Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase (original) (raw)
Abstract
Hepoxilins are lipid signaling molecules derived from arachidonic acid through the 12-lipoxygenase pathway. These _trans_-epoxy hydroxy eicosanoids play a role in a variety of physiological processes, including inflammation, neurotransmission, and formation of skin barrier function. Mammalian hepoxilin hydrolase, partly purified from rat liver, has earlier been reported to degrade hepoxilins to trioxilins. Here, we report that hepoxilin hydrolysis in liver is mainly catalyzed by soluble epoxide hydrolase (sEH): i) purified mammalian sEH hydrolyses hepoxilin A3 and B3 with a Vmax of 0.4–2.5 μmol/mg/min; ii) the highly selective sEH inhibitors N-adamantyl-N’-cyclohexyl urea and 12-(3-adamantan-1-yl-ureido) dodecanoic acid greatly reduced hepoxilin hydrolysis in mouse liver preparations; iii) hepoxilin hydrolase activity was abolished in liver preparations from sEH−/− mice; and iv) liver homogenates of sEH−/− mice show elevated basal levels of hepoxilins but lowered levels of trioxilins compared with wild-type animals. We conclude that sEH is identical to previously reported hepoxilin hydrolase. This is of particular physiological relevance because sEH is emerging as a novel drug target due to its major role in the hydrolysis of important lipid signaling molecules such as epoxyeicosatrienoic acids. sEH inhibitors might have undesired side effects on hepoxilin signaling.
Keywords: lipid metabolism, eicosanoid, 12-lipoxygenase
Epoxide hydrolases (EC 3.3.2.7-11) catalyze the hydrolysis of oxiranes to the corresponding vicinal diols. To date, a number of mammalian epoxide hydrolases have been characterized that play diverse roles in the organism (1).
The soluble epoxide hydrolase (sEH, EC 3.1.3.76; EC 3.3.2.10) is a bifunctional homodimeric enzyme composed of an epoxide hydrolase (EH) and a lipid phosphatase in each of its subunits (2–4). The sEH is abundantly expressed throughout the organism (5, 6) and accepts a broad range of substrates (7, 8), in particular, endogenous epoxides derived from unsaturated fatty acids such as epoxyeicosatrienoic acids (EETs) (9). The organism utilizes these lipid epoxides as important signaling molecules, which regulate a variety of physiological functions. EETs were identified as endothelium-derived hyperpolarizing factors (EDHFs) (10) acting on vascular smooth muscle cells leading to hyperpolarization and vasodilation (11, 12). Since then, several experimental hypertensive models confirmed a role for EETs in blood pressure regulation and end organ protection (13, 14). Further, EETs have anti-inflammatory and antinociceptive properties (15–17) and finally, seem to promote cell proliferation, migration, and angiogenesis (18–20).
The metabolism of these lipid epoxides by sEH to the corresponding diols is generally considered a deactivation process. For this reason, the sEH is a promising target for the treatment of hypertension, inflammatory diseases, pain, diabetes, and stroke (16, 21–25). A number of sEH inhibitors (sEHIs) have been developed (26, 27) for therapeutic applications. Yet the sEH also serves some function in xenobiotic metabolism by accepting certain _trans_-substituted epoxides, which are very poor substrates for microsomal epoxide hydrolase (mEH) (28, 29).
Other epoxide hydrolases with rather narrow substrate selectivity have been identified in mammals. Of those, hepoxilin A3 epoxide hydrolase (hepoxilin hydrolase, EC 3.3.2.7) was partly purified from rat liver cytosol and identified as the main hydrolase of the endogenous lipid hepoxilin A3. The authors further discriminated hepoxilin hydrolase from other EHs due to its size (53 kDa) and substrates preference for hepoxilin A3 compared with leukotriene or styrene oxide (30). To date, the enzyme is only incompletely characterized and no structural or sequence information is available.
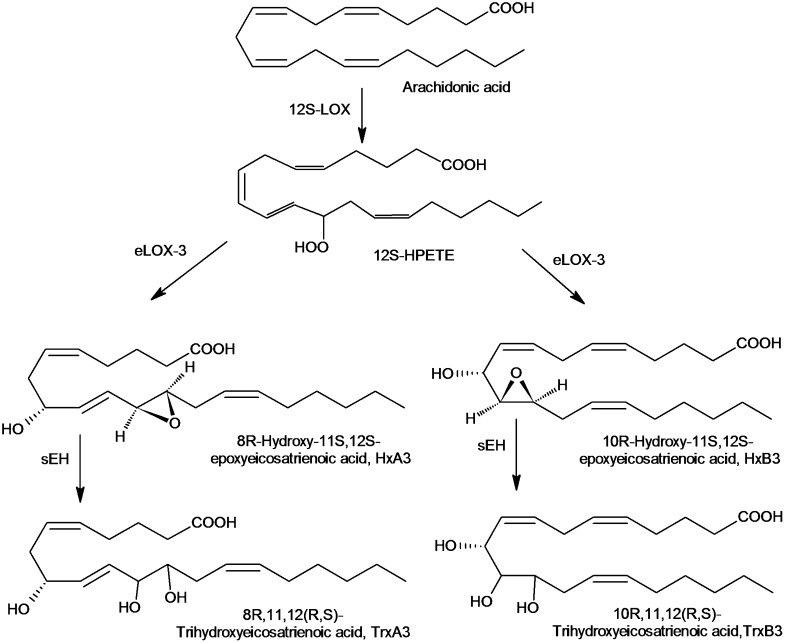
Most enzymatic-derived endogenous lipid epoxides are of _cis_-configuration, but also some _trans_-substitutes lipid epoxides are formed within the organism, such as the inflammatory mediator leukotriene A4. The _trans_-epoxy hydroxy eicosanoids hepoxilin A3 and B3 (HxA3 and HxB3) are formed from arachidonic acid through the 12-lipoxygenase (LOX) pathway (Fig. 1) in various organs like liver, brain, lung, pancreas, and skin (9, 31). They can be transformed to the corresponding trihydroxy metabolites (trioxilins, Trx) or glutathione conjugates (32).
Fig. 1.
Biosynthesis and metabolism of hepoxilins. Hepoxilins are synthesized from arachidonic acid by the action of 12S-lipoxygenase (12S-LOX) and epidermis-type lipoxygenase 3 (eLOX-3), leading to the regioisomers HxA3 and HxB3. The hepoxilins are turned over by sEH to the corresponding trihydroxy metabolites TrxA3 and B3. (Note that in the skin a specific 12R-LOX generates hepoxilins with R-configuration. This pathway plays a role in epidermal differentiation and skin barrier function.)
Early studies showed a hepoxilin-mediated increased insulin release from pancreatic islets (33). In the brain, hepoxilins modulate synaptic neurotransmission and neuronal excitability, mostly through stimulation of intracellular calcium release or increased calcium influx into the cell (34–37). Hepoxilins are considered pro-inflammatory because increased hepoxilin and trioxilin levels have been found in psoriatic lesions (38) and HxA3 was shown to regulate neutrophil migration in response to inflammation (39, 40). Several reports suggest an involvement of these lipid mediators in epidermal differentiation and skin barrier function (41). Interestingly, mutations in the hepoxilin-generating 12R-LOX pathway in the skin are associated with a congenital form of ichthyosis (42–46). A hepoxilin receptor has not been identified, but several reports (34, 47–49) support its existence.
Here, we report that 12S-LOX-derived HxA3 and HxB3 are efficiently converted to the corresponding trioxilins by sEHs. Our results suggest a biological relevance of sEH, rather than hepoxilin hydrolase, in hepoxilin metabolism, which opens a new branch in the numerous physiological functions of sEH.
METHODS
Enzyme assays
Human full length sEH containing an N-terminal Strep-Tag and rat sEH containing an N-terminal His-Tag were cloned, recombinantly expressed in Escherichia coli, and purified as described previously (3). Epoxide hydrolase activity was measured using 8(R,S)-Hydroxy-11S,12S-epoxyeicosa-5Z,9E,14Z-trienoic acid (HxA3) and 10(R,S)-Hydroxy-11S,12S-epoxyeicosa-5Z,8Z,14Z-trienoic acid (HxB3) as substrates by determining the formation of the corresponding diols 8(R,S)-Hydroxy-11,12-dihydroxyeicosa-5Z,9E,14Z-trienoic acid (TrxA3) and 10(R,S)-Hydroxy-11,12-dihydroxyeicosa-5Z,8Z,14Z-trienoic acid (TrxB3). Typically, 5–50 ng purified sEH or 10–100 µg organ extracts were incubated with various concentrations of HxA3 and HxB3 ranging from 0.1 µM to 30 µM with or without inhibitor in 50 mM Tris HCl, 50 mM NaCl, 2% glycerol, pH 7.4 in a final volume of 50 µl for 10 min at 37°C. The reaction was stopped by addition of 2 vols of methanol and samples were pelleted for 5 min at 13,000 rpm prior to LC-MS/MS analysis. Substrate turnover was determined using internal HxA3 and HxB3 standards. EH activity against EETs was performed as described previously (50). Kinetic constants were calculated by kinetic modeling based on the equation V = E × CS/(CS+Km) (with V = % turnover of Vmax, E = total amount of enzyme, CS = substrate concentration, and Km = Michaelis Menten constant). Variations were calculated from four to five independent experiments using freshly prepared enzyme preparations.
Lipid substrates were purchased from Biomol except for TrxA3 and TrxB3, which were synthesized biochemically using purified sEH. One microgram of HxA3 or HxB3 was turned over to the corresponding diol using 200 ng sEH in 50 mM Tris HCl, 50 mM NaCl, 2% glycerol, pH 7.4 in a final volume of 500 µl for 30 min at 37°C. The reaction products were extracted three times with 2 vols of ethyl acetate, dried under a stream of nitrogen, and reconstituted in methanol.
LC-MS/MS analysis
Separation of analytes was performed on an Agilent eclipse XDB-C18 reverse phase column (4.6 × 150 mm, 5 μm pore size) with a corresponding opti-gard precolumn using an Agilent 1100 liquid chromatography system. The mobile phase consisted of (A) 0.1% formic acid and (B) acetonitrile containing 0.1% formic acid at a flow rate of 400 μl/min using an injection volume of 20 μl. Starting conditions of 70% buffer B were maintained for 2 min followed by a linear gradient from 70 to 100% B within 7 min. An isocratic flow of 100% B was held for 1.5 min and finally the column was re-equilibrated for 2 min with 70% B. The HPLC system was coupled to a 4000 QTRAP hybrid quadrupole linear ion trap mass spectrometer (Applied Biosystems) equipped with a TurboV source and electrospray (ESI) interface. Analytes were recorded using multiple reaction monitoring in negative mode (−MRM) using the following source specific parameters: IS −4500V, TEM 450°C, curtain gas (CUR = 30), nebulizer gas (GS1 = 50), heater gas (GS2 = 70) and collision gas (CAD = 10). The compound specific parameters for the different substrates (as specified in supplemental material) were determined by direct infusion of standard solutions (100–300 ng/ml) in methanol at a flow rate of 10µl/min using the quantitative optimization function of the Analyst software 1.4.2. Samples were quantified by determining the peak area under the curve (AUC) with the quantification function of the Analyst software 1.4.2 using the transitions as specified in the supplementary online material. The background noise was assessed by analyzing blank samples and standard curves were generated using blank samples spiked with a series of control lipids ranging from 1 to 1,000 ng/ml. For HxA3, HxB3, TrxA3, and TrxB3, the limit of detection ranged from 4 to 20 pg and the limit of quantification from 15 to 65 pg, corresponding to a signal-to-noise ratio of 3 and 10, respectively.
Organ extracts
C57BL/6 mice were obtained from the Institute of Laboratory Animal Sciences, University of Zurich and C57BL/6 sEH−/− mice (51) were kindly provided by Dr. F. J. Gonzales (National Institutes of Health, Bethesda, MD). Six- to ten-week-old male mice were euthanized by cervical dislocation and livers were excised and homogenized in ice-cold phosphate buffered saline, pH 7.4. All subsequent steps were performed at 4°C. Cytosolic and microsomal fractions were prepared by ultracentrifugation of the 9,000 × g supernatant of the liver homogenates at 100,000 × g for 45 min.
Lipid extracts
Lipids were extracted from liver homogenates by addition of ethanol to a final concentration of 25% followed by solid phase extraction on C18 Bond Elut SPE columns (Varian, Palo Alto, CA). Extracts were applied to the SPE columns preconditioned with 2 ml acetonitrile and 2 ml ddH2O. Columns were washed with 1 ml ddH2O and evaporated to dryness. Lipids were eluted with 3 × 600 μl ethylacetate, dried under a stream of nitrogen, dissolved in acetonitrile, and pelleted for 2 min at 13,000 rpm prior to LC-MS/MS analysis as described above. In some cases, liver homogenates were treated with 30 μM arachidonic acid at 37°C for 30 min and lipids were isolated by solid phase extraction as described above.
Western blot
Protein samples in Laemmli buffer were subjected to SDS-PAGE and semi-dry blotting following standard procedures. Blots were incubated with polyclonal sEH rabbit antiserum for 2 h (dilution of 1:1000) in Tris-buffered saline containing 0.5% Tween-20. An alkaline phosphatase conjugated anti-rabbit antibody (Sigma Aldrich) was applied at a dilution of 1:30000 followed by colorimetric detection using 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium chloride (NBT).
RESULTS
sEH efficiently turns over HxA3 and HxB3
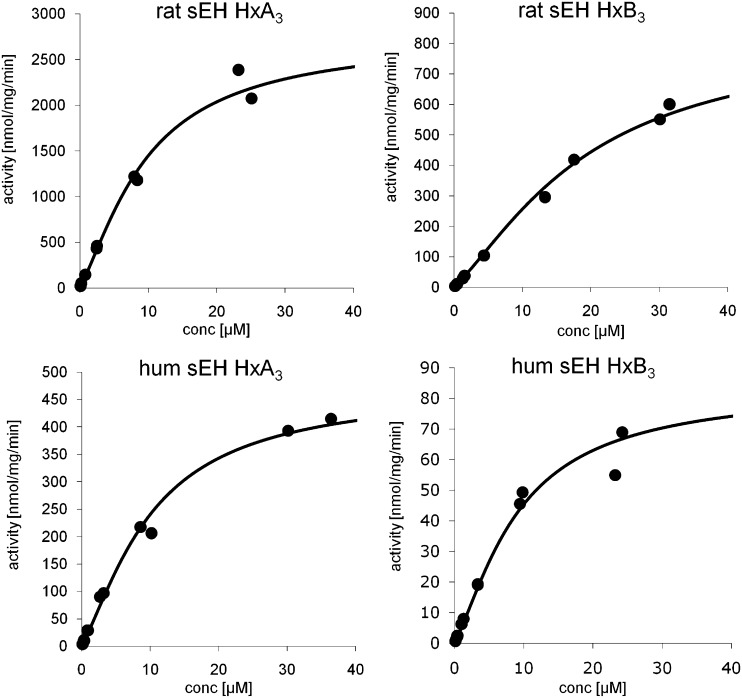
Human and rat sEH were cloned, recombinantly expressed in E. coli, and purified to homogeneity using affinity chromatography as described previously (3). To determine the effect of purified recombinant rat or human sEH on hepoxilin metabolism, we used LC-MS/MS analysis followed by kinetic evaluation. Human or rat sEH was incubated with various concentrations of HxA3 and HxB3 with or without inhibitor and samples were analyzed by LC-MS/MS. HxA3 was efficiently hydrolyzed by purified rat soluble epoxide hydrolase with a Vmax of 1.7 µmol/mg/min, a Km of 5 µM, and a catalytic efficiency of 4.5 × 105 as shown in Fig. 2. Both HxA3 and HxB3 are substrates for purified rat sEH and also human sEH (Fig. 2) and a summary of the kinetic parameters is presented in Table 1.
Fig. 2.
Kinetic analysis of hepoxilin turnover by sEH. Human and rat sEH were cloned, recombinantly expressed in E. coli, and purified as described above. Purified enzymes were incubated with various concentrations of substrate and samples were analyzed by LC-MS/MS. Substrate turnover was determined using internal HxA3 and HxB3 standards and the quantification function of the Analyst software 4.2.1. Kinetic constants were calculated by simulation of the Michaelis Menten kinetic as described in the Methods section.
TABLE 1.
Summary of kinetic parameters for hepoxilin turnover by sEH
| sEH Substrate | Vmax | Km | kcat | kcat/Km |
|---|---|---|---|---|
| nmol/mg/min | µM | s −1 | Ms −1 | |
| rsEH HxA3 | 1739 ± 539 | 4.6 ± 2.3 | 1.88 ± 0.58 | 4.5 x 10 ± 1.6 × 10 |
| rsEH HxB3 | 550 ± 261 | 14.7 ± 5.3 | 0.60 ± 0.28 | 5.1 x 10 ± 1.4 × 10 |
| hsEH HxA3 | 385 ± 95 | 7.3 ± 3.3 | 0.42 ± 0.10 | 5.8 x 10 ± 9.5 × 10 |
| hsEH HxB3 | 95 ± 43 | 10.8 ± 3.4 | 0.10 ± 0.05 | 1.2 x 10 ± 8.4 × 10 |
Hepoxilin hydrolase activity is linked to sEH presence
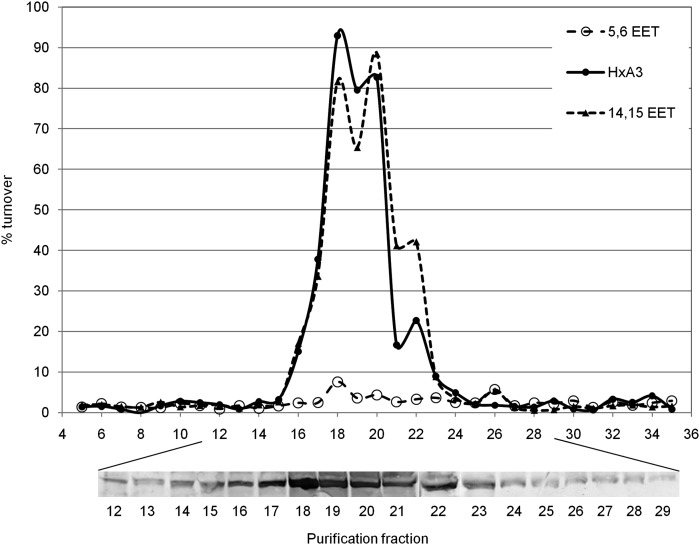
To evaluate the physiological contribution of sEH to hepoxilin metabolism, we separated mouse liver cytosolic fractions using gel permeation chromatography and tested each elution fraction for the metabolism of HxA3, 14,15-EET, and 5,6-EET. Each fraction was further assayed for the presence of sEH by Western blot analysis (Fig. 3). The hepoxilin hydrolase and sEH activities show 100% coelution from the column. Moreover, the fraction with the highest hepoxilin hydrolase activity also contains the highest amount of sEH (Fig. 3, lower panel).
Fig. 3.
Gel permeation chromatography. Five milligrams mouse liver cytosol was separated on a SE-1000 gel permeation column (Amersham biosciences) in phosphate buffered saline, pH 7.4. Each elution fraction was assayed for the metabolism of HxA3, 14,15-EET, and 5,6-EET (600 nM) as described in the Methods section. Each fraction was further analyzed for the presence of sEH protein by Western blot (lower panel).
Hepoxilin turnover is inhibited by sEH but not mEH inhibitors
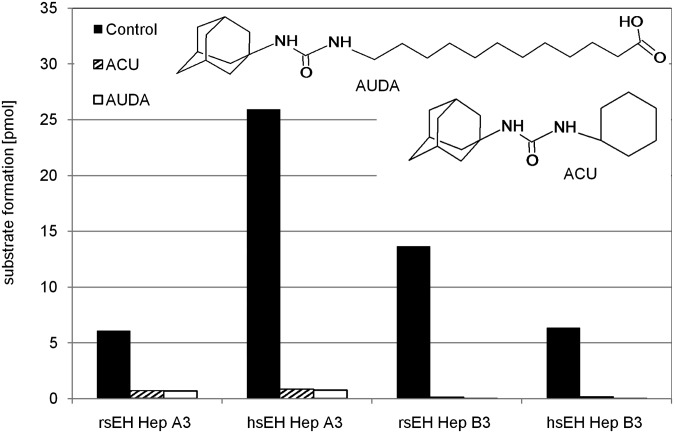
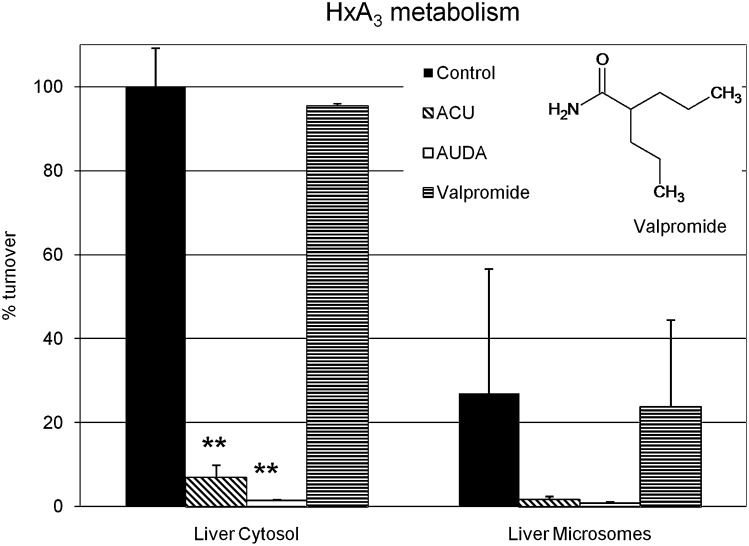
To characterize the physiological contribution of sEH to hepoxilin metabolism, we used a selection of epoxide hydrolase inhibitors. The hepoxilin turnover by purified sEH was effectively inhibited by sEHIs as shown in Fig. 4. Hepoxilin metabolism in liver protein extracts from wild-type (WT) animals was inhibited by ACU and AUDA but not the mEH inhibitor valpromide, as shown in Fig. 5. In addition, ACU inhibited hepoxilin metabolism by purified rat sEH and liver cytosolic preparations with an IC50 value of ∼1 nM (data not shown), which is in line with previously reported data (52). In microsomal preparations of sEH, WT mice hepoxilin turnover amounted to 30% compared with the cytosolic fraction. Western blot analysis of microsomal and cytosolic liver preparations confirmed the presence of sEH protein in both liver fractions, although to significantly lower amount in microsomes (data not shown). Furthermore, purified mEH, which is highly abundant in the liver, does not show any activity against hepoxilins (data not shown).
Fig. 4.
Inhibition of sEH. Rat and human sEH were incubated with 3 µM HxA3 and HxB3 in the presence of sEH selective inhibitors (2 µM ACU, 2 µM AUDA) and samples were analyzed by LC-MS/MS as described in the Methods section. Bars represent the amount of hepoxilin formed by sEH in the presence of inhibitors compared with the control without inhibitor. The inserted representations show the structures of the sEH inhibitors ACU, 1-Adamantyl-3-cyclohexylurea; AUDA, 12-(3-adamantan-1-yl-ureido)-dodecanoic acid.
Fig. 5.
Inhibition of hepoxilin metabolism. Protein extracts from liver (cytosolic and microsomal preparations) of WT mice were incubated with 3 µM HxA3 and HxB3 in the presence of inhibitors (2 µM ACU, AUDA, 2 mM valpromide), and samples were analyzed by LC-MS/MS. The representations show the fraction (%) of substrate turnover compared with the cytosolic preparation of WT animals, which is adjusted to 100% turnover. Error bars indicate SD. Unpaired, one-sided Student's _t_-tests were performed on each set of inhibited versus noninhibited samples. Two stars indicate a significant statistical difference with a _p_-value < 0.01.
sEH is responsible for hepoxilin metabolism
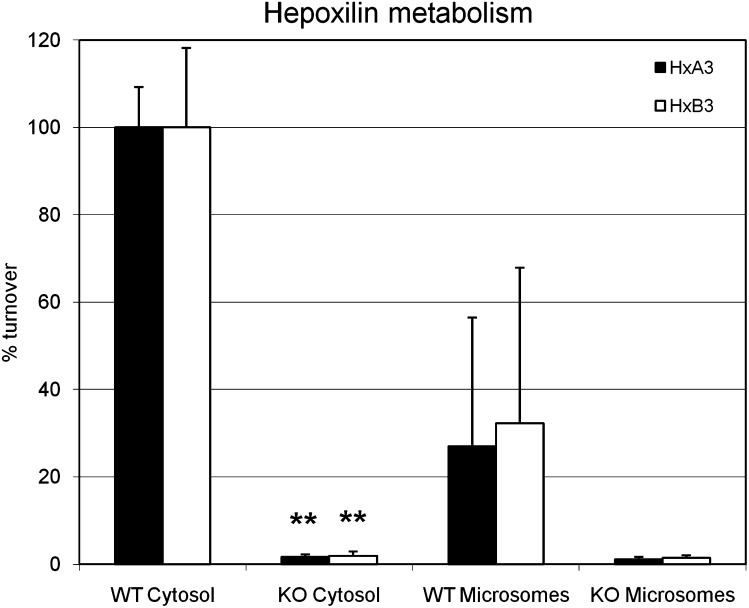
To investigate the quantitative contribution of sEH to hepoxilin turnover, we incubated protein extracts isolated from livers of sEH WT and sEH−/− mice with HxA3 and HxB3. Hepoxilin turnover to trioxilins was greatly abolished in sEH−/− mice compared with the WT mice (Fig. 6). Specifically, in both cytosolic and microsomal liver preparations of sEH−/− mice, the activity toward HxA3 and HxB3 was greatly reduced compared with the WT mice. Again, the activity toward hepoxilins in liver microsomal preparations of WT animals is explained by the presence of some sEH whereas no sEH protein was detectable in the sEH−/− mice by immunoblotting (data not shown).
Fig. 6.
Hepoxilin metabolism by sEH in WT and sEH−/− mice. Protein extracts (50 ug) from liver (cytosolic and microsomal preparations) of sEH−/− and WT mice were incubated with 3 µM HxA3 and HxB3 and samples were analyzed by LC-MS/MS. The representations show the fraction (%) of substrate turnover compared with the cytosolic preparation of WT animals, which is adjusted to 100% turnover. Error bars indicate SD. Unpaired, one-sided Student's _t_-tests were performed on each set of samples from WT versus knockout animals. Two stars indicate a significant statistical difference with a _p_-value < 0.01.
Hepoxilin/trioxilin contents of livers of WT and sEH−/− mice in vivo
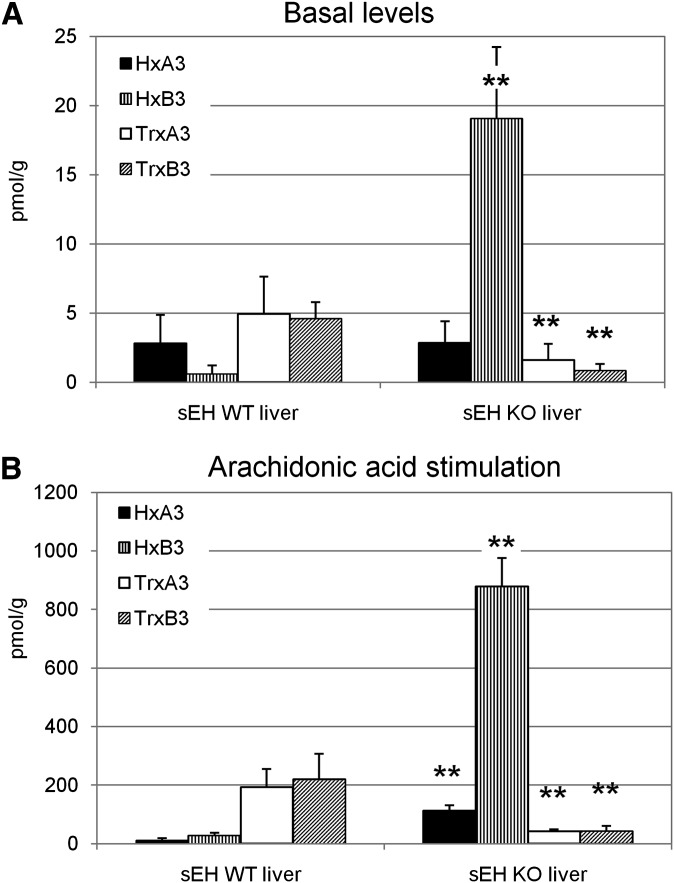
Lipids were extracted from liver organ preparations of sEH WT and sEH−/− mice with or without pretreatment with arachidonic acid followed by LC-MS/MS analysis of the lipids. Liver homogenates of sEH−/− mice show a significantly elevated basal level of HxB3 but lowered levels of trioxilins compared with the WT animals (Fig. 7A), where trioxilins are readily formed. After arachidonic acid treatment of organ extract, the synthesis of hepoxilin precursors is greatly induced (40-fold) and the accumulation of hepoxilins in liver homogenates from sEH−/− mice is even more pronounced (Fig. 7B).
Fig. 7.
Lipid extracts. A: Levels of hepoxilins and trioxilins in liver extracts of WT and sEH−/− mice. Liver samples of sEH WT and sEH−/− animals were homogenized, adjusted to a final concentration of 20% ethanol, and lipids were isolated by solid phase extraction. B: Levels of hepoxilins and trioxilins in liver extracts of WT and sEH−/− mice upon stimulation with exogenous arachidonic acid. Liver samples of sEH WT and sEH−/− animals were homogenized and treated with 30 µM arachidonic acid for 30 min. Samples were adjusted to a final concentration of 20% ethanol and lipids were isolated by solid phase extraction. Samples were analyzed for hepoxilin metabolism by LC-MS/MS. The values are presented in pmol lipid per g tissue. Error bars indicate SD. Unpaired, one-sided Student's _t_-tests were performed on each set of samples from WT versus KO animals. Two stars indicate a significant statistical difference with a _p_-value < 0.01.
DISCUSSION
Here, we report for the first time that _trans_-hydroxy-epoxy lipids, in particular the endogenous 12S-LOX-derived lipid mediators HxA3 and HxB3, are excellent substrates for mammalian sEH and converted to the corresponding trioxilins. 12R-LOX-derived hepoxilins, which are specifically generated in skin, are most likely preferred sEH substrates, although they have not been tested to date. sEH metabolizes hepoxilins with a catalytic efficiency that is within the range of turnover of its previously identified physiological substrates, EETs, which are among the best endogenous substrates for sEH. The activity of mammalian sEH against EETs lies in the range of 1–20 µmol/mg/min. We do not see a negative cooperativity with both hepoxilins, as has been suggested for the EET turnover by sEH (50). Human sEH turns over hepoxilins less efficiently (by a factor of three) than rat sEH. This has been seen for other substrates and might be explained by a compensatory effect due to the lower expression level of sEH in rat liver compared with human liver. HxA3 is a better substrate for mammalian sEH than HxB3. The hydroxy group positioned directly next to the epoxide might pose a sterical hindrance leading to less efficient turnover by sEH.
The sEH turns over hepoxilins orders of magnitude more efficiently than the previously reported hepoxilin hydrolase that displayed a specific activity of 0.2 nmol/mg/min. Hepoxilin hydrolase was partly purified from rat liver and suggested to be distinct from other mammalian EHs (mEH, sEH, leukotriene-A4 hydrolase) by its molecular weight as well as substrate and inhibitor spectrum. However, hepoxilin hydrolase is still only incompletely characterized and the amino acid sequence is not reported to date. The purification scheme used for the isolation of hepoxilin hydrolase (30) is quite similar to the procedure used for the isolation of rat liver sEH (53). We assume that the hepoxilin hydrolase activity in the enzyme preparation published previously is due to an invisible contamination by sEH. The assignment of the enzymatic activity to an incorrect polypeptide might be due to the obviously low abundance of sEH in livers of untreated rats, which would also explain the striking activity difference between the two enzymes.
These results suggested a physiological role of sEH in hepoxilin metabolism. Analysis of mouse liver cytosol by gel permeation chromatography followed by activity measurements against HxA3, 14,15-EET (an excellent sEH substrate), and 5,6-EET (a rather poor sEH substrate) and Western blot revealed that the hepoxilin hydrolase activity is linked to sEH presence, showing a perfect match. The double peak in the activity profile can be explained by the presence of monomeric and dimeric sEH in liver cytosol. These results suggested that mammalian sEH, rather than hepoxilin hydrolase, is the key enzyme responsible for hepoxilin metabolism in mouse liver.
To strengthen our hypothesis, we analyzed liver extracts from sEH WT and sEH−/− mice. Hepoxilin turnover was greatly abolished compared with the WT animals. The activity against hepoxilins found in the liver microsomal preparation of WT animals can be explained by the presence of sEH due to its partial peroxisomal localization in liver (54, 55), which we confirmed by Western blot analysis.
Only sEHIs but not mEHIs quantitatively inhibited hepoxilin turnover in cytosolic as well as microsomal liver preparations of WT animals. mEH shows a substrate preference for bulky, _cis_-substituted epoxides compared with the sEH, which accepts both _cis_- and _trans_-substituted epoxides. Indeed, we have shown that purified microsomal epoxide hydrolase does not turn over hepoxilins. In addition, ACU inhibited hepoxilin metabolism by purified rat sEH as well as liver cytosolic preparations with an IC50 value of ∼1 nM. These results are further supported by the LC-MS/MS analysis of lipid fractions prepared from organs of sEH WT and sEH−/− animals. The liver homogenates of sEH−/− mice show elevated basal levels of hepoxilins, particularly HxB3, whereas trioxilin levels are significantly decreased compared with the WT animals (Fig. 7A). The pretreatment of organ extracts with arachidonic acid presumably leads to a strong production of hepoxilin precursors such as 12-HPETE. In this case, both HxA3 and HxB3 significantly accumulate in the livers of sEH−/− animals and only a slow turnover to trioxilins is detected (Fig. 7B). An arachidonic acid pretreatment better reflects the actual enzyme capacity of the organ analyzed, whereas in the basal state, compensatory mechanisms of lipid metabolism might be of importance.
Quit unexpected were the large amounts of HxB3, particularly, in the livers of sEH−/− mice whereas HxA3 did not accumulate to that extent. HxA3 has been shown to be a substrate for glutathione-S-transferases and the glutathione-conjugated metabolite maintains biologic activity (32, 37). Due to the high expression level of GSTs in the liver, one would expect a lack of hepoxilin accumulation, which is only seen for the HxA3 regioisomer (Fig. 7). Therefore, glutathione conjugation of HxB3 does not seem to be an important pathway in the liver. Note that the glutathione derivative of HxB3 has not been detected in vivo to date. In contrast, HxA3 seem to be preferentially glutathionylated in livers of sEH−/− animals, which might also be the case in other organs, when the epoxide hydrolysis pathway is blocked.
Taken together, our results strongly suggest that mammalian sEH is the key enzyme responsible for hepoxilin metabolism and indeed, identical to previously reported hepoxilin hydrolase. Other mammalian epoxide hydrolase contributes, if at all, only partly to this metabolic pathway, depending on the tissue analyzed.
Our inhibitory analyses using sEHIs clearly show a complete block of hepoxilin hydrolysis in the liver. Therefore, possible undesirable effects of sEH inhibitors, which are in development for a number of applications, should be considered. Lipid signaling pathways other then the mostly targeted EET pathways might be affected with, to our knowledge, unknown consequences. This is even more important as EETs and hepoxilins seem to have somewhat opposing effects. Although the action of EETs are generally considered anti-inflammatory (15–17), hepoxilins instead are suspected to have pro-inflammatory effects. In psoriatic lesions, elevated levels of hepoxilins and trioxilins have been detected (38). Furthermore, HxA3 has recently been identified as a pathogen elicited epithelial chemoattractant. HxA3 leads to neutrophil migration across epithelial barriers in response to mucosal inflammation in the intestine or lung (39, 40). An inhibition of sEH might therefore cause enhanced release of hepoxilins at inflammatory sites. Such a deregulation might trigger pathophysiological effects of inflammation seen, for example, in inflammatory bowel disease, cystic fibrosis, or chronic obstructive pulmonary disease of the lung.
In conclusion, hepoxilins are excellent substrates for mammalian sEH in vitro and in vivo. Our findings suggest that sEH is identical to liver hepoxilin hydrolase and plays an important role in the physiological regulation of hepoxilins with important implications in particular for inflammatory diseases.
Supplementary Material
Supplemental Data
Acknowledgments
The authors cordially thank Julia Burgener for providing purified microsomal epoxide hydrolase.
Footnotes
Abbreviations:
ACU
N-adamantyl-N’-cyclohexyl urea
AUDA
12-(3-adamantan-1-yl-ureido) dodecanoic acid
EDHF
endothelium derived hyperpolarizing factor
EET
epoxyeicosatrienoic acid
EH
epoxide hydrolase
eLOX-3
epidermis-type lipoxygenase 3
Hx
hepoxilin
hepoxilin hydrolase
hepoxilin A3 epoxide hydrolase
LOX
lipoxygenase
mEH
microsomal epoxide hydrolase
sEH
soluble epoxide hydrolase
sEHI
soluble epoxide hydrolase inhibitor
Trx
trioxilin
WT
wild-type
This work was supported by the Swiss National Science Foundation (MA, No. 3100A0-108326). Some of the work described herein is part of the PhD thesis of M. Decker.
REFERENCES
- 1.Decker M., Arand M., Cronin A. 2009. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch. Toxicol. 83: 297–318. [DOI] [PubMed] [Google Scholar]
- 2.Cronin A., Homburg S., Durk H., Richter I., Adamska M., Frere F., Arand M. 2008. Insights into the catalytic mechanism of human sEH phosphatase by site-directed mutagenesis and LC-MS/MS analysis. J. Mol. Biol. 383: 627–640. [DOI] [PubMed] [Google Scholar]
- 3.Cronin A., Mowbray S., Durk H., Homburg S., Fleming I., Fisslthaler B., Oesch F., Arand M. 2003. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc. Natl. Acad. Sci. USA. 100: 1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman J. W., Morisseau C., Harris T. R., Hammock B. D. 2003. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc. Natl. Acad. Sci. USA. 100: 1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enayetallah A. E., French R. A., Thibodeau M. S., Grant D. F. 2004. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J. Histochem. Cytochem. 52: 447–454. [DOI] [PubMed] [Google Scholar]
- 6.Sura P., Sura R., Enayetallah A. E., Grant D. F. 2008. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 56: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summerer S., Hanano A., Utsumi S., Arand M., Schuber F., Blee E. 2002. Stereochemical features of the hydrolysis of 9,10-epoxystearic acid catalysed by plant and mammalian epoxide hydrolases. Biochem. J. 366: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeldin D. C., Kobayashi J., Falck J. R., Winder B. S., Hammock B. D., Snapper J. R., Capdevila J. H. 1993. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J. Biol. Chem. 268: 6402–6407. [PubMed] [Google Scholar]
- 9.Newman J. W., Morisseau C., Hammock B. D. 2005. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog. Lipid Res. 44: 1–51. [DOI] [PubMed] [Google Scholar]
- 10.Fisslthaler B., Popp R., Kiss L., Potente M., Harder D. R., Fleming I., Busse R. 1999. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 401: 493–497. [DOI] [PubMed] [Google Scholar]
- 11.Hu S., Kim H. S. 1993. Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. Eur. J. Pharmacol. 230: 215–221. [DOI] [PubMed] [Google Scholar]
- 12.Li P. L., Campbell W. B. 1997. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ. Res. 80: 877–884. [DOI] [PubMed] [Google Scholar]
- 13.Imig J. D., Zhao X., Capdevila J. H., Morisseau C., Hammock B. D. 2002. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 39: 690–694. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X., Yamamoto T., Newman J. W., Kim I. H., Watanabe T., Hammock B. D., Stewart J., Pollock J. S., Pollock D. M., Imig J. D. 2004. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J. Am. Soc. Nephrol. 15: 1244–1253. [PubMed] [Google Scholar]
- 15.Inceoglu B., Jinks S. L., Schmelzer K. R., Waite T., Kim I. H., Hammock B. D. 2006Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 79: 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmelzer K. R., Inceoglu B., Kubala L., Kim I. H., Jinks S. L., Eiserich J. P., Hammock B. D. 2006. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. USA. 103: 13646–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith K. R., Pinkerton K. E., Watanabe T., Pedersen T. L., Ma S. J., Hammock B. D. 2005. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc. Natl. Acad. Sci. USA. 102: 2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaelis U. R., Fisslthaler B., Barbosa-Sicard E., Falck J. R., Fleming I., Busse R. 2005. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J. Cell Sci. 118: 5489–5498. [DOI] [PubMed] [Google Scholar]
- 19.Potente M., Fisslthaler B., Busse R., Fleming I. 2003. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J. Biol. Chem. 278: 29619–29625. [DOI] [PubMed] [Google Scholar]
- 20.Sun J., Sui X., Bradbury J. A., Zeldin D. C., Conte M. S., Liao J. K. 2002. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ. Res. 90: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 21.Imig J. D. 2005. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am. J. Physiol. Renal Physiol. 289: F496–F503. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Zhang Y., Schmelzer K., Lee T. S., Fang X., Zhu Y., Spector A. A., Gill S., Morisseau C., Hammock B. D., et al. 2005. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA. 102: 16747–16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmelzer K. R., Kubala L., Newman J. W., Kim I. H., Eiserich J. P., Hammock B. D. 2005. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. USA. 102: 9772–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtoshi K., Kaneto H., Node K., Nakamura Y., Shiraiwa T., Matsuhisa M., Yamasaki Y. 2005. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem. Biophys. Res. Commun. 331: 347–350. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W., Koerner I. P., Noppens R., Grafe M., Tsai H. J., Morisseau C., Luria A., Hammock B. D., Falck J. R., Alkayed N. J. 2007.. Soluble epoxide hydrolase: a novel therapeutic target in stroke. _J. Cereb. Blood Flow Metab._27: 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisseau C., Newman J. W., Tsai H. J., Baecker P. A., Hammock B. D. 2006. Peptidyl-urea based inhibitors of soluble epoxide hydrolases. Bioorg. Med. Chem. Lett. 16: 5439–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisseau C., Goodrow M. H., Newman J. W., Wheelock C. E., Dowdy D. L., Hammock B. D. 2002. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem. Pharmacol. 63: 1599–1608. [DOI] [PubMed] [Google Scholar]
- 28.Morisseau C., Hammock B. D. 2005. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu. Rev. Pharmacol. Toxicol. 45: 311–333. [DOI] [PubMed] [Google Scholar]
- 29.Arand M., Cronin A., Oesch F., Mowbray S. L., Jones T. A. 2003. The telltale structures of epoxide hydrolases. Drug Metab. Rev. 35: 365–383. [DOI] [PubMed] [Google Scholar]
- 30.Pace-Asciak C. R., Lee W. S. 1989. Purification of hepoxilin epoxide hydrolase from rat liver. J. Biol. Chem. 264: 9310–9313. [PubMed] [Google Scholar]
- 31.Nigam S., Zafiriou M. P., Deva R., Ciccoli R., Roux-Van der Merwe R. 2007. Structure, biochemistry and biology of hepoxilins: an update. FEBS J. 274: 3503–3512. [DOI] [PubMed] [Google Scholar]
- 32.Laneuville O., Corey E. J., Couture R., Pace-Asciak C. R. 1991. Hepoxilin A3 (HxA3) is formed by the rat aorta and is metabolized into HxA3-C, a glutathione conjugate. Biochim. Biophys. Acta. 1084: 60–68. [DOI] [PubMed] [Google Scholar]
- 33.Pace-Asciak C. R., Martin J. M. 1984. Hepoxilin, a new family of insulin secretagogues formed by intact rat pancreatic islets. Prostaglandins Leukot. Med. 16: 173–180. [DOI] [PubMed] [Google Scholar]
- 34.Reynaud D., Demin P. M., Sutherland M., Nigam S., Pace-Asciak C. R. 1999. Hepoxilin signaling in intact human neutrophils: biphasic elevation of intracellular calcium by unesterified hepoxilin A3. FEBS Lett. 446: 236–238. [DOI] [PubMed] [Google Scholar]
- 35.Derewlany L. O., Pace-Asciak C. R., Radde I. C. 1984. Hepoxilin A, hydroxyepoxide metabolite of arachidonic acid, stimulates transport of 45Ca across the guinea pig visceral yolk sac. Can. J. Physiol. Pharmacol. 62: 1466–1469. [DOI] [PubMed] [Google Scholar]
- 36.Carlen P. L., Gurevich N., Zhang L., Wu P. H., Reynaud D., Pace-Asciak C. R. 1994. Formation and electrophysiological actions of the arachidonic acid metabolites, hepoxilins, at nanomolar concentrations in rat hippocampal slices. Neuroscience. 58: 493–502. [DOI] [PubMed] [Google Scholar]
- 37.Pace-Asciak C. R., Laneuville O., Su W. G., Corey E. J., Gurevich N., Wu P., Carlen P. L. 1990. A glutathione conjugate of hepoxilin A3: formation and action in the rat central nervous system. Proc. Natl. Acad. Sci. USA. 87: 3037–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anton R., Puig L., Esgleyes T., de Moragas J. M., Vila L. 1998. Occurrence of hepoxilins and trioxilins in psoriatic lesions. J. Invest. Dermatol. 110: 303–310. [DOI] [PubMed] [Google Scholar]
- 39.Mrsny R. J., Gewirtz A. T., Siccardi D., Savidge T., Hurley B. P., Madara J. L., McCormick B. A. 2004. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc. Natl. Acad. Sci. USA. 101: 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick B. A. 2007. Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. FEBS J. 274: 3513–3518. [DOI] [PubMed] [Google Scholar]
- 41.Brash A. R., Yu Z., Boeglin W. E., Schneider C. 2007. The hepoxilin connection in the epidermis. FEBS J. 274: 3494–3502. [DOI] [PubMed] [Google Scholar]
- 42.Laneuville O., Corey E. J., Couture R., Pace-Asciak C. R. 1991. Hepoxilin A3 increases vascular permeability in the rat skin. Eicosanoids. 4: 95–97. [PubMed] [Google Scholar]
- 43.Epp N., Furstenberger G., Muller K., de Juanes S., Leitges M., Hausser I., Thieme F., Liebisch G., Schmitz G., Krieg P. 2007. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 177: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Z., Schneider C., Boeglin W. E., Brash A. R. 2007. Epidermal lipoxygenase products of the hepoxilin pathway selectively activate the nuclear receptor PPARalpha. Lipids. 42: 491–497. [DOI] [PubMed] [Google Scholar]
- 45.Furstenberger G., Epp N., Eckl K. M., Hennies H. C., Jorgensen C., Hallenborg P., Kristiansen K., Krieg P. 2007. Role of epidermis-type lipoxygenases for skin barrier function and adipocyte differentiation. Prostaglandins Other Lipid Mediat. 82: 128–134. [DOI] [PubMed] [Google Scholar]
- 46.Yu Z., Schneider C., Boeglin W. E., Brash A. R. 2005. Mutations associated with a congenital form of ichthyosis (NCIE) inactivate the epidermal lipoxygenases 12R-LOX and eLOX3. Biochim. Biophys. Acta. 1686: 238–247. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland M., Schewe T., Nigam S. 2000. Biological actions of the free acid of hepoxilin A3 on human neutrophils. Biochem. Pharmacol. 59: 435–440. [DOI] [PubMed] [Google Scholar]
- 48.Reynaud D., Demin P., Pace-Asciak C. R. 1996. Hepoxilin A3-specific binding in human neutrophils. Biochem. J. 313: 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nigam S., Nodes S., Cichon G., Corey E. J., Pace-Asciak C. R. 1990. Receptor-mediated action of hepoxilin A3 releases diacylglycerol and arachidonic acid from human neutrophils. Biochem. Biophys. Res. Commun. 171: 944–948. [DOI] [PubMed] [Google Scholar]
- 50.Marowsky A., Burgener J., Falck J. R., Fritschy J. M., Arand M. 2009. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 163: 646–661. [DOI] [PubMed] [Google Scholar]
- 51.Sinal C. J., Miyata M., Tohkin M., Nagata K., Bend J. R., Gonzalez F. J. 2000. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J. Biol. Chem. 275: 40504–40510. [DOI] [PubMed] [Google Scholar]
- 52.Hwang S. H., Tsai H. J., Liu J. Y., Morisseau C., Hammock B. D. 2007. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J. Med. Chem. 50: 3825–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schladt L., Hartmann R., Worner W., Thomas H., Oesch F. 1988. Purification and characterization of rat-liver cytosolic epoxide hydrolase. Eur. J. Biochem. 176: 31–37. [DOI] [PubMed] [Google Scholar]
- 54.Arand M., Knehr M., Thomas H., Zeller H. D., Oesch F. 1991. An impaired peroxisomal targeting sequence leading to an unusual bicompartmental distribution of cytosolic epoxide hydrolase. FEBS Lett. 294: 19–22. [DOI] [PubMed] [Google Scholar]
- 55.Mullen R. T., Trelease R. N., Duerk H., Arand M., Hammock B. D., Oesch F., Grant D. F. 1999. Differential subcellular localization of endogenous and transfected soluble epoxide hydrolase in mammalian cells: evidence for isozyme variants. FEBS Lett. 445: 301–305.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data