Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies (original) (raw)
Abstract
Objective To investigate the association between intake of dietary fibre and whole grains and risk of colorectal cancer.
Design Systematic review and meta-analysis of prospective observational studies.
Data sources PubMed and several other databases up to December 2010 and the reference lists of studies included in the analysis as well as those listed in published meta-analyses.
Study selection Prospective cohort and nested case-control studies of dietary fibre or whole grain intake and incidence of colorectal cancer.
Results 25 prospective studies were included in the analysis. The summary relative risk of developing colorectal cancer for 10 g daily of total dietary fibre (16 studies) was 0.90 (95% confidence interval 0.86 to 0.94, I2=0%), for fruit fibre (n=9) was 0.93 (0.82 to 1.05, I2=23%), for vegetable fibre (n=9) was 0.98 (0.91 to 1.06, I2=0%), for legume fibre (n=4) was 0.62 (0.27 to 1.42, I2=58%), and for cereal fibre (n=8) was 0.90 (0.83 to 0.97, I2=0%). The summary relative risk for an increment of three servings daily of whole grains (n=6) was 0.83 (0.78 to 0.89, I2=18%).
Conclusion A high intake of dietary fibre, in particular cereal fibre and whole grains, was associated with a reduced risk of colorectal cancer. Further studies should report more detailed results, including those for subtypes of fibre and be stratified by other risk factors to rule out residual confounding. Further assessment of the impact of measurement errors on the risk estimates is also warranted.
Introduction
Colorectal cancer is the third most common type of cancer, with 1.2 million new cases diagnosed in 2008 worldwide, accounting for about 9.7% of all cases of cancer.1 Evidence from ecological studies, migrant studies, and secular trend studies suggest that environmental risk factors are of major importance in the cause of colorectal cancer.2 3 4 Dietary factors have been suspected as important, but only intakes of red and processed meat and alcohol are considered to be convincing dietary risk factors for colorectal cancer.5
In the 1970s, Burkitt proposed the hypothesis that dietary fibre reduces the risk of colorectal cancer, based on the observation of low rates of such cancer among rural Africans who ate a diet with a high fibre content.6 Several plausible mechanisms have been proposed to explain the hypothesis, including increased stool bulk and dilution of carcinogens in the colonic lumen, reduced transit time, and bacterial fermentation of fibre to short chain fatty acids.7 However, although many epidemiological studies have investigated the association between fibre intake and risk of colorectal cancer, the results have not been consistent and the possibility of residual confounding by folate intake remains a controversial issue.8 Case-control studies have generally shown a protective association,9 10 whereas the results from cohort studies have been mixed.8 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 In addition, it is not clear whether only specific types or sources of fibre are associated with the risk. Although initial cohort studies generally reported no significant association between fibre intake and risk of colorectal cancer, the hypothesis regained interest when the European Prospective Investigation into Cancer and Nutrition (EPIC) study reported a linear decrease in the risk of colorectal cancer with increasing fibre intake.19 A subsequent pooled analysis of 13 North American and European cohort studies (not including the EPIC study) reported an 18% increased risk of colorectal cancer with low fibre intake (<10 g/day v 10-15 g/day), but no further reductions in risk were observed with higher intake.31 More recently, results from additional large cohort studies23 24 25 26 27 28 29 30 have been published and, together with the EPIC study, included more than 1.7 million participants and 12 000 cases and included several studies from Asian populations.23 24 26 30 With such a large number of additional studies we had sufficient statistical power to clarify the dose-response relation between fibre intake and risk of colorectal cancer. In addition we examined whether specific types of fibre are associated with risk.
Whole grains are a major source of dietary fibre and contain germ, endosperm, and bran, in contrast with refined grains that contain only the endosperm. The germ and bran contain numerous nutrients, which are removed during the refining process. In addition, whole grains are a major source of several vitamins, minerals, and phytochemicals, which have anticancer properties and could plausibly influence the risk of colorectal cancer by several potential mechanisms.32 An earlier review and meta-analysis of case-control studies of whole grain intake and colorectal cancer and polyps reported a summary odds ratio of 0.79 for the highest versus the lowest intake.33 However, the interpretation of case-control studies is hampered by possible recall and selection biases, which make it difficult to draw firm conclusions. Over the past decade results from several cohort studies have been published on whole grain intake and risk of colorectal cancer, with mixed results.16 20 25 27 34 35 36 37 38 39 Some studies suggested no association,16 20 34 36 whereas others reported an inverse association with higher whole grain intake.25 27 35 37 38 39 To clarify the association between dietary fibre and whole grain intake and risk of colorectal cancer we carried out a systematic review and meta-analysis of published prospective studies. We also did meta-regression and sensitivity analyses to evaluate potential sources of heterogeneity in the analyses.
Methods
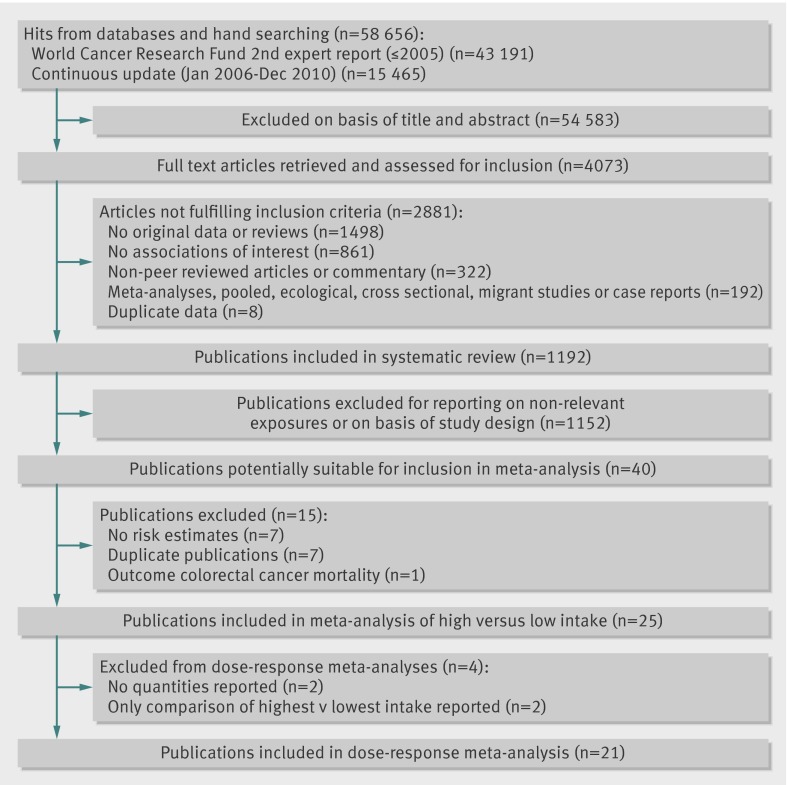
Several reviewers at Wageningen University carried out the literature search and extracted data up to December 2005. They searched several databases, including PubMed, Embase, CAB Abstracts, ISI Web of Science, BIOSIS, Latin American and Caribbean Center on Health Sciences Information, Cochrane library, Cumulative Index to Nursing and Allied Health Literature, the Allied and Complementary Medicine Database, National Research Register, and In Process Medline. As all the relevant prospective studies were identified by the PubMed searches the protocol was modified and only PubMed was used for the updated searches from January 2006 to December 2010. No language restrictions were imposed. This review was done as part of the Continuous Update Project of the World Cancer Research Fund and has been published online (www.wcrf.org/PDFs/Colorectal-cancer-CUP-report-2010.pdf). A predefined protocol was used for the review (www.dietandcancerreport.org/downloads/SLR_Manual.pdf) and we followed standard criteria for meta-analyses of observational studies.40 Abstracts, grey literature, and unpublished results or information were not included. We also searched the reference lists of the studies that were included in our analysis as well as those listed in the published meta-analyses.33 41
Study selection
To be included studies had to have a prospective cohort, case-cohort, or nested case-control design and investigate the association between dietary fibre or whole grain intake and incidence of colorectal cancer. We excluded retrospective case-control studies and cross sectional studies. The publication had to include estimates of the relative risk (hazard ratio, risk ratio) with the 95% confidence intervals. For the dose-response analysis, a quantitative measure of intake and the total number of cases and person years had to be provided. When several publications were from the same study we selected the publication with the largest number of cases. We identified 40 potentially relevant full text publications.8 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 34 35 36 37 38 39 42 43 44 45 46 47 48 49 50 51 52 53 54 We excluded seven that reported mean exposure only,42 43 44 45 46 47 48 seven that were duplicate publications,19 49 50 51 52 53 54 and one that was on mortality from colorectal cancer.34 Two publications that were included in the dietary fibre analyses reported on specific whole grain foods, not overall intake, and we excluded these from the whole grain analysis.16 20 For the dose-response analysis we further excluded two publications that reported results only for the highest versus the lowest levels of intake,30 37 and two publications that did not report quantities of intake.11 15
Data extraction
From each study we extracted data on the first author’s last name, year of publication, country where the study was done, study name, follow-up period, sample size, sex, age, number of cases, method of dietary assessment (type, number of food items, and whether the assessment method had been validated), exposure (by type of outcome), quantity of intake, relative risks and 95% confidence intervals for the highest versus the lowest intake, and variables adjusted for in the analysis. Several reviewers at Wageningen University carried out the search and extracted data of articles published up to December 2005 during the systematic literature review for the World Cancer Research Fund and Association for International Cancer Research report (www.dietandcancerreport.org/downloads/SLR/Colon_and_Rectum_SLR.pdf). Two of the authors (DSMC and RL) did the search from January 2006 to December 2010. Three authors (DSMC, RL, and DA) extracted the data into a database, and two authors (TN and DA) checked these for accuracy.
Statistical analysis
We used random effects models to calculate summary relative risks and 95% confidence intervals for the highest versus the lowest levels of dietary fibre and whole grain intake and for the dose-response analysis. The average of the natural logarithm of the relative risks was estimated and we weighted the relative risk from each study by the inverse of its variance. A two tailed P<0.05 was considered statistically significant. For studies that reported results separately for colon and rectal cancer or for men and women separately, we combined the estimates using a fixed effects model to obtain an overall estimate for colorectal cancer or both sexes combined.
We used a previously described method55 for the dose-response analysis and computed study specific slopes (linear trends) and 95% confidence intervals from the natural logs of the relative risks and confidence intervals across categories of dietary fibre and whole grain intake. The method requires that the distribution of cases and person years or non-cases and the relative risks with the variance estimates are known for at least three quantitative categories of use. We estimated the distribution of cases or person years in studies that did not report these but reported the total number of cases or person years if the results were analysed by quantiles (and could be approximated)—for example, the total number of person years was divided by 5 when data were analysed by quintiles to derive the number of person years in each fifth. We assigned the median or mean level of dietary fibre or whole grain intake in each category to the corresponding relative risk for each study. For studies that reported the intake by ranges of intake we estimated the midpoint in each category by calculating the average of the lower and upper bound. When the highest category was open ended we assumed the length of the open ended interval to be the same as that of the adjacent interval. When the lowest category was open ended we set the lower boundary to zero. If the intakes were reported in densities (servings per 1000 kcal) we recalculated the reported intakes to absolute intakes using the mean or median energy intake.18 21 27 28 In studies that reported the whole grain intake in grams daily we used 30 g as a serving size for recalculation of the intakes to a common scale (servings daily). The dose-response results in the forest plots are presented for a 10 g daily increment for dietary fibre and for an increment of three servings daily (90 g) for whole grains. We examined a potential non-linear dose-response relation between dietary fibre and whole grain intake and colorectal cancer by using fractional polynomial models.56 We determined the best fitting second order fractional polynomial regression model, defined as the one with the lowest deviance. A likelihood ratio test was used to assess the difference between the non-linear and linear models to test for non-linearity.57
Heterogeneity between studies was assessed by the Q test and I2 statistic.58 I2 is the amount of total variation that is explained by variation between studies. We did not use a score to assess study quality but in subgroup analyses we determined whether indicators of study quality, such as study size, number of cases, duration of follow-up, and adjustment for confounders modified the results. Heterogeneity between subgroups was evaluated by meta-regression.
Publication bias was assessed with Egger’s test59 and Begg’s test,60 with the results considered to indicate publication bias when P<0.10. In addition, we visually explored funnel plots for asymmetry. We carried out sensitivity analyses excluding one study at a time to explore whether the results were driven by one large study or by a study with an extreme result.
Results
Dietary fibre
Twenty one prospective studies8 11 12 13 14 15 16 17 18 20 21 22 23 24 25 26 27 28 29 30 36 were identified and included in the analysis of the highest versus the lowest intake of dietary fibre and risk of colorectal cancer, 18 of which8 12 13 14 16 17 18 20 21 22 23 24 25 26 27 28 29 36 were included in the dose-response analyses (table 1, fig 1). Twelve of the studies were from the United States, five from Europe, and four from Asia. Table 1 summarises the characteristics of the included studies. The ranges of intake varied: 6.3-21.4 g/day for total dietary fibre, 1.8-15.5 g/day for fruit fibre, 1.9-16.8 g/day for vegetable fibre, 3.0-16.9 g/day for cereal fibre, and 1.3-3.8 g/day for legume fibre (results not shown).
Table 1.
Prospective studies of dietary fibre intake and incidence of colorectal cancer
| Study, country | Study name | Follow-up period | Study size, sex, age, No of cases* | Diet assessment, No of items, fibre definition | Exposure | Quantity | Relative risk (95% CI) | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|
| Kabat 200829, USA | Women’s Health Initiative | 1993-8, 7.9 years | 158 800 women, age 50-79, 1476 cases | Validated food frequency questionnaire, 122 food items, NA | Total fibre | ≥21.2 v <9.9 g/day | 1.06 (0.67 to 1.70) | Age, education, daily cigarette consumption, body mass index, height, HRT, diabetes mellitus, family history of colorectal cancer, physical activity, observational study participant, energy, dietary calcium |
| Butler 200830, Singapore | Singapore Chinese Health Study | 1993-2005, 9.8 years | 61 321 men and women, age 45-74, 961 cases | Validated food frequency questionnaire, 165 food items, NA | Dietary fibre | Fourths: 4 v 1 | 0.98 (0.81 to 1.19) | Age, sex, dialect group, interview year, diabetes mellitus, smoking, body mass index, alcohol, education, physical activity, family history of colorectal cancer, energy |
| Nomura 200728, USA | Multiethnic Cohort Study | 1993-2001, 7.3 years | 85 903 men and 105 108 women, age 45-75, 1138/972 cases | Validated food frequency questionnaire, 180 food items, AOAC method | Dietary fibre, men | 16.5 v 6.1 g/1000 kcal/day | 0.62 (0.48 to 0.79) | Age, ethnicity, time since cohort entry, family history of colorectal cancer, history of colorectal polyps, pack years of cigarette smoking, body mass index, hours of vigorous activity, aspirin use, multivitamin use, HRT, alcohol, red meat, folate, vitamin D, calcium, energy |
| Fruit fibre, men | 12.6 v 0.9 g/1000 kcal/day | 0.78 (0.63 to 0.97) | ||||||
| Vegetable fibre, men | 18.4 v 3.0 g/1000 kcal/day | 0.78 (0.62 to 0.97) | ||||||
| Grain fibre, men | 15.6 v 2.8 g/1000 kcal/day | 0.86 (0.69 to 1.07) | ||||||
| Legume fibre, men | 7.6 v 0.3 g/1000 kcal/day | 0.87 (0.68 to 1.10) | ||||||
| Dietary fibre, women | 18.6 v 7.5 g/1000 kcal/day | 0.88 (0.67 to 1.14) | ||||||
| Fruit fibre, women | 14.0 v 1.2 g/1000 kcal/day | 0.82 (0.64 to 1.05) | ||||||
| Vegetable fibre, women | 17.2 v 3.0 g/1000 kcal/day | 0.95 (0.75 to 1.20) | ||||||
| Grain fibre, women | 14.0 v 2.4 g/1000 kcal/day | 1.00 (0.78 to 1.27) | ||||||
| Legume fibre, women | 5.8 v 0.2 g/1000 kcal/day | 1.16 (0.90 to 1.49) | ||||||
| Schatzkin 200727, USA | NIH-AARP Diet and Health Study | 1995-2000, 4.5 years | 291 988 men and 197 623 women, age 50-71, 2974 cases | Validated food frequency questionnaire, 124 food items, AOAC method | Dietary fibre | 15.9 v 6.6 g/1000 kcal/day | 0.99 (0.85 to 1.15) | Age, sex, physical activity, smoking, HRT (women), red meat, dietary calcium, dietary folate, energy |
| Grain fibre | 5.7 v 1.7 g/1000 kcal/day | 0.86 (0.76 to 0.98) | ||||||
| Fruit fibre | 4.8 v 0.5 g/1000 kcal/day | 1.08 (0.95 to 1.23) | ||||||
| Vegetable fibre | 6.0 v 1.7 g/1000 kcal/day | 1.01 (0.89 to 1.15) | ||||||
| Bean fibre | 2.3 v 0.2 g/1000 kcal/day | 0.93 (0.83 to 1.04) | ||||||
| Wakai 200726, Japan | Japan Collaborative Cohort Study | 1988-97, 7.6 years | 43 115 men and women, age 40-79, 443 cases | Validated food frequency questionnaire, 40 food items, AOAC method | Total dietary fibre | 13.4/13.4 v 6.7/7.4 g/d men and women | 0.73 (0.51 to 1.03) | Age, sex, area, education, family history of colorectal cancer, alcohol, smoking, body mass index, walking, exercise, sedentary work, beef/pork, energy, folate, calcium, vitamin D |
| McCarl 200625, USA | Iowa Women’s Health Study | 1986-2001, 15 years | 35197 women, age 55-69, 954 cases | Validated food frequency questionnaire, 131 food items, NA | Fibre | ≥25.4 v ≤13.2 g/day | 0.75 (0.61 to 0.92) | Age |
| Shin 200623, China | Shanghai Women’s Health Study | 1997-2004, 5.74 years | 73 314 women, age 40-70, 283 cases | Validated food frequency questionnaire, 77 food items, NA | Fibre | Fifths: 5 v 1 | 1.1 (0.6 to 1.8) | Age, menopausal status, education, smoking, alcohol, exercise, family history of colorectal cancer, energy, vitamin supplements |
| Otani 200624, Japan | Japan Public Health Center-based Prospective Study | Cohort 1: 1995-2002; Cohort 2: 1998-2002, 5.8 years | 78 326 men and women, age 40-59 (cohort 1) and 40-69 (cohort 2), 522 cases | Validated food frequency questionnaire 138 food items, AOAC method | Dietary fibre, men | 18.7 v 6.4 g/day | 0.85 (0.53 to 1.4) | Age, alcohol, smoking, body mass index, physical exercise, folate, calcium, vitamin D, red meat, study area, energy |
| Dietary fibre, women | 20.0 v 8.3 g/day | 0.58 (0.31 to 1.1) | ||||||
| Bingham 20058, Europe | European Prospective Investigation into Cancer and Nutrition | 1992-2004, 6.2 years | 519 978 men and women, age 25-70, 1721 cases | Validated food frequency questionnaire, 300-350 food items, diet records, Englyst method (UK) | Dietary fibre | 30.1/24.3 v 18.2/15.9 g/day, men and women | 0.79 (0.63 to 0.99) | Age, sex, energy from non-fat sources, energy from fat sources, height, weight, folate, red and processed meat, physical activity, alcohol, smoking status, educational level |
| Fruit fibre | 5.3/5.4 v 2.7/2.8 g/day | 0.81 (0.68 to 0.97) | ||||||
| Cereal fibre | 13.1/9.2 v 6.6/4.9 g/day | 0.93 (0.76 to 1.15) | ||||||
| Vegetable fibre | 5.3/5.4 v 2.7/2.8 g/day | 0.94 (0.76 to 1.16) | ||||||
| Legume fibre | 1.9/1.0 v 0 g/day | 0.98 (0.82 to 1.17) | ||||||
| Michels 200521, USA | Nurses’ Health Study | 1984-2000, 16 years | 76 947 women, age 38-63, 919 cases | Validated food frequency questionnaire, 131 food items, AOAC method | Dietary fibre | >14.0 v <8.0 g/1000 kcal/day | 0.98 (0.70 to 1.37) | Age, time period, family history of colorectal cancer, sigmoidoscopy or colonoscopy, height, body mass index, physical activity, aspirin use and duration, pack years of early onset smoking, multivitamins, energy, alcohol, dietary folate, calcium, red meat, processed meat, glycaemic load, methionine, HRT (women), menopausal status (women) |
| Cereal fibre | 11.45 v 2.8 g/1000 kcal/day | 0.79 (0.60 to 1.05) | ||||||
| Fruit fibre | 9.3 v 1.4 g/1000 kcal/day | 0.92 (0.68 to 1.23) | ||||||
| Vegetable fibre | 12.2 v 3.6 g/1000 kcal/day | 1.09 (0.83 to 1.42) | ||||||
| Health Professionals Follow-up Study | 1986-2000, 14 years | 47 279 men, age 40-75, 593 cases | Validated food frequency questionnaire, 131 food items, AOAC method | Dietary fibre | >14.0 v <8.0 g/1000 kcal/day | 0.91 (0.65 to 1.28) | ||
| Cereal fibre | 8.0 v 2.3 g/1000 kcal/day | 0.89 (0.71 to 1.12) | ||||||
| Fruit fibre | 7.3 v 1.4 g/1000 kcal/day | 0.88 (0.68 to 1.13) | ||||||
| Vegetable fibre | 10.0 v 3.6 g/1000 kcal/day | 1.20 (0.94 to 1.56) | ||||||
| Lin 200522, USA | Women’s Health Study | 1993-2003, 10 years | 39 976 women, age ≥45, 223 cases | Validated food frequency questionnaire, 131 food items, AOAC method | Total fibre | 26 v 12 g/day | 0.75 (0.47 to 1.18) | Age, body mass index, randomised treatment assignment, family history of colorectal cancer, colon polyps, physical activity, smoking status, aspirin, red meat, alcohol, energy, menopausal status, HRT |
| Fruit fibre | 6.0 v 2.5 g/day | 1.00 (0.67 to 1.49) | ||||||
| Vegetable fibre | 8.0 v 5.9 g/day | 1.00 (0.65 to 1.56) | ||||||
| Cereal fibre | 6.1 v 3.1 g/day | 0.97 (0.66 to 1.42) | ||||||
| Legume fibre | 1.8 v 0.4 g/day | 0.60 (0.40 to 0.91) | ||||||
| Sanjoaquin 200420, England | Oxford Vegetarian Study | 1980-9, 17 years | 10 998 men and women, age 16-89: 95 cases | Validated food frequency questionnaire, NA | Total dietary fibre | 36.7 v 17.0 g/day | 0.82 (0.43 to 1.56) | Age, sex, alcohol, smoking |
| McCullough 200336, USA | Cancer Prevention Study 2 Nutrition Cohort | 1992-7, 4.5 years | 62 609 men and 70 554 women, age 50-74, 298 and 210 cases | Validated food frequency questionnaire, 68 food items, NA | Dietary fibre, men | ≥16.6 v <9.3 g/day | 1.11 (0.72 to 1.70) | Age, exercise metabolic equivalent of tasks, body mass index, aspirin, smoking, family history of colorectal cancer, education, energy, multivitamins, total calcium, red meat intake, and HRT (women) |
| Dietary fibre, women | ≥14.4 v <8.0 g/day | 0.86 (0.52 to 1.42) | ||||||
| Mai 200318, USA | Breast Cancer Detection Demonstration Project | 1987-8, 8.5 years | 45 491 women, mean age 62, 487 cases | Validated food frequency questionnaire, 62 food items, NA | Total fibre | >12 v <6.3 g/1000 kcal/day | 0.94 (0.70 to 1.26) | Age, non-steroidal anti-inflammatory drugs, smoking, alcohol, calcium, vitamin D, red meat, height, body mass index, education |
| Fruit fibre | >3.57 v <0.90 g/1000 kcal/day | 1.10 (0.83 to 1.46) | ||||||
| Vegetable fibre | >3.48 v <1.44 g/1000 kcal/day | 0.92 (0.69 to 1.21) | ||||||
| Bean fibre | >1.38 v <0.20 g/1000 kcal/day | 0.84 (0.63 to 1.10) | ||||||
| Grain fibre | >4.75 v <1.80 g/1000 kcal/day | 1.02 (0.76 to 1.37) | ||||||
| Terry 200117, Sweden | Swedish Mammography Cohort Study | 1987-98, 9.6 years | 61 463 women, age 40-74, 460 cases | Food frequency questionnaire, 67 items, AOAC method | Cereal fibre | 13.6 v 5.7 g/day | 0.91 (0.69 to 1.20) | Age, red meat, dairy products, energy |
| Total dietary fibre | 21.8 v 12.3 g/day | 0.96 (0.70 to 1.33) | ||||||
| Fruit fibre | 5.2 v 0.8 g/day | 0.97 (0.69 to 1.38) | ||||||
| Vegetable fibre | 2.5 v 0.6 g/day | 1.17 (0.85 to 1.61) | ||||||
| Pietinen 199916, Finland | ATBC Cancer Prevention Study | 1985-95, 8 years | 27 111 male smokers, age 50-69, 185 cases | Validated food frequency questionnaire, 276 items, Englyst method | Dietary fibre | 34.1 v 16.0 g/day | 1.0 (0.6 to 1.5) | Age, tobacco years, body mass index, alcohol, education, physical activity, calcium, energy |
| Kato 199715, USA | New York University Women’s Cohort Study | 1985-94, mean 7.1 years | 15 785 women, age 34-65, 100 cases | Food frequency questionnaire, 70 items, NA | Dietary fibre | Fourths: 4 v 1 | 1.51 (0.85 to 2.68) | Age, energy, place at enrolment, highest level of education |
| Gaard 199614, Norway | Norwegian National Health Screening Study | 1977-91, 11.4 years | 505 35 men and women, age 20-54, 143 cases of colon cancer | Validated food frequency questionnaire, 80 food items, NA | Fibre, men | ≥17.9 v ≤13.5 g/day | 0.82 (0.46 to 1.46) | Age, body mass index, height, smoking status, energy |
| Fibre, women | ≥11.3 v 8.5 g/day | 2.10 (0.90 to 4.87) | ||||||
| Steinmetz 199413, USA | Iowa Women’s Health Study | 1986-91, 5 years | 41 837 women, age 55-69, 212 cases of colon cancer | Validated food frequency questionnaire, 127 food items, NA | Dietary fibre | >24.7 v <14.5 g/day | 0.80 (0.49 to 1.31) | Age, energy |
| Heilbrun 198912, USA | Honolulu Heart Program | 1965-85, 16 years | 8006 American Japanese men: 102 cases of colon cancer, 60 cases of rectal cancer, 361 controls | Dietary recall, 24 hour, 54 food items, NA | Dietary fibre, colon cancer | ≥14.80 v <7.50 g/day | 0.71 (0.38 to 1.32) | Age, alcohol intake |
| Dietary fibre, rectal cancer | ≥14.80 v <7.50 g/day | 1.20 (0.51 to 2.83) | ||||||
| Wu 198711, USA | Leisure World Cohort Study | 1981-5, 3.5 years | 11 564 men and women, age ≤64 to ≥85, 58 and 68 cases | Validated food frequency questionnaire, 56 food items, NA | Dietary fibre, men | Thirds: 3 v 1 | 1.13 (0.60 to 2.10) | Age |
| Dietary fibre, women | Thirds: 3 v 1 | 0.64 (0.40 to 1.20) |
Fig 1 Flow chart of publications included in systematic review
Total dietary fibre
High versus low intake
Nineteen prospective studies (18 publications) were included in the analysis of high versus low intake of total dietary fibre and risk of colorectal cancer (table 1).8 11 12 15 16 17 18 20 21 22 23 24 25 26 27 28 29 30 The summary relative risk was 0.88 (95% confidence interval 0.82 to 0.94), with no evidence of heterogeneity (I2=0%, P=0.48, see web extra figure 1a).
Dose-response analysis
Sixteen prospective studies (15 publications)8 12 16 17 18 20 21 22 23 24 25 26 27 28 29 were included in the dose-response analysis, with 14 514 cases among 1 985 552 participants. The summary relative risk was 0.90 (0.86 to 0.94) for each 10 g/day intake, with no significant heterogeneity (I2=0%, P=0.48, fig 2). A statistically significant inverse association was seen for colon cancer8 12 13 14 21 23 24 26 27 28 29 36 (13 studies, summary relative risk 0.89, 0.81 to 0.97, I2=35%, P=0.11) but not for rectal cancer8 12 21 23 24 26 27 28 29 (10 studies, 0.91, 0.83 to 1.03, I2=15%, P=0.31), although evidence was lacking for heterogeneity between subsites (P=0.86, see table 3). Publication bias was not evident with either Egger’s test (P=0.62) or Begg’s test (P=0.56). In a sensitivity analysis excluding one study at a time, the summary relative risk for colorectal cancer ranged from 0.89 (0.85 to 0.93) when the National Institutes of Health-American Association for Retired Persons (NIH-AARP) Diet and Health Study was excluded to 0.91 (0.88 to 0.96) when the EPIC study was excluded. A non-linear association was not evident between intake of total dietary fibre and risk of colorectal cancer (P=0.32 for non-linearity, fig 2).
Fig 2 Dose-response analyses between dietary fibre and risk of colorectal cancer. NHS=Nurses’ Health Study; HPFS=Health Professionals Follow-up Study
Fruit fibre
High versus low intake
Nine cohort studies (eight publications)8 17 18 21 22 26 27 28 were included in the analysis of high versus low intake of fruit fibre and risk of colorectal cancer. The summary relative risk was 0.94 (0.85 to 1.04; see also web extra figure 2a), with little evidence of heterogeneity (I2=39%, P=0.11).
Dose-response analysis
Nine cohort studies (eight publications)8 17 18 21 22 26 27 28 were included in the dose-response analysis of fruit fibre and risk of colorectal cancer, with 9930 cases among 1 514 871 participants. The summary relative risk for each 10 g/day intake was 0.93 (0.82 to 1.05, fig 3), with little evidence of heterogeneity (I2=23%, P=0.24). Publication bias was not evident with Egger’s test (P=0.83) or Begg’s test (P=0.47). The summary relative risk ranged from 0.87 (0.78 to 0.96) when the NIH-AARP Diet and Health Study was excluded to 0.95 (0.84 to 1.07) when the Nurses’ Health Study was excluded.
Fig 3 Risk of colorectal cancer according to fibre types. NHS=Nurses’ Health Study; HPFS=Health Professionals Follow-up Study
Vegetable fibre
High versus low intake
Nine cohort studies (eight publications)8 17 18 21 22 26 27 28 were included in the analysis of high versus low intake of vegetable fibre and risk of colorectal cancer. The summary relative risk was 0.98 (0.91 to 1.06, also see web extra figure 2b), with no evidence of heterogeneity (I2=0%, P=0.48).
Dose-response analysis
Nine cohort studies (eight publications)8 17 18 21 22 26 27 28 were included in the dose-response analysis of vegetable fibre and risk of colorectal cancer, with 9930 cases among 1 514 871 participants. The summary relative risk for each 10 g/day intake was 0.98 (0.91 to 1.06, fig 3), with no evidence of heterogeneity (I2=0%, P=0.60). Publication bias was not evident with Egger’s test (P=0.51) or Begg’s test (P=0.92). The summary relative risk ranged from 0.96 (0.89 to 1.04) when the Nurses’ Health Study was excluded to 1.02 (0.94 to 1.10) when the Multiethnic Cohort Study was excluded.
Legume fibre
High versus low intake
Four cohort studies8 18 22 27 were included in the analysis of high versus low intake of legume fibre and risk of colorectal cancer. The summary relative risk was 0.89 (0.78 to 1.02, see also web extra figure 2c), with moderate heterogeneity (I2=40.8%, P=0.17).
Dose-response analysis
Four cohort studies8 18 22 27 were included in the dose-response analysis of legume fibre intake and risk of colorectal cancer, with 5405 cases among 1 095 056 participants. The summary relative risk for each 10 g/day intake was 0.62 (0.27 to 1.42, fig 3), with moderate to high heterogeneity (I2=58%, P=0.07). The summary relative risk ranged from 0.38 (0.08 to 1.87) when excluding the NIH-AARP Diet and Health Study to 0.84 (0.65 to 1.09) when excluding the Women’s Health Study.
Cereal fibre
High versus low intake
Eight cohort studies (seven publications)8 17 18 21 22 27 28 were included in the analysis of high versus low intake of cereal fibre and risk of colorectal cancer. The summary relative risk was 0.90 (0.83 to 0.96, also see web extra figure 2d), with no significant heterogeneity (I2=0%, P=0.94).
Dose-response analysis
Eight cohort studies (seven publications)8 17 18 21 22 27 28 were included in the dose-response analysis of cereal fibre and risk of colorectal cancer, with 9487 cases among 1 471 756 participants. The summary relative risk for each 10 g/day intake was 0.90 (0.83 to 0.97, fig 3), with no evidence of heterogeneity (I2=0%, P=0.78). Publication bias was not evident with Egger’s test (P=0.90) or Begg’s test (P=1.00). The summary relative risk ranged from 0.85 (0.76 to 0.95) when the Multiethnic Cohort Study was excluded to 0.93 (0.85 to 1.03) when the NIH-AARP Diet and Health Study was excluded.
Whole grains
Seven cohort studies were included in the analysis of total whole grain intake and risk of colorectal cancer (table 2, fig 1).25 27 35 36 37 38 39 Two studies were from Europe and the other five from the United States (table 2). Total whole grains included whole grain rye breads, whole grain breads, oatmeal, whole grain cereals, high fibre cereals, brown rice, and porridge. The range of whole grain intake varied from and 61-128 g/day (results not shown).
Table 2.
Prospective studies of whole grain intake and incidence of colorectal cancer
| Study, country | Study name | Follow-up period | Study size, sex, age, No of cases* | Diet assessment, No of items | Exposure | Quantity | Relative risk (95% CI) | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|
| Fung 201039, USA | Nurses’ Health Study | 1980-2006, 26 years | 87 256 women, age 34-59, 1432 cases | Validated food frequency questionnaire, 61-116 food items | Whole grains | Per serving/day | 0.95 (0.89 to 1.02) | Age, body mass index, alcohol, family history of colorectal cancer, physical activity, aspirin, colonoscopy, history of polyps, pack years of smoking, energy, multivitamins |
| Fung 201039, USA | Health Professionals Follow-Up Study | 1986-2006, 20 years | 45 490 men, age 40-75, 1032 cases | Validated food frequency questionnaire, about 140 food items | Whole grains | Per serving/day | 0.94 (0.88 to 0.99) | Age, body mass index, alcohol, family history of colorectal cancer, physical activity, aspirin, colonoscopy, history of polyps, pack years of smoking, energy, multivitamins |
| Egeberg 201038, Denmark | The Diet Cancer and Health Cohort Study | 1993-2006, 10.2 years | 26 630 men and 29 189 women, age 50-64, 461 cases of colon cancer and 283 cases of rectal cancer | Validated food frequency questionnaire, 192 food items | Whole grains, colon cancer, men | >160 v ≤75 g/day | 0.61 (0.43 to 0.86) | Age, body mass index, alcohol intake, school education, red and processed meat, HRT (women), leisure time physical activity |
| Whole grains, rectal cancer, men | >160 v ≤75 g/day | 0.88 (0.57 to 1.36) | ||||||
| Whole grains, colon cancer, women | >160 v ≤75 g/day | 0.92 (0.63 to 1.35) | ||||||
| Whole grains, rectal cancer, women | >160 v ≤75 g/day | 0.81 (0.50 to 1.30) | ||||||
| Schatzkin 200727, USA | NIH-AARP Diet and Health Study | 1995-2000, 5 years | 291 988 men and 197 623 women, age 50-71, 2974 cases | Validated food frequency questionnaire, 124 food items | Whole grains | 1.3 v 0.2 serv/1000 kcal/day | 0.79 (0.70 to 0.89) | Age, sex, physical activity, smoking, HRT (women), red meat, dietary calcium, dietary folate, energy |
| McCarl 200625, USA | Iowa Women’s Health Study | 1986-2000, 14 years | 35 197 women, age 55-69, 954 cases | Validated food frequency questionnaire, 127 food items | Whole grains | ≥19 v ≤3.5 servings/week | 0.81 (0.66 to 0.99) | Age |
| Larsson 200535, Sweden | Swedish Mammography Cohort Study | 1987-2004, 14.8 years | 61 433 women, age 40-76, 805 cases | Validated food frequency questionnaire, 67 food items | Whole grain | ≥4.5 v <1.5 servings/day | 0.80 (0.60 to 1.06) | Age, body mass index, education, energy, saturated fat, calcium, red meat, fruits and vegetables |
| Wu 200437, USA | Health Professional’s Follow-up Study | 1986-2000, 14 years | 47 311 men, age 45-75, 561 cases of colon cancer | Validated food frequency questionnaire, 131 food items | Whole grain | Fifths: 5 v 1 | 0.75 (0.57 to 1.00) | Age, family history of colorectal cancer in first degree relative, history of endoscopy, physical activity, pack years of smoking before age 30, race, aspirin use, energy |
| McCullough 200336, USA | Cancer Prevention Study 2 | 1992-7, 4.5 years | 62 609 men and 70 554 women, age 50-74, 298/210 cases of colon cancer | Validated food frequency questionnaire, 68 items | Whole grains, men | ≥11.0 v. <2.0 servings/week | 0.95 (0.64 to 1.42) | Age, exercise metabolic equivalent of tasks, aspirin, smoking, family history of colorectal cancer, body mass index, education, energy, multivitamin use, total calcium, red meat intake, and HRT (women) |
| Whole grains, women | ≥11.2 v <2.5 servings/week | 1.17 (0.73 to 1.87) |
High versus low intake
Four cohort studies25 27 35 38 were included in the analysis of high versus low intake of whole grains and risk of colorectal cancer. The summary relative risk was 0.79 (0.72 to 0.86), with no evidence of heterogeneity (I2=0%, P=0.98, see web extra figure 1b). The results for colon and rectal cancer were similar: summary relative risks 0.82 (0.72 to 0.92, I2=23%, P=0.27)27 35 36 37 38 and 0.80 (0.59 to 1.07, I2=58%, P=0.10).27 35 38 The results for rectal cancer were, however, not statistically significant.
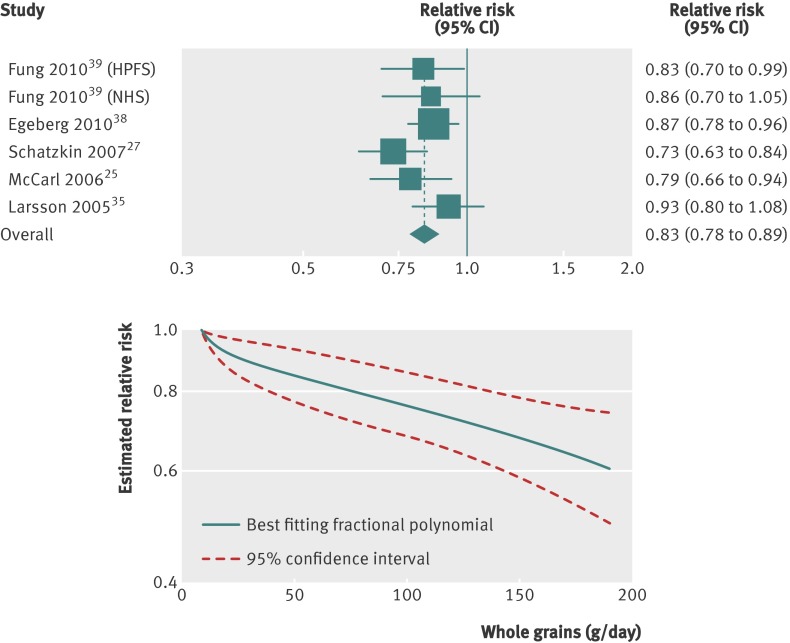
Dose-response analysis
Six studies (five publications)25 27 35 38 39 were included in the dose-response analysis, with a total of 7941 cases among 774 806 participants. The summary relative risk for colorectal cancer with an increment of three servings daily (90 g/day) of whole grains was 0.83 (0.78 to 0.89, fig 4), with no evidence of heterogeneity (I2=18%, P=0.30). The summary relative risk for colon cancer27 35 36 38 was 0.86 (0.79 to 0.94), with no evidence of heterogeneity (I2=0%, P=0.42), and for rectal cancer27 35 38 was 0.80 (0.56 to 1.14), with substantial heterogeneity (I2=91%, P<0.001, table 4). In a sensitivity analysis excluding one study at a time, no particular study explained the results for colorectal cancer; the summary relative risk ranged from 0.82 (0.77 to 0.88) when the Swedish Mammography Study was excluded to 0.86 (0.80 to 0.92) when the NIH-AARP Diet and Health Study was excluded. Publication bias was not evident with Egger’s test (P=0.54) or Begg’s test (P=1.00), although the number of studies was low. However, the funnel plots did not suggest asymmetry. A non-linear association between whole grain intake and risk of colorectal cancer was not indicated (P=0.26, fig 4).
Fig 4 Dose-response analyses between whole grains and risk of colorectal cancer. NHS=Nurses’ Health Study; HPFS=Health Professionals Follow-up Study
Subgroup, sensitivity, and meta-regression analyses
In subgroup analyses defined by sex, subsite, adjustment for confounders, number of cases, duration of follow-up, geographical location, and range of intake, total dietary fibre intake was inversely associated with risk of colorectal cancer in most subgroups, with no evidence of significant heterogeneity between subgroups with meta-regression analyses (table 3). Similar results were observed for intake of cereal fibre and whole grains (table 4). Intake of fruit fibre was not significantly associated with risk of colorectal cancer in most subgroup analyses. In the subgroups of studies that adjusted for alcohol intake and body mass index or weight, however, inverse associations were significant, with evidence of heterogeneity between subgroups (P=0.04, table 3). When stratified by the range of intake, an inverse association was observed for intake of fruit fibre in studies with a range of 10 g/day or more but not among studies with a range of 10 g/day or less (P=0.04 for heterogeneity), but evidence of a difference in the results for the other fibre types was lacking when stratified by the range of intake (tables 3 and 4). Intake of vegetable fibre consistently was not associated with risk of colorectal cancer in subgroup analyses (table 3). Too few studies of legume fibre precluded any meaningful subgroup analyses.
Table 3.
Subgroup analyses of fibre intake and risk of colorectal cancer, dose-response analysis
| Subgroups | Total dietary fibre | Fruit fibre | Vegetable fibre | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Relative risk (95% CI) | I2 (%) | P for heterogeneity | No of studies | Relative risk (95% CI) | I2 (%) | P for heterogeneity | No of studies | Relative risk (95% CI) | I2 (%) | P for heterogeneity | ||||
| * | † | * | † | * | † | ||||||||||
| All studies | 16 | 0.90 (0.86 to 0.94) | 0 | 0.48 | — | 9 | 0.93 (0.82 to 1.05) | 23 | 0.24 | — | 9 | 0.98 (0.91 to 1.06) | 0 | 0.60 | — |
| Duration of follow-up: | |||||||||||||||
| <10 years | 10 | 0.91 (0.84 to 0.97) | 33.2 | 0.14 | 0.77 | 6 | 0.97 (0.82 to 1.14) | 37.6 | 0.16 | 0.26 | 6 | 0.96 (0.88 to 1.04) | 0 | 0.51 | 0.27 |
| ≥10 years | 6 | 0.91 (0.85 to 0.98) | 0 | 0.97 | 3 | 0.80 (0.64 to 1.00) | 0 | 0.91 | 3 | 1.10 (0.90 to 1.35) | 0 | 0.74 | |||
| Sex: | |||||||||||||||
| Men | 7 | 0.92 (0.82 to 1.03) | 54.4 | 0.04 | 0.72 | 2 | 0.83 (0.72 to 0.97) | 0 | 0.80 | 0.45 | 2 | 0.94 (0.78 to 1.14) | 44.3 | 0.18 | 0.34 |
| Women | 11 | 0.94 (0.89 to 0.99) | 0 | 0.74 | 5 | 0.91 (0.78 to 1.06) | 0 | 0.80 | 5 | 1.02 (0.89 to 1.17) | 0 | 0.54 | |||
| Subsite: | |||||||||||||||
| Colon | 13 | 0.90 (0.83 to 0.98) | 33.9 | 0.11 | 0.86 | 3 | 0.90 (0.34 to 2.38) | 59.2 | 0.42 | 0.85 | 3 | 0.89 (0.57 to 1.40) | 30.9 | 0.24 | 0.24 |
| Rectum | 10 | 0.91 (0.83 to 1.03) | 14.7 | 0.31 | 1 | 1.26 (0.09 to 18.24) | — | — | 1 | 6.40 (0.97 to 42.34) | — | — | |||
| Geographical location: | |||||||||||||||
| Europe | 4 | 0.87 (0.78 to 0.96) | 9.2 | 0.35 | 0.74 | 2 | 0.75 (0.46 to 1.23) | 0 | 0.39 | 0.34 | 2 | 1.30 (0.35 to 4.84) | 58.2 | 0.12 | 0.70 |
| USA | 9 | 0.92 (0.88 to 0.96) | 0 | 0.69 | 6 | 0.93 (0.81 to 1.07) | 38.0 | 0.15 | 6 | 0.98 (0.91 to 1.06) | 0 | 0.61 | |||
| Asia | 3 | 0.78 (0.60 to 1.03) | 24.7 | 0.29 | 1 | 1.90 (0.40 to 9.04) | — | — | 1 | 0.71 (0.26 to 1.91) | — | — | |||
| No of cases: | |||||||||||||||
| <500 | 8 | 0.92 (0.82 to 1.03) | 0 | 0.64 | 0.35 | 4 | 1.08 (0.73 to 1.60) | 0 | 0.87 | 0.98 | 4 | 1.05 (0.61 to 1.79) | 8.0 | 0.35 | 0.37 |
| 500-1499 | 5 | 0.92 (0.87 to 0.99) | 0 | 0.68 | 2 | 0.80 (0.64 to 1.00) | 0 | 0.70 | 2 | 1.09 (0.89 to 1.34) | 0 | 0.93 | |||
| ≥1500 | 3 | 0.88 (0.80 to 0.97) | 64.0 | 0.06 | 3 | 0.94 (0.75 to 1.17) | 71.4 | 0.03 | 3 | 0.96 (0.88 to 1.04) | 0 | 0.41 | |||
| Range of intake: | |||||||||||||||
| <15 to <10 g/day‡ | 11 | 0.90 (0.84 to 0.96) | 11.9 | 0.33 | 0.80 | 6 | 1.07 (0.94 to 1.23) | 0 | 0.61 | 0.04 | 6 | 1.03 (0.89 to 1.18) | 0 | 0.58 | 0.54 |
| ≥15 to ≥10 g/day‡ | 5 | 0.90 (0.85 to 0.95) | 0 | 0.51 | 3 | 0.86 (0.77 to 0.96) | 0 | 0.71 | 3 | 0.96 (0.88 to 1.05) | 0 | 0.38 | |||
| Adjustment for confounders | |||||||||||||||
| Alcohol: | |||||||||||||||
| Yes | 12 | 0.87 (0.83 to 0.92) | 0 | 0.63 | 0.08 | 7 | 0.86 (0.78 to 0.96) | 0 | 0.75 | 0.04 | 7 | 0.95 (0.87 to 1.04) | 0 | 0.75 | 0.32 |
| No | 4 | 0.95 (0.88 to 1.01) | 0 | 0.51 | 2 | 1.10 (0.95 to 1.28) | 0 | 0.75 | 2 | 1.34 (0.54 to 3.34) | 45.0 | 0.18 | |||
| Smoking: | |||||||||||||||
| Yes | 13 | 0.90 (0.85 to 0.95) | 15.7 | 0.29 | 0.84 | 8 | 0.92 (0.81 to 1.05) | 32.5 | 0.17 | 0.89 | 8 | 0.98 (0.90 to 1.05) | 0 | 0.74 | 0.20 |
| No | 3 | 0.95 (0.88 to 1.01) | 0 | 0.51 | 1 | 0.97 (0.45 to 2.09) | — | — | 1 | 3.15 (0.63 to 15.64) | — | — | |||
| Body mass index, weight, waist to hip ratio: | |||||||||||||||
| Yes | 10 | 0.89 (0.83 to 0.95) | 19.4 | 0.26 | 0.22 | 7 | 0.86 (0.78 to 0.96) | 0 | 0.75 | 0.04 | 7 | 0.95 (0.87 to 1.04) | 0 | 0.75 | 0.32 |
| No | 6 | 0.93 (0.87 to 1.00) | 0 | 0.91 | 2 | 1.10 (0.95 to 1.28) | 0 | 0.75 | 2 | 1.34 (0.54 to 3.34) | 45.0 | 0.18 | |||
| Physical activity: | |||||||||||||||
| Yes | 11 | 0.90 (0.85 to 0.96) | 27.7 | 0.18 | 0.70 | 7 | 0.91 (0.79 to 1.05) | 40.0 | 0.13 | 0.56 | 7 | 0.95 (0.87 to 1.04) | 0 | 0.75 | 0.77 |
| No | 5 | 0.92 (0.85 to 0.99) | 0 | 0.97 | 2 | 1.06 (0.68 to 1.65) | 0 | 0.79 | 2 | 1.34 (0.54 to 3.34) | 45.0 | 0.18 | |||
| Red, processed meat: | |||||||||||||||
| Yes | 10 | 0.89 (0.84 to 0.95) | 21.3 | 0.25 | 0.32 | 9 | 0.93 (0.82 to 1.05) | 23 | 0.24 | NC | 9 | 0.98 (0.91 to 1.06) | 0 | 0.60 | NC |
| No | 6 | 0.93 (0.87 to 1.00) | 0 | 0.86 | 0 | — | — | — | 0 | — | — | — | |||
| Dairy products, calcium: | |||||||||||||||
| Yes | 10 | 0.93 (0.87 to 0.98) | 9.6 | 0.35 | 0.20 | 7 | 0.94 (0.82 to 1.07) | 32.2 | 0.18 | 0.35 | 7 | 0.98 (0.91 to 1.06) | 0 | 0.51 | 0.81 |
| No | 6 | 0.87 (0.82 to 0.92) | 0 | 0.74 | 2 | 0.69 (0.40 to 1.19) | 0 | 0.58 | 2 | 0.94 (0.42 to 2.07) | 8.0 | 0.30 | |||
| Folate: | |||||||||||||||
| Yes | 7 | 0.89 (0.82 to 0.95) | 41.3 | 0.12 | 0.27 | 6 | 0.90 (0.77 to 1.06) | 49.9 | 0.08 | 0.60 | 6 | 0.98 (0.90 to 1.05) | 0 | 0.62 | 0.59 |
| No | 9 | 0.93 (0.87 to 1.00) | 0 | 0.94 | 3 | 1.04 (0.69 to 1.56) | 0 | 0.93 | 3 | 1.33 (0.61 to 2.87) | 21.7 | 0.28 | |||
| Energy intake: | |||||||||||||||
| Yes | 11 | 0.90 (0.84 to 0.96) | 27.3 | 0.18 | 0.62 | 9 | 0.93 (0.82 to 1.05) | 23 | 0.24 | NC | 9 | 0.98 (0.91 to 1.06) | 0 | 0.60 | NC |
| No | 5 | 0.92 (0.86 to 0.98) | 0 | 0.98 | 0 | — | — | — | 0 | — | — | — |
Table 4.
Subgroup analyses of cereal fibre and whole grain intake and risk of colorectal cancer, dose-response analysis
| Subgroups | Cereal fibre | Whole grains | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Relative risk (95% CI) | I2 (%) | P for heterogeneity* | P for heterogeneity† | No of studies | Relative risk (95% CI) | I2 (%) | P for heterogeneity* | P for heterogeneity† | |
| All studies | 8 | 0.90 (0.83 to 0.97) | 0 | 0.78 | — | 6 | 0.83 (0.78 to 0.89) | 18.2 | 0.30 | — |
| Duration of follow-up: | ||||||||||
| <10 years | 5 | 0.90 (0.82 to 0.98) | 0 | 0.42 | 0.84 | 1 | 0.73 (0.63 to 0.84) | — | — | 0.12 |
| ≥10 years | 3 | 0.87 (0.71 to 1.08) | 0 | 0.99 | 5 | 0.86 (0.80 to 0.92) | 0 | 0.72 | ||
| Sex: | ||||||||||
| Men | 2 | 0.92 (0.80 to 1.06) | 0 | 0.60 | 0.69 | 3 | 0.79 (0.72 to 0.87) | 0 | 0.44 | 0.14 |
| Women | 5 | 0.96 (0.83 to 1.11) | 0 | 0.98 | 5 | 0.88 (0.81 to 0.95) | 0 | 0.58 | ||
| Subsite: | ||||||||||
| Colon | 3 | 1.03 (0.80 to 1.32) | 0 | 0.45 | 0.45 | 4 | 0.86 (0.79 to 0.94) | 0 | 0.58 | 0.53 |
| Rectum | 1 | 1.39 (0.78 to 2.48) | — | — | 3 | 0.80 (0.56 to 1.14) | 90.5 | <0.001 | ||
| Geographical location: | ||||||||||
| Europe | 2 | 0.94 (0.73 to 1.21) | 0 | 0.48 | 0.70 | 2 | 0.87 (0.78 to 0.96) | 58.8 | 0.12 | 0.13 |
| USA | 6 | 0.89 (0.82 to 0.97) | 0 | 0.65 | 4 | 0.79 (0.72 to 0.86) | 0 | 0.57 | ||
| Asia | 0 | — | — | — | 0 | — | — | — | ||
| No of cases: | ||||||||||
| <500 | 3 | 1.01 (0.77 to 1.32) | 0 | 0.99 | 0.48 | 0 | 0.12 | |||
| 500-1499 | 2 | 0.87 (0.71 to 1.08) | 0 | 0.92 | 5 | 0.86 (0.80 to 0.92) | 0 | 0.72 | ||
| ≥1500 | 3 | 0.88 (0.77 to 1.00) | 35.1 | 0.21 | 1 | 0.73 (0.63 to 0.84) | — | — | ||
| Range of intake: | ||||||||||
| <7 to <90 g/day‡ | 3 | 0.91 (0.68 to 1.21) | 0 | 0.85 | 0.90 | 1 | 0.73 (0.63 to 0.84) | 0.18 | ||
| ≥7 to ≥90 g/day‡ | 5 | 0.89 (0.82 to 0.97) | 0 | 0.45 | 3 | 0.87 (0.80 to 0.94) | 0 | 0.39 | ||
| Adjustment for confounders | ||||||||||
| Alcohol: | ||||||||||
| Yes | 6 | 0.93 (0.84 to 1.02) | 0 | 0.98 | 0.27 | 3 | 0.86 (0.79 to 0.93) | 0 | 0.92 | 0.51 |
| No | 2 | 0.86 (0.68 to 1.08) | 41.9 | 0.19 | 3 | 0.81 (0.70 to 0.93) | 60.6 | 0.08 | ||
| Smoking: | ||||||||||
| Yes | 7 | 0.89 (0.82 to 0.97) | 0 | 0.76 | 0.47 | 3 | 0.79 (0.71 to 0.87) | 1.6 | 0.36 | 0.21 |
| No | 1 | 1.02 (0.73 to 1.43) | — | — | 3 | 0.87 (0.80 to 0.94) | 0 | 0.39 | ||
| Body mass index, weight, waist to hip ratio: | ||||||||||
| Yes | 7 | 0.89 (0.82 to 0.97) | 0 | 0.76 | 0.27 | 4 | 0.87 (0.81 to 0.94) | 0 | 0.82 | 0.09 |
| No | 1 | 1.02 (0.73 to 1.43) | — | — | 2 | 0.75 (0.67 to 0.84) | 0 | 0.52 | ||
| Physical activity: | ||||||||||
| Yes | 6 | 0.89 (0.81 to 0.96) | 0 | 0.68 | 0.38 | 4 | 0.82 (0.76 to 0.89) | 20.1 | 0.29 | 0.60 |
| No | 2 | 1.02 (0.77 to 1.34) | 0 | 0.97 | 2 | 0.86 (0.73 to 1.01) | 47.5 | 0.17 | ||
| Red, processed meat: | ||||||||||
| Yes | 8 | 0.90 (0.83 to 0.97) | 0 | 0.78 | NC | 3 | 0.84 (0.74 to 0.95) | 64.0 | 0.06 | 0.81 |
| No | 0 | — | — | — | 3 | 0.82 (0.74 to 0.91) | 0 | 0.81 | ||
| Dairy products, calcium: | ||||||||||
| Yes | 6 | 0.90 (0.83 to 0.98) | 0 | 0.56 | 0.82 | 2 | 0.82 (0.65 to 1.03) | 79.7 | 0.03 | 0.79 |
| No | 2 | 0.85 (0.59 to 1.23) | 0 | 0.87 | 4 | 0.84 (0.78 to 0.91) | 0 | 0.81 | ||
| Folate: | ||||||||||
| Yes | 5 | 0.88 (0.81 to 0.96) | 0 | 0.54 | 0.38 | 1 | 0.73 (0.63 to 0.84) | 0.12 | ||
| No | 3 | 1.01 (0.77 to 1.32) | 0 | 0.99 | 5 | 0.86 (0.80 to 0.92) | 0 | 0.72 | ||
| Energy intake: | ||||||||||
| Yes | 6 | 0.90 (0.82 to 0.98) | 0 | 0.56 | 0.83 | 4 | 0.83 (0.74 to 0.92) | 41.1 | 0.17 | 0.91 |
| No | 2 | 0.87 (0.71 to 1.08) | 0 | 0.92 | 2 | 0.85 (0.77 to 0.93) | 0 | 0.35 |
In addition, the effect on the results of excluding studies from the dose-response analysis was explored. When the analysis of high versus low intake was restricted to the studies that were included in the dose-response analysis of total dietary fibre, the summary relative risk was 0.86 (0.80 to 0.92, I2=0%, P=0.46 for heterogeneity), similar to the original analysis including all studies.
The influence on the results of the method used to estimate total fibre intake was assessed. For the eight studies17 21 22 24 26 27 28 using the Association of Official Analytical Chemists method, the summary relative risk was 0.91 (0.85 to 0.97, I2=13.3%, P=0.33 for heterogeneity), for the four studies8 16 21 using the Englyst method it was 0.91 (0.81 to 1.02, I2=37.0%, P=0.19 for heterogeneity), and for the six studies12 18 20 23 25 29 with an unknown method it was 0.93 (0.86 to 1.00, I2=0%, P=0.89 for heterogeneity). In this sensitivity analysis no heterogeneity was found between subgroups (P=0.39 for heterogeneity). In addition, in one study the results did not differ materially between the two methods.21
Discussion
Our meta-analysis supports an inverse association between intake of dietary fibre, cereal fibre, and whole grains and risk of colorectal cancer, but we found no significant evidence for an association with intake of fibre from fruit, vegetables, or legumes.
Comparison with other studies
Our results for total dietary fibre are consistent with a previous meta-analysis of case-control studies, which found an inverse association between fibre intake and risk of colorectal cancer. Our results, based on prospective studies, are not, however, as strong as the previous results from case-control studies.9 The size of the summary estimates from our analyses is more in line with those of a pooled analysis of cohort studies,31 which found an 18% increased risk among people with a low intake of dietary fibre (<10 g/day). In that analysis, however, no further reduction in risk occurred with higher intake of fibre, whereas we observed a linear inverse association with increasing intake, such as shown in the EPIC study.8 Several differences between our analysis and the pooled analysis could explain the differences between the results. For example, although some overlap occurs between the studies included in the two analyses, some differences also exist. Our dose-response analysis included results from seven16 17 18 21 22 25 of the 13 studies in the Pooling Project of Prospective Studies of Diet and Cancer, but included nine additional studies8 12 20 23 24 26 27 28 29 not included in the pooled analysis, some of which were large. Thus our analysis included more than 14 000 cases among 1.9 million participants compared with 8000 cases among 700 000 participants in the pooled analysis. It is therefore possible that these additional studies contributed to a better assessment of the dose-response relation between fibre intake and risk of colorectal cancer. In line with the pooled analysis we found no evidence for an association between fruit or vegetable fibre and risk of colorectal cancer. However, in a previous meta-analysis of prospective studies we showed a reduction in risk with high intake of fruit and vegetables,61 suggesting the potential role of components other than fibre in fruits and vegetables in explaining this result. In addition, we cannot exclude the possibility that the range of fruit fibre intake was too low to detect an inverse association in the overall analysis, although no difference in the summary estimates was observed for the other fibre types when stratified by the range of intake. Inverse associations were evident between intakes of cereal fibre and whole grain and risk of colorectal cancer in our analysis, and the results for whole grain intake are consistent with a previous meta-analysis of case-control studies, which reported a 20% reduction in risk with high whole grain intake.33 The pooled analysis found a marginally significant inverse association between whole grain intake and colorectal cancer: pooled relative risk 0.92 (95% confidence interval 0.84 to 1.00).31 In contrast to our results, the Women’s Health Initiative Trial did not find a reduction in risk of colorectal cancer among participants who were randomised to an intervention with increased intakes of fruits, vegetables, grains, and fibre and reductions in fat intake.62 However, fibre intake increased by only 2.5 g/day from baseline to the three year follow-up, from 15.4 to 17.9 g/day, whereas the intake in the comparison group did not materially change (from 15.4 to 14.8 g/day). Thus the changes in fibre intake in that trial may have been too small to significantly reduce the risk of colorectal cancer. Given that our results show a 10% reduction in risk of colorectal cancer for each 10 g intake of fibre daily, only a 2-3% reduction in risk would be expected with such a small increase in fibre intake.
Limitations of the study
Our meta-analysis has limitations that affect the interpretation of the results. It is possible that the weak inverse associations between dietary fibre or whole grain intake and risk of colorectal cancer could result from unmeasured or residual confounding by other dietary or lifestyle factors. Higher intakes of dietary fibre and whole grain are typically associated with other health behaviours, such as higher intakes of calcium and folate; higher levels of physical activity; lower prevalence of smoking, overweight, or obesity; and lower intakes of alcohol and red and processed meat.22 27 63 Many but not all of the studies adjusted for potential confounding factors, although not all potential confounders were adjusted for in every study. In analyses stratified by adjustment for confounding factors, however, we found that the association between dietary fibre, cereal fibre, and whole grains persisted in most subgroups, with adjustment for potential confounding factors. In addition, in meta-regression analyses evidence that the results for these exposures differed significantly whether confounders had been adjusted for or not was lacking. Only in the analysis of fruit fibre was heterogeneity evident between studies that did or did not adjust for body mass index or weight and alcohol intake, with significant inverse associations among the studies with such adjustments. None of the included studies reported results stratified by alcohol, smoking, body mass index, or meat intake. Any further studies should report analyses stratified by other risk factors to better be able to rule out residual confounding.
Although publication bias can be a problem in meta-analyses of published literature we found no evidence of such bias in this analysis. In addition, the few studies that were excluded from the dose-response analysis of dietary fibre are unlikely to have altered the results because the results from the analyses of high versus low intake were similar when we repeated the analyses with the same dataset as in the dose-response analysis.
Accurate assessment of dietary fibre intake and other food constituents is a challenge. The definition of dietary fibre may differ between studies and may contribute to heterogeneity in the results. Some studies used the Englyst definition of fibre, which distinguishes non-starch polysaccharides from starch, whereas other studies calculated fibre intake using the Association of Official Analytical Chemists method, which includes some starch as dietary fibre. The summary relative risks were generally similar, however, no matter which method was used, and there was no evidence of heterogeneity between subgroups when stratified by the method used to calculate fibre intake.
Most studies carried out to date have used food frequency questionnaires to assess dietary intake. Concern is, however, increasing that measurement errors associated with the use of food frequency questionnaires may obscure associations between dietary intake and risk of chronic disease.64 Few studies have reported results corrected for measurement errors. In the EPIC study the relative risk of colorectal cancer was 0.75 (95% confidence interval 0.59 to 0.95) for the highest compared with lowest fifths of fibre intake, and after calibration with more detailed data the relative risk was 0.58 (0.41 to 0.85).19 In the Pooling Project of Prospective Studies the adjusted relative risk for less than 10 g/day compared with 10 g/day or more was 1.22 (1.10 to 1.35), but this increased to 2.16 (1.12 to 4.16) after correction for measurement error.31 In a pooled analysis of seven UK based cohort studies, a stronger association was observed when food diaries were used to assess dietary fibre intake; an odds ratio of 0.66 (95% confidence interval 0.45 to 0.96) for the highest versus lowest fifths of fibre density compared with 0.88 (0.57 to 1.36) when food frequency questionnaires were used to measure dietary intake.65 The latter was of similar size to our summary estimate for the highest versus lowest intake (summary relative risk 0.88, 95% confidence interval 0.82 to 0.94). The results using food diaries were further strengthened when corrected for measurement errors: odds ratio 0.68 (95% confidence interval 0.48 to 0.96) for a 0.7 g/MJ increase in fibre intake (uncorrected odds ratio 0.83, 95% confidence interval 0.70 to 0.97).65 The results from these studies suggest that our results for dietary fibre and risk of colorectal cancer are likely to be conservative estimates of the true underlying risk and that any further studies should incorporate correction for measurement error in the analyses.
Strengths of the study
Our meta-analysis also has several strengths. Because we based our analysis on prospective studies, our findings are unlikely to be explained by recall bias and selection bias. Our meta-analysis included a large number of studies and more than 14 500 cases, and almost two million participants in the dietary fibre analysis. Thus we had adequate statistical power to clarify the shape of the dose-response relation between dietary fibre intake and risk of colorectal cancer and to detect moderate reductions in risk. We also carried out sensitivity analyses to investigate whether any particular study explained the results, but the findings were generally robust. We quantified the association between intake of dietary fibre and whole grain and risk of colorectal cancer by carrying out linear and non-linear dose-response analyses.
Mechanisms
A protective effect of dietary fibre and whole grain consumption on risk of colorectal cancer is biologically plausible. Whole grain foods are important sources of dietary fibre and may decrease the risk of colorectal cancer by increasing stool bulk, diluting faecal carcinogens, and decreasing transit time, thus reducing the contact between carcinogens and the lining of the colorectum.7 In addition, bacterial fermentation of fibre results in the production of short chain fatty acids, which may have protective effects against colorectal cancer.66 Other components of whole grains may also protect against colorectal cancer, including antioxidants, vitamins, trace minerals, phytate, phenolic acids, lignans, and phytoestrogens.66 67 68 Whole grains have a high content of folate and magnesium, which have been associated with a reduced risk of colorectal cancer.69 70 71 Higher intakes of dietary fibre and whole grain also protect against weight gain72 73 and type 2 diabetes,74 75 and it is possible that part of the potential effect of fibre intake is mediated through improved weight control and reduced insulin resistance, although these may not be the main mechanisms. However, the results persisted in studies that adjusted for both folate and body mass index, suggesting an association independent of folate and body mass index.
Conclusions and policy implications
Our results indicate a 10% reduction in risk of colorectal cancer for each 10 g/day intake of total dietary fibre and cereal fibre and a about a 20% reduction for each three servings (90 g/day) of whole grain daily, and further reductions with higher intake. These findings thus have important public health implications. Our results provide further support for public health recommendations to increase the intake of dietary fibre in the prevention of colorectal cancer. However, they suggest a particular benefit of increasing cereal fibre and whole grain intake. Increasing the intake of dietary fibre and whole grains is also likely to reduce the risk of cardiovascular disease,76 77 78 type 2 diabetes,74 75 overweight and obesity,72 73 and possibly overall mortality,76 78 thus there are several health benefits by increasing fibre intake and replacing refined grains with whole grains.
In summary, our meta-analysis suggests that a high intake of dietary fibre, particularly from cereal and whole grains, is associated with a reduced risk of colorectal cancer. Further studies should report more detailed results, including those for subtypes of fibre, stratify the results by subsites within the colorectum, and stratify the results by other risk factors to be able to rule out residual confounding. Further assessment of the impact of measurement errors on the risk estimates is also warranted.
What is already known on this topic
- Colorectal cancer is the third most common cancer worldwide, with 1.2 million new cases annually
- Intake of dietary fibre and whole grains has been established as protective against cardiovascular disease, but the association with colorectal cancer is not convincing
- It is unclear whether only specific types of fibre or sources of fibre are associated with the risk of colorectal cancer
What this study adds
- Intakes of dietary fibre, cereal fibre, and whole grains are associated with linear decreases in the risk of colorectal cancer
- Evidence of an association between intake of fruit, vegetable, or legume fibre and risk of colorectal cancer was lacking
- Intake of dietary fibre, particularly cereal fibre and whole grains, was associated with a small reduction in the risk of colorectal cancer
We thank the systematic literature review team at Wageningen University for their contributions to the colorectal cancer database.
Contributors: The systematic literature review team at Wageningen University carried out the search, and selected and extracted data up to the end of December 2005. RV developed and managed the database for the Continuous Update Project. RL and DSMC did the updated literature search. RL, DSMC, and DA did the updated data extraction. DA and DSMC selected the studies and carried out the statistical analyses. DCG was statistical adviser and contributed to the statistical analyses. All authors revised the manuscript. EK was principal investigator of the Systematic Literature Reviews at Wageningen University. TN is principal investigator of the Continuous Update Project and wrote the protocol and implemented the study. DA wrote the first draft of the original manuscript. All authors had full access to all of the data in the study and prepared the manuscript. DA is guarantor. The sponsor of this study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The views expressed in this review are the opinions of the authors. They may not represent the views of the World Cancer Research Fund International/American Institute for Cancer Research and may differ from those in future updates of the evidence related to food, nutrition, physical activity, and cancer risk.
Funding: This work was funded by the World Cancer Research Fund (grant No 2007/SP01) as part of the Continuous Update Project.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2011;343:d6617
Web Extra. Extra material supplied by the author
High versus low intake of dietary fibre and whole grains and risk of colorectal cancer
High versus low intake of fruit, vegetable, legume, and cereal fibre and risk of colorectal cancer
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 1975;15:617-31. [DOI] [PubMed] [Google Scholar]
- 3.Kolonel LN. Cancer patterns of four ethnic groups in Hawaii. J Natl Cancer Inst 1980;65:1127-39. [PubMed] [Google Scholar]
- 4.Kono S. Secular trend of colon cancer incidence and mortality in relation to fat and meat intake in Japan. Eur J Cancer Prev 2004;13:127-32. [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: a global perspective. AICR, 2007.
- 6.Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer 1971;28:3-13. [DOI] [PubMed] [Google Scholar]
- 7.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr 1999;19:545-86. [DOI] [PubMed] [Google Scholar]
- 8.Bingham SA, Norat T, Moskal A, Ferrari P, Slimani N, Clavel-Chapelon F, et al. Is the association with fiber from foods in colorectal cancer confounded by folate intake? Cancer Epidemiol Biomarkers Prev 2005;14:1552-6. [DOI] [PubMed] [Google Scholar]
- 9.Howe GR, Benito E, Castelleto R, Cornee J, Esteve J, Gallagher RP, et al. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst 1992;84:1887-96. [DOI] [PubMed] [Google Scholar]
- 10.Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst 1990;82:650-61. [DOI] [PubMed] [Google Scholar]
- 11.Wu AH, Paganini-Hill A, Ross RK, Henderson BE. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. Br J Cancer 1987;55:687-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilbrun LK, Nomura A, Hankin JH, Stemmermann GN. Diet and colorectal cancer with special reference to fiber intake. Int J Cancer 1989;44:1-6. [DOI] [PubMed] [Google Scholar]
- 13.Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women’s Health Study. Am J Epidemiol 1994;139:1-15. [DOI] [PubMed] [Google Scholar]
- 14.Gaard M, Tretli S, Loken EB. Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev 1996;5:445-54. [PubMed] [Google Scholar]
- 15.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer 1997;28:276-81. [DOI] [PubMed] [Google Scholar]
- 16.Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1999;10:387-96. [DOI] [PubMed] [Google Scholar]
- 17.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 2001;93:525-33. [DOI] [PubMed] [Google Scholar]
- 18.Mai V, Flood A, Peters U, Lacey JV Jr, Schairer C, Schatzkin A. Dietary fibre and risk of colorectal cancer in the Breast Cancer Detection Demonstration Project (BCDDP) follow-up cohort. Int J Epidemiol 2003;32:234-9. [DOI] [PubMed] [Google Scholar]
- 19.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003;361:1496-501. [DOI] [PubMed] [Google Scholar]
- 20.Sanjoaquin MA, Appleby PN, Thorogood M, Mann JI, Key TJ. Nutrition, lifestyle and colorectal cancer incidence: a prospective investigation of 10998 vegetarians and non-vegetarians in the United Kingdom. Br J Cancer 2004;90:118-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels KB, Fuchs CS, Giovannucci E, Colditz GA, Hunter DJ, Stampfer MJ, et al. Fiber intake and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev 2005;14:842-9. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Zhang SM, Cook NR, Rexrode KM, Liu S, Manson JE, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control 2005;16:225-33. [DOI] [PubMed] [Google Scholar]
- 23.Shin A, Li H, Shu XO, Yang G, Gao YT, Zheng W. Dietary intake of calcium, fiber and other micronutrients in relation to colorectal cancer risk: results from the Shanghai Women’s Health Study. Int J Cancer 2006;119:2938-42. [DOI] [PubMed] [Google Scholar]
- 24.Otani T, Iwasaki M, Ishihara J, Sasazuki S, Inoue M, Tsugane S. Dietary fiber intake and subsequent risk of colorectal cancer: the Japan Public Health Center-based prospective study. Int J Cancer 2006;119:1475-80. [DOI] [PubMed] [Google Scholar]
- 25.McCarl M, Harnack L, Limburg PJ, Anderson KE, Folsom AR. Incidence of colorectal cancer in relation to glycemic index and load in a cohort of women. Cancer Epidemiol Biomarkers Prev 2006;15:892-6. [DOI] [PubMed] [Google Scholar]
- 26.Wakai K, Date C, Fukui M, Tamakoshi K, Watanabe Y, Hayakawa N, et al. Dietary fiber and risk of colorectal cancer in the Japan collaborative cohort study. Cancer Epidemiol Biomarkers Prev 2007;16:668-75. [DOI] [PubMed] [Google Scholar]
- 27.Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, et al. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2007;85:1353-60. [DOI] [PubMed] [Google Scholar]
- 28.Nomura AM, Hankin JH, Henderson BE, Wilkens LR, Murphy SP, Pike MC, et al. Dietary fiber and colorectal cancer risk: the multiethnic cohort study. Cancer Causes Control 2007;18:753-64. [DOI] [PubMed] [Google Scholar]
- 29.Kabat GC, Shikany JM, Beresford SA, Caan B, Neuhouser ML, Tinker LF, et al. Dietary carbohydrate, glycemic index, and glycemic load in relation to colorectal cancer risk in the Women’s Health Initiative. Cancer Causes Control 2008;19:1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler LM, Wang R, Koh WP, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer 2008;99:1511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA 2005;294:2849-57. [DOI] [PubMed] [Google Scholar]
- 32.Slavin JL, Martini MC, Jacobs DR Jr, Marquart L. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr 1999;70:S459-63. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs DR Jr, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer 1998;30:85-96. [DOI] [PubMed] [Google Scholar]
- 34.Appleby PN, Key TJ, Burr ML, Thorogood M. Mortality and fresh fruit consumption. IARC Sci Publ 2002;156:131-3. [PubMed] [Google Scholar]
- 35.Larsson SC, Giovannucci E, Bergkvist L, Wolk A. Whole grain consumption and risk of colorectal cancer: a population-based cohort of 60,000 women. Br J Cancer 2005;92:1803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCullough ML, Robertson AS, Chao A, Jacobs EJ, Stampfer MJ, Jacobs DR, et al. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control 2003;14:959-70. [DOI] [PubMed] [Google Scholar]
- 37.Wu K, Hu FB, Fuchs C, Rimm EB, Willett WC, Giovannucci E. Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States). Cancer Causes Control 2004;15:853-62. [DOI] [PubMed] [Google Scholar]
- 38.Egeberg R, Olsen A, Loft S, Christensen J, Johnsen NF, Overvad K, et al. Intake of wholegrain products and risk of colorectal cancers in the Diet, Cancer and Health cohort study. Br J Cancer 2010;103:730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010;92:1429-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [DOI] [PubMed] [Google Scholar]
- 41.Haas P, Machado MJ, Anton AA, Silva AS, De Francisco A. Effectiveness of whole grain consumption in the prevention of colorectal cancer: Meta-analysis of cohort studies. Int J Food Sci Nutr 2009;Mar 21:1-13. [Epub ahead of print.] [DOI] [PubMed]
- 42.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol 1993;137:1302-17. [DOI] [PubMed] [Google Scholar]
- 43.Glynn SA, Albanes D, Pietinen P, Brown CC, Rautalahti M, Tangrea JA, et al. Alcohol consumption and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1996;7:214-23. [DOI] [PubMed] [Google Scholar]
- 44.Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control 1997;8:615-25. [DOI] [PubMed] [Google Scholar]
- 45.Colbert LH, Hartman TJ, Malila N, Limburg PJ, Pietinen P, Virtamo J, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev 2001;10:265-8. [PubMed] [Google Scholar]
- 46.Konings EJ, Goldbohm RA, Brants HA, Saris WH, van den Brandt PA. Intake of dietary folate vitamers and risk of colorectal carcinoma: results from The Netherlands Cohort Study. Cancer 2002;95:1421-33. [DOI] [PubMed] [Google Scholar]
- 47.Wong HL, Seow A, Arakawa K, Lee HP, Yu MC, Ingles SA. Vitamin D receptor start codon polymorphism and colorectal cancer risk: effect modification by dietary calcium and fat in Singapore Chinese. Carcinogenesis 2003;24:1091-5. [DOI] [PubMed] [Google Scholar]
- 48.Koh WP, Yuan JM, van den BD, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer 2004;90:1760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 1990;323:1664-72. [DOI] [PubMed] [Google Scholar]
- 50.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res 1994;54:2390-7. [PubMed] [Google Scholar]
- 51.Sellers TA, Bazyk AE, Bostick RM, Kushi LH, Olson JE, Anderson KE, et al. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States). Cancer Causes Control 1998;9:357-67. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Stampfer MJ, Rosner B, et al. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med 1999;340:169-76. [DOI] [PubMed] [Google Scholar]
- 53.Higginbotham S, Zhang ZF, Lee IM, Cook NR, Giovannucci E, Buring JE, et al. Dietary glycemic load and risk of colorectal cancer in the Women’s Health Study. J Natl Cancer Inst 2004;96:229-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst 2005;97:906-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301-9. [DOI] [PubMed] [Google Scholar]
- 56.Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 2000;19:1831-47. [DOI] [PubMed] [Google Scholar]
- 57.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 2004;159:1077-86. [DOI] [PubMed] [Google Scholar]
- 58.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 59.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed] [Google Scholar]
- 61.Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011;141:106-18. [DOI] [PubMed] [Google Scholar]
- 62.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative randomized controlled dietary modification trial. JAMA 2006;295:643-54. [DOI] [PubMed] [Google Scholar]
- 63.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 2003;158:14-21. [DOI] [PubMed] [Google Scholar]
- 65.Dahm CC, Keogh RH, Spencer EA, Greenwood DC, Key TJ, Fentiman IS, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst 2010;102:614-26. [DOI] [PubMed] [Google Scholar]
- 66.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr 2000;19:S300-7. [DOI] [PubMed] [Google Scholar]
- 67.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr 2006;136:3046-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer 2005;51:117-31. [DOI] [PubMed] [Google Scholar]
- 69.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 2005;113:825-8. [DOI] [PubMed] [Google Scholar]
- 70.Folsom AR, Hong CP. Magnesium intake and reduced risk of colon cancer in a prospective study of women. Am J Epidemiol 2006;163:232-5. [DOI] [PubMed] [Google Scholar]
- 71.Larsson SC, Bergkvist L, Wolk A. Magnesium intake in relation to risk of colorectal cancer in women. JAMA 2005;293:86-9. [DOI] [PubMed] [Google Scholar]
- 72.Bazzano LA, Song Y, Bubes V, Good CK, Manson JE, Liu S. Dietary intake of whole and refined grain breakfast cereals and weight gain in men. Obes Res 2005;13:1952-60. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 2003;78:920-7. [DOI] [PubMed] [Google Scholar]
- 74.De Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 2007;167:956-65. [DOI] [PubMed] [Google Scholar]
- 76.Jacobs DR Jr, Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am J Clin Nutr 2007;85:1606-14. [DOI] [PubMed] [Google Scholar]
- 77.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659-69. [DOI] [PubMed] [Google Scholar]
- 78.Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP Diet and Health Study. Arch Intern Med 2011;171:1061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High versus low intake of dietary fibre and whole grains and risk of colorectal cancer
High versus low intake of fruit, vegetable, legume, and cereal fibre and risk of colorectal cancer