (Figure 1, A), recurrence-free survival (Figure 1, B), and recurrence-free interval (Figure 1, C) show that there was no evidence of heterogeneity of the effect of race across studies for any of these endpoints analyzed (race–study interaction: for overall survival, _P_interaction = .59; for recurrence-free survival, _P_interaction = .41; and for recurrence-free interval, _P_interaction = .71). We next analyzed the effect of race on our three endpoints after stratifying for study and controlling for sex, stage, age, and treatment type. All Kaplan–Meier estimates are adjusted for study, sex, stage, age, and type of treatment to control for possible confounding from these factors. As shown in the adjusted Kaplan–Meier plots of overall survival by race (Figure 2, A), overall survival (time to death from any cause) in black patients was worse than in white patients (5539 deaths, HR of death = 1.22, 95% confidence interval [CI] = 1.11 to 1.34, P < .001). Table 3 shows the results from Cox models testing the effect of race on our three endpoints. The 5-year adjusted Kaplan–Meier estimates of survival rates for black vs white patients were 68.2% vs 72.8%, respectively (data not shown in table or figure). Overall survival was consistently worse in black patients in subsets defined by sex, stage, and age (data not shown). As shown in the adjusted Kaplan–Meier plots of recurrence-free survival by race (Figure 2, B), recurrence-free survival (time to recurrence or death without recurrence) results were attenuated compared with overall survival, but recurrence-free survival remained statistically significantly worse in black patients (6231 events, HR of recurrence or death = 1.14, 95% CI = 1.04 to 1.24, P = .0045). The 3-year adjusted Kaplan–Meier estimates of recurrence-free survival rates for black vs white patients were 68.4% vs 72.1%, respectively (data not shown in table or figure). As shown in the adjusted Kaplan–Meier plots of recurrence-free interval by race (Figure 2, C), recurrence-free interval (time to recurrence censoring for death without recurrence) did not differ statistically significantly by race (4734 recurrences, HR of recurrence = 1.08, 95% CI = 0.97 to 1.19, P = .15). The 3-year adjusted Kaplan–Meier estimates of recurrence-free interval rates for black vs white patients were 71.3% vs 74.2%, respectively (data not shown in table or figure). This analysis had 76% power to detect a hazard ratio of 1.15 for recurrence-free interval (roughly equivalent to a 3.2% detriment for blacks in 3-year recurrence-free interval assuming 72.4% recurrence-free interval for whites; survival follows an exponential distribution).
Figure 1.
Forest plots of hazard ratios (black divided by white) for death (overall survival [OS]), recurrence or death (recurrence-free survival [RFS]), and recurrence (recurrence-free interval [RFI]). The vertical lines indicate a hazard ratio of 1.0 (no difference between black and white race), values less than 1.0 favor black patients, and values greater than 1.0 favor white patients. Solid rectangles represent the hazard ratio of each single randomized controlled trial; the area of each rectangle is proportional to the inverse of the variance of the estimate. The horizontal line represents the 95% confidence interval and arrowheads indicate that the confidence interval extends beyond the scale of the plot. The solid diamonds represent the overall estimated hazard ratio based on multivariable Cox models of overall survival, recurrence-free survival, and recurrence-free interval controlling for sex, stage, age, and treatment, either not controlling for study (pooled) or stratifying by study (stratified); the diamond’s width represents the 95% confidence interval of the hazard ratio. A) OS. B) RFS. C) RFI. CALGB = Cancer And Leukemia Group B; CI = confidence interval; INT = Intergroup; NCCTG = North Central Cancer Treatment Group; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Figure 2.
Kaplan–Meier estimates adjusted by the method of Xie and Liu (21) for study, sex, stage, age, and type of treatment. A) Overall survival. B) Recurrence-free survival. C) Recurrence-free interval. Life tables for the number of patients at risk are presented below each graph. In a matrix within each graph, the number of patients and events are provided by race along with the hazard ratio and P value from a multivariable Cox model stratified by study and controlling for sex, stage, age, and treatment. The P values come from the two-sided Wald test.
Table 3.
Multivariable Cox models stratified by study*
| Factor | No. | OS | RFS | RFI | |
|---|---|---|---|---|---|
| HR (95% CI) | P† | HR (95% CI) | P† | HR (95% CI) | P† |
| Cancer stage‡ | <.001 | <.001 | <.001 | ||
| II | 4792 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| III | 9819 | 1.97 (1.85 to 2.10) | 1.96 (1.84 to 2.08) | 2.56 (2.37 to 2.75) | |
| Sex | <.001 | <.001 | 0.27 | ||
| Women | 6697 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| Men | 7914 | 1.18 (1.12 to 1.25) | 1.14 (1.08 to 1.20) | 1.03 (0.98 to 1.10) | |
| Age, y | <.001 | <.001 | 0.40 | ||
| <50 | 2708 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| 50–59 | 3996 | 1.14 (1.04 to 1.24) | 1.09 (1.00 to 1.18) | 1.00 (0.91 to 1.08) | |
| ≥60 | 7907 | 1.52 (1.41 to 1.65) | 1.32 (1.23 to 1.42) | 0.96 (0.88 to 1.03) | |
| Race | <.001 | 0.0045 | 0.15 | ||
| White | 13 393 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| Black | 1218 | 1.22 (1.11 to 1.34) | 1.14 (1.04 to 1.24) | 1.08 (0.97 to 1.19) |
Discussion
In this cohort of 14 611 colon cancer patients treated on clinical trials of adjuvant chemotherapy, 1218 patients were black and 13 393 patients were white. We analyzed individual patient data from 12 clinical trials included in the ACCENT collaborative database. We found statistically significantly shorter overall survival for black patients compared with white patients with a 4.6% detriment in 5-year survival. Recurrence-free survival was also statistically significantly shorter for black patients compared with white patients with a 3.7% detriment in 3-year outcomes. Finally, we found no statistically significant difference in recurrence-free interval (time to recurrence) with a non-statistically significant detriment of 2.9% observed for 3-year outcomes.
The aim of adjuvant chemotherapy for colon cancer is to extend patient survival by delaying or preventing recurrence of colon cancer. Of the three endpoints considered in this analysis, recurrence-free interval (time to recurrence) is the most sensitive measure of the intended effect of chemotherapy. Unlike overall and recurrence-free survival, this endpoint would not be biased as a result of disparity in treatment for recurrent colon cancer, disparate care for comorbid conditions, or differential rates of death from causes unrelated to colon cancer. In this study, recurrence-free interval for black and white patients did not differ statistically significantly by race (HR of recurrence = 1.08, 3-year difference 2.9%, 4734 recurrences). The similar outcomes for recurrence-free interval suggest that equivalent treatment in the setting of a controlled clinical trial produces similar outcomes in both black and white patients with regard to tumor recurrence. Any differential response to adjuvant chemotherapy must be small.
The second endpoint considered in this study was recurrence-free survival (time to recurrence or death). In this analysis, black patients were found to have slightly worse recurrence-free survival compared with white patients (HR of recurrence or death = 1.14, 3-year difference 3.7%, 6231 events [recurrences or deaths]). Every patient who has an event (recurrence) for recurrence-free interval also has an event for recurrence-free survival; however, patients who die without recurrence are counted as an event for recurrence-free survival but not recurrence-free interval. These additional deaths (which were not preceded by recurrence, N = 6231–4734 = 1497) are likely unrelated to colon cancer and unlikely to be affected by adjuvant chemotherapy. Racial differences in comorbidity or general life expectancy are potential explanations for the statistically significant difference observed on this endpoint. Because life expectancy differs by race independent of a cancer diagnosis (22), it is reasonable to conclude that some of the disparity in recurrence-free survival observed in this study is because of factors unrelated to adjuvant treatment for colon cancer.
Overall survival (time to death from any cause) was the final endpoint considered in this study. Despite similar stages of disease and equivalent adjuvant therapy, our analysis of the survival endpoint found a statistically significant difference by race with worse survival for black patients (HR of death = 1.22, 5-year difference 4.6%, 5539 deaths) when compared with white patients. The overall survival endpoint includes the same 1497 deaths not preceded by recurrence (27% of all deaths) included as events for recurrence-free survival, and overall survival is similarly susceptible to confounding by racial differences in comorbidity or general life expectancy. Additionally, the remaining 4042 deaths (73% of all deaths) were preceded by recurrence, and time to death for these patients may have been impacted by salvage treatment for recurrent disease. Because treatment for recurrent disease was outside the research protocols of the studies included in this analysis, racial differences in care for recurrent disease are also a potential confounder of overall survival.
There was no heterogeneity of the effect of race across studies for any of the endpoints considered. The effect of 5-FU treatment appears to be similar by race for the endpoints considered. The effect of race was also similar for our three endpoints by stage of disease, sex, and age.
Colorectal cancer remains a leading cause of cancer-related deaths in the United States. Across all stages of disease, survival for black patients continues to lag behind that reported for nonblack patients (1). There have been a number of potential reasons proposed for this observation including later stage at diagnosis, differences in treatment, and the postulate that black patients have a biologically more aggressive disease or have a poorer response to currently used chemotherapy agents. Although these factors are important in patient outcome, perhaps the most relevant hypothesis is that African American patients are less likely to have access to routine cancer care, resulting in a poorer patient condition (eg, greater weight loss and poorer performance status) and later stage at diagnosis. In support of this postulate are data from the Black/White Cancer Survival Study Group (23–27). Using the ACCENT clinical trial database, we have attempted to investigate this hypothesis. The ACCENT adjuvant colon cancer database offers several advantages over the use of other data sources. First, stage of disease is comparable within the constraints of the protocol entry criteria. Second, treatment and follow-up care are conducted according to a uniform standard.
This study has a few limitations. The ACCENT database does not include data on toxicity, comorbid conditions, and treatment for recurrent disease. The lack of these important data hinders our investigation of some important questions related to disparities in outcomes. Black vs white race is clearly not a randomized comparison, so we are forced to attempt to control for as many potentially prognostic covariates as possible to avoid a biased comparison by race.
The reason for the inferior overall survival for the black patients in this analysis warrants further consideration. In addition to the potential for noncancer deaths to contribute to differences in overall survival, the literature would support the notion that black patients may have a genetically unique response or toxicity to treatment compared with white patients. In the adjuvant trials included in this analysis, all patients receiving chemotherapy were treated with 5-FU either alone or in combination with other agents. 5-FU must first be activated by binding to methylenetetrahydrofolate reductase (MTHFR), a cellular molecule present at varying concentrations among patients(28). It is known that the MTHFR gene has several polymorphisms among populations, different MTHFR polymorphisms confer different levels of sensitivity to chemotherapy (28), and that the polymorphisms are influenced by race. In a related finding, the pharmacogenetic syndrome, dihydropyrimidine dehydrogenase (DPD) deficiency, has been shown to predispose cancer patients to severe 5-FU toxicity (29). As reported by Mattison et al. (30), the prevalence of DPD deficiency was threefold higher in black healthy volunteers when compared with white volunteers; 8.0% and 2.8%, respectively. An analysis of 1402 patients receiving chemotherapy for metastatic colorectal cancer (3) found that black patients had a different frequency of polymorphisms in key genes coding for enzymes involved in drug activation, metabolism, and disposition. The unavailability of toxicity data for the current analysis limits our ability to explore this question fully, but the similarities of recurrence-free interval outcomes by race suggest similar response to adjuvant chemotherapy.
The ability for patients to receive or to respond to salvage chemotherapy and/or surgery subsequent to recurrence could clearly impact on overall survival without affecting recurrence-free interval. The disparity in access to medical care between black and white patients is real (31) but incompletely understood. Further study is warranted with the goal of improving overall survival in black patients with stage II and III colon cancer.
Black patients with resected stage II and III colon cancer treated with identical adjuvant therapy experienced poorer overall and recurrence-free survival but similar recurrence-free interval compared with white patients. The overall and recurrence-free survival endpoints include deaths not preceded by cancer recurrence and likely unrelated to colon cancer. This suggests that overall and recurrence-free survival differences may be largely because of factors unrelated to the patients’ adjuvant colon cancer treatment. Biological differences, differences in general health, and disparities in health care outside the clinical trial are possible explanations for these findings but will need to be pursued in other studies where data on patient care for recurrent cancer or comorbid conditions are available.
Funding
Public Health Service Grants U10CA-12027, U10CA-69974, U10CA-37377, U10CA-69651, and U24-CA-114732 (National Surgical Adjuvant Breast and Bowel Project); CA 25224 (North Central Cancer Treatment Group) from the National Cancer Institute, Department of Health and Human Services.
Appendix
The Adjuvant Colon Cancer ENdpoinT (ACCENT) Collaborative Group consists of D. J. Sargent, E. Green, A. Grothey, S. R. Alberts, B. Bot, M. Campbell, Q. Shi (Mayo Clinic, Rochester, MN); G. Yothers, M. J. O’Connell, N. Wolmark (National Surgical Adjuvant Breast and Bowel Project Biostatistical and Operations Centers, Pittsburgh, PA; Allegheny General Hospital, Pittsburgh, PA [NW]); A. de Gramont (Hôpital Saint Antoine, Paris, France); R. Gray, D. Kerr (QUASAR Collaborative Group, Birmingham and Oxford, UK); D. G. Haller (Abramson Cancer Center at the University of Pennsylvania, Philadelphia, PA); J. Benedetti (Southwest Oncology Group Statistical Center, Seattle, WA); M. Buyse (International Drug Development Institute, Louvain-la-Neuve, Belgium); R. Labianca (Ospedali Riuniti, Bergamo, Italy); J. F. Seitz (University of the Mediterranean, Marseilles, France); C. J. O’Callaghan (National Cancer Institute of Canada Clinical Trials Group, Queens University, Kingston, ON, Canada); G. Francini (University of Siena, Siena, Italy); P. J. Catalano (Eastern Cooperative Oncology Group Statistical Center, Boston, MA); C. D. Blanke (British Columbia Cancer Agency, Vancouver, British Columbia, Canada); T. Andre (Groupe hospitalier de la Pitié-Salpétrière, Paris, France); R. M. Goldberg, H. Sanoff (University of North Carolina, Chapel Hill, NC); A. Benson (Northwestern University, Chicago, IL).
Footnotes
Clinical Trials registration—CALGB 89803: NCT00003835; INT 0035: Not applicable; NCCTG 894651: Not applicable; NCCTG 914653: NCCTG 914653; S9415: NCT00002593; NSABP C-01: NCT00427570; NSABP C-02: NCT00427310; NSABP C-03: NSABP-C-03; NSABP C-04: NCT00425152; NSABP C-05: NSABP-C-05; NSABP C-06: NCT00004931; NSABP C-07: NCT00096278.
The authors were responsible for all decisions related to analysis and interpretation of the data, writing of this report, and the decision to submit for publication. The study designs and collection of data for the included trials were the responsibility of the original authors of the Adjuvant Colon Cancer ENdpoinT (ACCENT) Adjuvant Trials.
GY had full access to the primary data and performed the analyses. AWB conceived the idea for these analyses and was instrumental in an early draft of this report. Specific contributions were as follows: GY, DJS, NW, JJD, and AWB were responsible for conception and study design: GY, DJS, RG, LBS were responsible for acquisition of data; GY, DJS, RG, MJO, JKB, and AWB were responsible for analysis and interpretation of data; GY, DJS, and AWB were responsible for writing the article; DJS, NW, RG, MJO, JKB, LBS, and JJD were responsible for critical revisions of the draft; GY, DJS, and JKB were responsible for statistical analysis; DJS was responsible for obtaining the funding. All authors contributed to decisions on analyses to be performed, interpretation of the results, and the writing and editing of the report. GY, AWB, DJS, and RMG determined submission. Administrative, technical, or material support was provided by GY, DJS, NW, and LBS. GY, DJS, RG, and LBS were responsible for supervision.
Conflicts of interest: NW declares funding from sanofi-aventis to NSABP through a business contract for C-07 only, for certain costs not covered by the NCI, for non-standard-of-care procedures required for the study. Also, consultancy from sanofi-aventis (C-07 only), honoraria to the NSABP for NW's participation on the advisory board, and reasonable travel and accommodations for the sanofi-aventis board meeting. There are no other potential conflicts.
References
- 1.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 19752006. Bethesda, MD: National Cancer Institute; 2008. http://seer.cancer.gov/csr/1975_2006/. Accessed June 1, 2010. Based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- 2.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clin North Am. 2005;89(4):771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Sanoff H, Sargent DJ, Green EM, McLeod HM, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27:4109–4115. doi: 10.1200/JCO.2009.21.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25(23):3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322(6):352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16(1):295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 7.O’Connell MJ, Sargent DJ, Windschitl HE, et al. Randomized clinical trial of high-dose levamisole combined with 5-fluorouracil and leucovorin as surgical adjuvant therapy for high-risk colon cancer. Clin Colorectal Cancer. 2006;6(2):133–139. doi: 10.3816/ccc.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 8.Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol. 2005;23(9):1819–1825. doi: 10.1200/JCO.2005.04.169. [DOI] [PubMed] [Google Scholar]
- 9.Wolmark N, Fisher B, Rockette H, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst. 1988;80(1):30–36. doi: 10.1093/jnci/80.1.30. [DOI] [PubMed] [Google Scholar]
- 10.Wolmark N, Rockette H, Wickerham DL, et al. Adjuvant therapy of Dukes’ A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C-02. J Clin Oncol. 1990;8:1466–1475. doi: 10.1200/JCO.1990.8.9.1466. [DOI] [PubMed] [Google Scholar]
- 11.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from NSABP protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 12.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17(11):3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 13.Wolmark N, Bryant J, Smith R, et al. Adjuvant 5-fluorouracil and leucovorin with or without interferon alfa- 2a in colon carcinoma: National Surgical Adjuvant Breast and Bowel Project protocol C-05. J Natl Cancer Inst. 1998;90(23):1810–1816. doi: 10.1093/jnci/90.23.1810. [DOI] [PubMed] [Google Scholar]
- 14.Lembersky BC, Wieand HS, Petrelli NJ, et al. Oral uracil-tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24(13):2059–2064. doi: 10.1200/JCO.2005.04.7498. [DOI] [PubMed] [Google Scholar]
- 15.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus 5-fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP protocol C-07. J Clin Oncol. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- Public Law 103-43. National Institutes of Health Revitalization Act of 1993. 42 USC 289 (a)(1). http://history.nih.gov/research/downloads/PL103-43.pdf. Accessed June 13, 2011. [Google Scholar]
- 17.Dignam JJ, Colangelo L, Tian W, et al. Outcomes among African-Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst. 1999;91(22):1933–1940. doi: 10.1093/jnci/91.22.1933. [DOI] [PubMed] [Google Scholar]
- 18.McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in African-American and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94(15):1160–1167. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 20.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 21.Xie J, Liu C. Adjusted Kaplan–Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 22.Harper S, Lynch J, Burris S, Davey Smith G. Trends in the black-white life expectancy gap in the United States, 1983-2003. JAMA. 2007;297(11):1224–1232. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- 23.Howard J, Hankey BF, Greenberg RS, et al. A collaborative study of differences in the survival rates of black patients and white patients with cancer. Cancer. 1992;69(9):2349–2360. doi: 10.1002/1097-0142(19920501)69:9<2349::aid-cncr2820690925>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272(12):947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 25.Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87(22):1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 26.Hill HA, Eley JW, Harlan LC, Greenberg RS, Barrett RJ, II, Chen VW. Racial differences in endometrial cancer survival: the Black/White Cancer Survival Study. Obstet Gynecol. 1996;88(6):919–926. doi: 10.1016/s0029-7844(96)00341-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen VW, Fenoglio-Preiser CM, Wu XC, et al. Aggressiveness of colon carcinoma in blacks and whites. National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1997;6(12):1087–1093. [PubMed] [Google Scholar]
- 28.Etienne MC, Formento JL, Chazal M, et al. Methylenetetrahydrofolate reductase gene polymorphisms and response to fluorouracil-based treatment in advanced colorectal cancer patients. Pharmacogenetics. 2004;14(12):785–792. doi: 10.1097/00008571-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Yen JL, McLeod HL. Should DPD analysis be required prior to prescribing fluoropyrimidines? Eur J Cancer. 2007;43(6):1011–1016. doi: 10.1016/j.ejca.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Mattison LK, Fourie J, Desmond RA, Modak A, Saif MW, Diasio RB. Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with Caucasians. Clin Cancer Res. 2006;12(18):5491–5495. doi: 10.1158/1078-0432.CCR-06-0747. [DOI] [PubMed] [Google Scholar]
- 31.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]