Neurocognitive and Motor Deficits in HIV-Infected Ugandan Children With High CD4 Cell Counts (original) (raw)
Human immunodeficiency virus (HIV)-infected antiretroviral therapy-naive Ugandan children with CD4 cell counts of ∼350 cells/μL and percentages of >15% have significant motor and cognitive deficits compared with HIV-uninfected children. Study of whether early initiation of treatment could prevent or reverse such deficits is needed.
Abstract
(See the Editorial Commentary by Wagner and Frenkel, on pages 1010–2.)
Background. Human immunodeficiency virus (HIV) infection causes neurocognitive or motor function deficits in children with advanced disease, but it is unclear whether children with CD4 cell measures above the World Health Organization (WHO) thresholds for antiretroviral therapy (ART) initiation suffer significant impairment.
Methods. The neurocognitive and motor functions of HIV-infected ART-naive Ugandan children aged 6–12 years with CD4 cell counts of >350 cells/μL and CD4 cell percentage of >15% were compared with those of HIV-uninfected children, using the Test of Variables of Attention (TOVA), the Kaufman Assessment Battery for Children, second edition (KABC-2), and the Bruininks-Oseretsky Test of Motor Proficiency, second edition (BOT-2).
Results. Ninety-three HIV-infected children (median CD4 cell count, 655 cells/μL; plasma HIV RNA level, 4.7 log10 copies/mL) were compared to 106 HIV-uninfected children. HIV-infected children performed worse on TOVA visual reaction times (multivariate analysis of covariance; P = .006); KABC-2 sequential processing (P = .005), simultaneous processing (P = .039), planning/reasoning (P = .023), and global performance (P = .024); and BOT-2 total motor proficiency (P = .003). High plasma HIV RNA level was associated with worse performance in 10 cognitive measures and 3 motor measures. In analysis of only WHO clinical stage 1 or 2 HIV-infected children (n = 68), significant differences between the HIV-infected and HIV-uninfected groups (P < .05) remained for KABC-2 sequential processing, KABC-2 planning/reasoning, and BOT-2 motor proficiency.
Conclusions. Significant motor and cognitive deficits were found in HIV-infected ART-naive Ugandan children with CD4 cell counts of ∼350 cells/μL and percentages of >15%. Study of whether early initiation of ART could prevent or reverse such deficits is needed.
Human immunodeficiency virus (HIV) has been shown to cause both cognitive and motor dysfunction in perinatally infected children [1–4]. Severe compromise of the immune system from HIV is associated with high rates of neurodevelopmental disability [5], but little is known about the function of HIV-infected children before they have experienced a significant decrease in CD4 lymphocyte cell counts.
Studies of neurodevelopmental function in HIV-infected African children have been small and have generated conflicting results [6–9]. One report of 26 Congolese HIV-infected school-aged children demonstrated cognitive, language, and motor impairment using the first-edition Kaufman Assessment Battery for Children (KABC), compared with HIV-uninfected exposed and control children [10]. In contrast, Bagenda et al [11] reported no significant impairment among 28 HIV-infected ART-naive Ugandan school children, except in one KABC subscale of hand movements and in the Wide Range Achievement Test - Third Edition of academic achievement. Neither study was able to investigate the degree of impairment in relation to quantitative measures of disease stage such as HIV RNA level or CD4 cell count.
As access to HIV testing and care expands throughout Africa, increasing numbers of school-age HIV-infected children are being identified. Many of these children are ineligible for antiretroviral therapy (ART) because of high CD4 cell counts. It is important to know whether these children suffer impairment of their neurocognitive or motor development.
In this study, we sought to determine whether HIV-infected ART-naive Ugandan children with CD4 cell measures that were above World Health Organization (WHO) [12] thresholds for ART initiation exhibited neurocognitive or motor deficits compared with HIV-uninfected controls from 2 observational cohorts in Kampala, Uganda [12]. We also examined the associations of WHO clinical stage, CD4 cell count and percentage, and plasma HIV RNA level with neurodevelopmental deficits.
METHODS
Study Participants
HIV-infected participants were enrolled from an observational cohort of 300 HIV-infected children aged 2–12 years in Kampala, Uganda [13]. For this study, only children >6 years old with CD4 cell counts of >350 cells/μL and CD4 cell percentages of ≥15% (the thresholds for ART initiation according to contemporaneous Ugandan and 2006 WHO treatment guidelines) were included [12]. The neuropsychological testing results from some subjects were included in a prior study investigating differences in neuropsychological function by HIV subtype [14]. HIV-uninfected children, matched for age, were enrolled from a separate cohort of 601 children from the same urban community [15]. Informed written consent was obtained from parents or guardians with additional assent from children >7 years of age. Institutional review board approval for this study was obtained from Makerere University, the Uganda National Council for Science and Technology, Michigan State University, and the University of California, San Francisco.
Clinical and Laboratory Data
Clinical and laboratory data were obtained from the respective study cohort databases, including height and weight. For HIV-infected children, WHO clinical stage, plasma HIV RNA level (range of detection, 400–750 000 copies/mL; Amplicor version 1.5; Roche, Pleasanton, CA), and CD4 cell count and percentage (FACSCalibur; Becton Dickinson, San Jose, CA) were also analyzed.
In-Home Assessment
A socioeconomic status (SES) score was calculated for each child’s household, using a scale that assesses physical features of the child’s home environment, including the presence of electricity, shoes, radio, television, bicycle, motorcycle, motor vehicle, and cows. The middle childhood version of the Caldwell Home Observation for the Measurement of the Environment (HOME) was also performed to assess the stimulation and learning opportunities in the home [16]. HOME scores correlate strongly with neurodevelopmental outcomes in school-age periurban Ugandan children and were included in analyses to control for the effects of environmental stimulation on neurocognitive outcomes [17].
Neuropsychological Testing
Children were tested using the Test of Variables of Attention (TOVA), a modified Kaufman Assessment Battery for Children (KABC-2), and the Bruininks-Oseretsky Test of Motor Proficiency, second edition (BOT-2). Neuropsychological assessments were conducted in a private room that afforded quiet and privacy. Native speakers conducted assessments in Luganda, the children’s primary language. Testing was deferred in children experiencing acute illness. Examiners were blinded to the HIV status of study children.
Test of Variables of Attention
The TOVA (TOVA Company, Los Alamitos, CA) generates 6 visual and 5 auditory performance indices that also capture dysfunction in attention and impulsivity. The TOVA, performed first in our assessments, also served as a screening test for children with severe vision and hearing deficits that would preclude further neuropsychological assessment.
Kaufman Assessment Battery for Children, Second Edition
The KABC-2 (Pearson Assessments, Minneapolis, MN) assesses cognitive function and has been used to study the cognitive function of HIV-infected American children [2], HIV-infected Congolese children [18], and Ugandan school-aged children. We utilized 15 individual subtests and generated global composite scores in sequential processing (memory), simultaneous processing (visual-spatial processing and problem solving), planning (executive reasoning), and learning (immediate and delayed memory). We did not administer the knowledge (crystallized intelligence) portion of this test because of problems in cultural suitability.
Bruininks-Oseretsky Test for Motor Proficiency, Second Edition
The BOT-2 (Pearson Assessments) is a comprehensive assessment of motor function that generates scores in 8 different domains of movement and coordination. The test comprises game-like tasks that are engaging for the child and are easy to convey in the child’s local language.
Statistical Analyses
Nonparametric tests were used to compare subject characteristics between all HIV-infected children, HIV-infected WHO stages 1 and 2 children, and HIV-uninfected children. Raw scores from neuropsychological testing in the individual modules were compared using a Student t test and by multivariate analysis of covariance (ANCOVA) that included age, sex, SES score, and HOME z score (standardized from the entire study sample) as covariates. Global composite scores were then generated using the total of the raw scores of the individual testing modules, and compared by Student t test and multivariate ANCOVA that included age, sex, SES score, and HOME z score as covariates. Correlations of scores with CD4 cell count, CD4 cell percentage, and plasma HIV RNA level were assessed using multivariate linear regression that included age, sex, SES score, and HOME z score. To further examine whether children with high plasma HIV RNA levels performed worse than other children, we compared the scores from the subset of HIV-infected children who had HIV RNA levels above the median with the scores from HIV-infected children with HIV RNA levels below the median, using multivariate ANCOVA. Between-group comparisons were also repeated, excluding the WHO clinical stage 3 HIV-infected children who would have been eligible for ART according to the revised 2010 WHO guidelines [19]. Analyses were performed with SPSS (version 17.0; (IBM, Chicago, IL) and STATA (version 10; StataCorp) software.
RESULTS
A total of 93 HIV-infected and 106 HIV-uninfected subjects with a median age of 8.7 years (interquartile range [IQR], 7.4–10.1 years) were studied (Table 1). HIV-infected children were similar to HIV-uninfected children in age and weight-for-age z scores (standardized using the 2000 Centers for Disease Control and Prevention [20] norms from Epi Info), but HIV-infected children were more commonly female (62% vs 42%). By in-home assessment, HIV-infected children had comparable Caldwell HOME scores but lower SES scores. The HIV-infected children had plasma HIV RNA levels of 2.7–5.9 log10 copies/mL. Most HIV-infected children were at WHO clinical stage 1 or 2 (n = 68; 73%). The 25 children who were at WHO clinical stage 3 had histories of pulmonary tuberculosis (n = 22) and oral candidiasis (n = 3), conditions not considered indications for ART in the 2006 WHO guidelines.
Table 1.
Characteristics of HIV-Infected and HIV-Uninfected Children in Uganda
| Characteristic | HIV-Infected Subjects N = 93 | HIV-Infected Subjects at WHO Stages 1 and 2 N = 68 | HIV-Uninfected Subjects N = 106 | Pa | Pb |
|---|---|---|---|---|---|
| Age, years | 8.7 (7.4–10.0) | 9.1 (7.6–10.5) | 8.7 (7.4 to 10.1) | .906 | .499 |
| Female sex, n (%) | 58 (62%) | 48 (63%) | 44 (42%) | .003c | .005c |
| Caldwell HOME z scoree | −0.1 (−0.7 to 0.6) | 0.0 (−0.7 to 0.8) | 0.0 (−0.6 to 0.7) | .267 | .524 |
| SES scoree | 8 (6–12) | 8 (6–13) | 11 (7–13) | .014d | .034d |
| Weight-for-age z score | −1.3 (−1.9 to −0.6) | −1.1 (−1.9 to −0.2) | −1 (−1.5 to −0.2) | .028d | .324 |
| Height-for-age z score | −1.1 (−1.7 to −0.4) | −0.9 (−1.6 to −0.2) | −0.7 (−1.5 to 0.1) | .018d | .386 |
| CD4 cell count, cells/μL | 655 (507–921) | 648 (515–908) | … | … | … |
| CD4 cell percentage, % | 27 (23–34) | 27 (23–34) | … | … | … |
| Plasma HIV RNA level, log10 copies/mL | 4.7 (4.2–5.1) | 4.7 (4.1–5.1) | … | … | … |
| <400 | 0 | 0 | … | … | … |
| 400–10 000 | 16 | 14 | … | … | … |
| >10 000–100 000 | 45 | 31 | … | … | … |
| >100 000–750 000 | 30 | 21 | … | … | … |
| >750 000 | 2 | 2 | … | … | … |
| WHO stage | |||||
| 1 | 18 | 18 | … | … | … |
| 2 | 50 | 50 | … | … | … |
| 3f | 25 | 0 | … | … | … |
| 4 | 0 | 0 | … | … | … |
Test of Variables of Attention
HIV-infected children performed comparably to control children in 9 of 11 visual and auditory TOVA measures, but HIV-infected children had significantly poorer scores in the attention deficit (unadjusted t score) and visual signal modules (Table 2). No child demonstrated vision or hearing deficits that would have interfered with other testing. Plasma HIV RNA levels were correlated with D-prime signal detection (coefficient, −2.2; P = .03) but not with other TOVA scores; neither CD4 cell count nor CD4 cell percentage was significantly correlated with individual TOVA module scores. Compared with HIV-infected children with HIV RNA levels below the median, children with HIV RNA levels above the median performed worsein the visual TOVA in percentage of omission errors (inattention; P = .023), overall reaction time (P = .001), and attention deficit hyperactivity disorder score (P = .035) as well as in reaction time for the auditory TOVA (P = .002), by multivariate analysis of variance adjusting for age, SES score, Caldwell HOME score, and sex.
Table 2.
Comparison of Composite Global Neuropsychological Raw Score Measures for HIV-Infected and HIV-Uninfected Children in Uganda
| Neuropsychological Testa | HIV-Infected Subjects (N = 93) | HIV-Infected Subjects at WHO Stages 1 and 2 (N = 68) | HIV-Uninfected Subjects (N = 106) | Pb | Pc | Adjusted Pd | Adjusted Pe | Observed Powerf | Effect Size (SE)g |
|---|---|---|---|---|---|---|---|---|---|
| TOVA visual D-prime score—attention | 2.62 (0.91) | 2.74 (0.90) | 2.63 (0.93) | .96 | .446 | .838 | .757 | 0.061 | 0.04 (0.13) |
| ADHD score—visual | −4.55 (2.92) | −4.40 (3.15) | −3.60 (2.64) | .021h | .090 | .084 | .283 | 0.188 | 0.5 (0.5) |
| Omission errors—visual, % | 17.5 (16.7) | 15.9 (15.9) | 15.0 (13.8) | .261 | .728 | .378 | .734 | 0.063 | 0.7 (2.1) |
| Commission errors—visual, % | 9.2 (7.7) | 8.5 (6.8) | 9.9 (5.8) | .463 | .159 | .710 | .387 | 0.147 | 0.9 (0.9) |
| Reaction time—visual, msec | 658 (144) | 637 (147) | 604 (130) | .006i | .129 | .006i | .085 | 0.407 | 29 (17) |
| TOVA auditory D-prime score—attention | 1.03 (1.78) | 1.06 (1.81) | 0.82 (2.04) | .442 | .419 | .611 | .771 | 0.060 | 0.1 (0.3) |
| Omission errors—auditory, % | 36.8 (29.3) | 36.6 (29.6) | 39.8 (28.9) | .463 | .488 | .597 | .760 | 0.061 | 1.4 (4.6) |
| Commission errors—auditory, % | 35.0 (31.5) | 34.9 (31.7) | 38.6 (33.5) | .442 | .476 | .667 | .849 | 0.054 | 1.0 (5.1) |
| Reaction time—auditory, msec | 775 (200) | 766 (209) | 746 (183) | .297 | .521 | .283 | .308 | 0.174 | 30 (29) |
| KABC-2 cognitive raw score total | 184.7 (63.7) | 193.6 (64.4) | 200.6 (68.7) | .093 | .198 | .024h | .113 | 0.354 | 12.5 (7.8) |
| Sequential processing score | 29.6 (6.4) | 30.3 (6.7) | 31.5 (5.3) | .023h | .196 | .005i | .029h | 0.591 | 1.6 (0.7) |
| Simultaneous processing score | 41.0 (15.6) | 42.8 (15.8) | 45.9 (18.2) | .043h | .241 | .039h | .110 | 0.359 | 3.0 (1.9) |
| Planning and reasoning score | 11.5 (8.0) | 12.4 (8.2) | 13.8 (9.6) | .068 | .294 | .023h | .049h | 0.505 | 2.1 (1.1) |
| Learning score | 102.1 (41.7) | 108.1 (42.6) | 109.1 (45.2) | .258 | .878 | .106 | .326 | 0.165 | 5.9 (6.0) |
| BOT-2 motor proficiency raw score total | 187.8 (34.3) | 191.6 (34.3) | 198.3 (33.9) | .026h | .215 | .003i | .020h | 0.648 | 9.6 (4.1) |
Kaufman Assessment Battery for Children, Second Edition
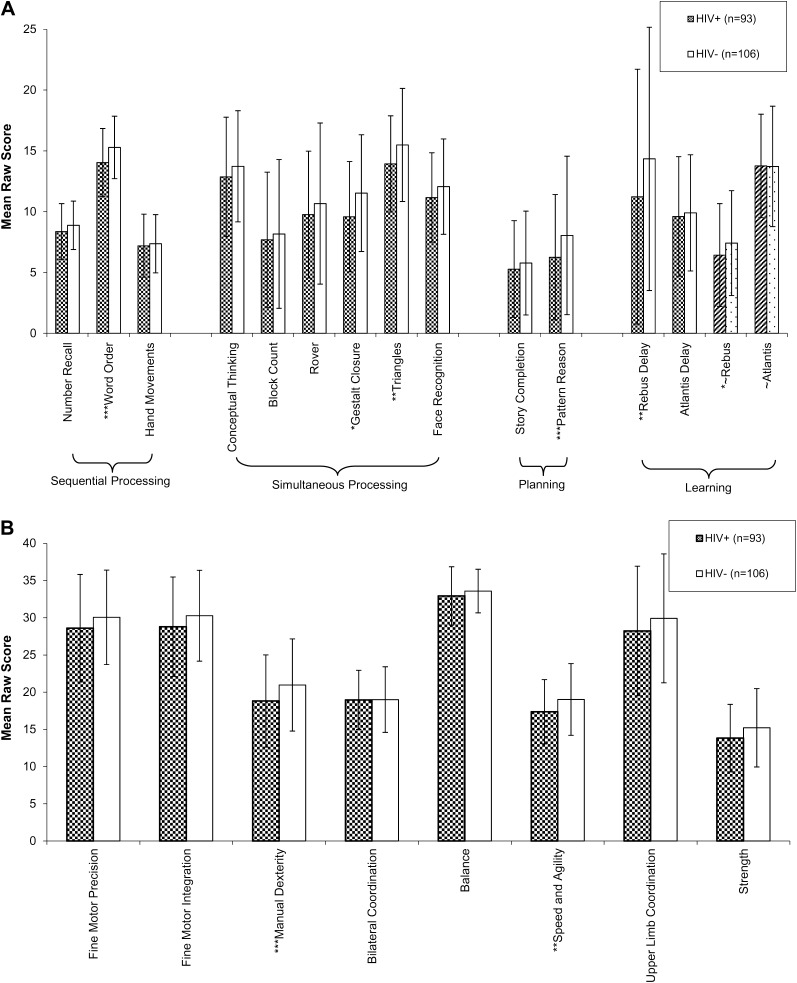
HIV-infected children performed significantly worse that HIV-uninfected children in 5 KABC-2 subscale raw score measures, including word order, gestalt closure, triangles, rebus, rebus delay, and pattern reasoning (Figure 1_A_), with at least a trend toward poorer performance in 14 of 15 KABC-2 modules. Among the global raw scores, HIV-infected children performed worse in sequential processing, simultaneous processing, planning/reasoning, and total KABC-2 score compared with HIV-uninfected children with use of an ANCOVA between-group comparison controlling for age, sex, SES score, and Caldwell HOME score (Table 2).
Figure 1.
Mean raw scores of Kaufman Assessment Battery for Children (second edition; KABC-2) (A) and Bruininks-Oseretsky Test of Motor Proficiency (second edition) (B) of human immunodeficiency virus (HIV)–positive (HIV+) and HIV-negative (HIV−) children in Uganda, compared by analysis of covariance with age, sex, total socioeconomic status score, and Caldwell Home Observation for the Measurement of the Environment (HOME) score [16]. Error bars represent standard deviation. KABC-2 rebus and Atlantis scores were divided by 4 to fit in the figure scale. *P < .05; **P < .01; ***P < .001.
In multivariate linear regression, increasing plasma HIV RNA level was associated with poorer performance in the global scale of simultaneous processing (coefficient, −4.5; P = .015) as well as its individual modules of gestalt closure (coefficient, −1.3; P = .044), triangles (coefficient, −1.2; P = .024), and rover (coefficient, −1.7; P = .021). Poor performance was associated with high plasma HIV RNA level in the learning subscale of Atlantis (coefficient, −5.9; P = .023), with trends toward associations in word order (coefficient, −0.74; P = .058) and pattern reasoning (coefficient, −1.3; P = .064). No individual or composite KABC-2 score was significantly correlated with CD4 cell count or CD4 cell percentage among HIV-infected children.
The subset of 46 children with HIV RNA levels above the median (4.73 log10 copies/mL) performed significantly worse than children with HIV RNA levels below the median in 10 of the 14 individual KABC-2 measures, including number recall (P = .05), conceptual thinking (P = .04), rover (P = .038), word order (P < .001), gestalt closure (P < .001), triangles (P = .004), rebus (P = .031), rebus delay (P = .012), and pattern reasoning (P = .007) as well as the composite measures of sequential processing (P = .012), simultaneous processing (P < .001), and planning (P = .018).
Bruininks-Oseretsky Test for Motor Proficiency, Second Edition
HIV-infected children demonstrated significantly lower scores in 2 measures—manual dexterity and speed/agility—with a trend toward poorer performance in fine motor precision, fine motor integration, balance, upper limb coordination, and strength (Figure 1_B_). Bilateral coordination was equivalent between groups. HIV-infected children had a significantly lower BOT-2 motor proficiency total score than did HIV-negative children (Table 2). Among the HIV-infected children overall, BOT-2 performance measures were not significantly correlated with HIV RNA level, CD4 cell count, or CD4 cell percentage. However, the subset of 46 children with HIV RNA levels above the median performed significantly worse in 3 of 8 measures—manual dexterity (P < .001), speed/agility (P = .015), and upper limb coordination (P = .05)—compared with children with HIV RNA levels below the median.
Comparisons Including WHO Clinical Stage 1 or 2 HIV-Infected Children
Compared with HIV-uninfected children, HIV-infected children of WHO stages 1 or 2 (n = 68) had worse mean scores in composite measures of TOVA auditory, KABC-2 cognitive, and BOT-2 motor testing (Table 2). In multivariate ANCOVA, statistically significant (P < .05) impairment among HIV-infected WHO stages 1 and 2 children was noted in the measures of KABC-2 sequential processing, KABC-2 planning/reasoning, and BOT-2 motor proficiency total scores. Within KABC-2 subscales, HIV-infected WHO stages 1 and 2 children had worse performance in triangles (P = .005), word order (P = .003), pattern reasoning (P = .005), and rebus delayed recall (P = .046). The overall TOVA performance measure (D-prime signal detection) did not differ significantly between the HIV-infected and HIV-uninfected children on either the visual or auditory tests in multivariate ANCOVA analysis (Table 2).
DISCUSSION
In this study, we found that HIV-infected Ugandan school-age children with CD4 cell counts and percentages above WHO thresholds for ART had significant cognitive and motor deficits compared with HIV-uninfected control children. Higher plasma HIV RNA level was associated with poorer cognitive and motor functioning among these children with CD4 cell counts of >350 cells/μL and percentages of >15%. This result suggests that in children, HIV infection has direct neuropathogenic effects that operate independently of the severe immunocompromise or inflammation associated with advanced HIV disease. Some data from studies of dementia in HIV-infected adults also support mediation of neuropathology by direct viral effects. HIV-1 replication in the central nervous system of adults correlates with gliosis and neuronal loss in a region-specific manner [22]. Progressive encephalopathy in adults seems to be driven by HIV glycoprotein gp120-mediated decreases in neural progenitor cell proliferation and neurogenesis [23]. However, there have been few data from HIV-infected children who demonstrate distinct motor and cognitive dysfunction while they progress through key stages of neurocognitive development [1].
The mechanisms of HIV neuropathogenesis may differ between residents of Africa and those of the United States and Europe, where different viral strains are in circulation. Although HIV subtype B predominates in the United States and Europe, HIV subtypes A, D, and C predominate in East Africa. Even small genetic differences between HIV variants can alter neurotoxic potential [24–28]. There is evidence that different HIV subtypes are associated with different rates of HIV-associated dementia in adults [29–31]; differences in neuropsychological impairment by HIV subtype among the same cohort of ART-naive HIV-infected Ugandan children were recently documented [32]. These results suggest that clinical guidelines for the management of neurodevelopmental impairment in African children must not solely rely on results from the United States and Europe but should be based on data from children in Africa.
Neurocognitive deficits in HIV-infected African children likely relate not only to the pathophysiology of HIV infection but also to poor nutrition and alterations in the home environment that result from HIV illness among providers and caregivers [10, 33]. We included measures of home environment in terms of physical resources (SES) and caregiving (HOME) in our analyses, but these may not have captured all the ways in which HIV illness can affect families. Malaria can also lead to neurocognitive impairment [34], but the HIV-infected and HIV-uninfected children in this study experienced low and comparable malaria incidence in the year prior to testing (data not shown).
Our results have potential implications for the decision of when to initiate ART in HIV-infected children. Because the children in this study had relatively high CD4 cell counts and percentages, they were ineligible for ART according to current Ugandan and 2006 WHO guidelines [12]. HIV encephalopathy is a WHO stage 4 event that is considered to be an indication for the initiation of ART. But the WHO definition of encephalopathy requires progressive change over at least 2 months in 1 of the following areas: (1) failure to attain, or loss of, developmental milestones, or loss of intellectual ability; (2) progressive impaired brain growth demonstrated by stagnation of head circumference; or (3) acquired symmetric motor deficit accompanied by 2 or more of the following: paresis, pathological reflexes, ataxia, or gait disturbances. Thus, for children to be recognized as having HIV encephalopathy, they must either have severe disability or have had sophisticated serial neurodevelopmental testing, which is not possible in most of Africa. Of note, none of the children in the current study had been considered to be encephalopathic by their physicians. According to the more recent 2010 WHO guidelines [19], the 25 children in our study who were at WHO clinical stage 3 would have been eligible for ART. However, when we restricted the analysis to only WHO stages 1 and 2 HIV-infected children, we still found a significant pattern of impairment, suggesting that even under the new guidelines, many children with impairment will remain off ART (Table 2).
There are additional issues worth considering in assessing whether these observations of neurodevelopmental impairment support the initiation of ART at higher CD4 cell counts in HIV-infected children. First, although statistically significant impairment among HIV-infected children was observed in this and prior studies, data that translate performance in these testing modules to measures of daily function such as school or work performance are limited. Second, the extent to which ART can interrupt or reverse the neuropathologic effects of HIV infection in children remains unclear. Some studies from the United States showed modest improvements in motor and cognitive function in HIV-infected children following therapy [35–37], but other studies described persistent behavioral and cognitive impairment [38]. One study of 35 Congolese infants and children showed that initiation of ART led to significant cognitive improvement only in children >30 months old [18]. It may be that ART must be initiated soon after infection and before CD4 cell counts decrease to prevent irreversible impairment at critical stages in neurodevelopment. In a recent randomized trial of Thai and Cambodian children aged 1–12 years, the initiation of ART at CD4 cell percentages of 15%–25% compared with <15% was not associated with improved neurodevelopment outcomes in the Berry visual-motor integration test [39]. Additional research including African children with longer follow-up time is indicated. It may also be that the use of ART with excellent penetration into the central nervous system is important; recent data from adults showed differences in neurocognitive recovery that were associated with the ability of particular ART medications to penetrate the central nervous system [40].
In summary, we report that HIV-infected Ugandan children with CD4 cell counts of >350 cells/μL demonstrate significant cognitive and motor deficits that correlate with HIV plasma RNA levels. Given the lifelong implications of neurodevelopmental impairment for HIV-infected children, further studies investigating the mechanisms of HIV neuropathogenesis as well as treatment strategies to prevent or reverse impairment are needed.
Notes
Financial support.
This work was supported by the National Institutes of Health (grant R21 MH083573 to J. K. W., M. J. B., and T. D. R.; grant R01 NS051132 to J. K. W.; grant U0 1 AI052142 to P. J. R.; grant U01 AI062677 to D. V. H.; and grants P30 AI027763, TL1 RR024129, and K23 HD604592 to T. D. R.); the Doris Duke Foundation (support for H. E. B. and P. J. R. who is a Distinguished Clinical Investigator); and a Fulbright African Regional Research award (to M. J. B.).
Potential conflicts of interest.
J. K. W. has served on the Scientific Advisory Board of Abbott Laboratories and as a consultant for Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nozyce M, Hittelman J, Muenz L, Durako SJ, Fischer ML, Willoughby A. Effect of perinatally acquired human immunodeficiency virus infection on neurodevelopment in children during the first two years of life. Pediatrics. 1994;94:883–91. [PubMed] [Google Scholar]
- 2.Diamond GW, Kaufman J, Belman AL, Cohen L, Cohen HJ, Rubinstein A. Characterization of cognitive functioning in a subgroup of children with congenital HIV infection. Arch Clin Neuropsychol. 1987;2:245–56. [PubMed] [Google Scholar]
- 3.Belman AL, Diamond G, Dickson D, et al. Pediatric acquired immunodeficiency syndrome: neurologic syndromes. Am J Dis Child. 1988;142:29–35. doi: 10.1001/archpedi.1988.02150010039017. [DOI] [PubMed] [Google Scholar]
- 4.Cooper ER, Hanson C, Diaz C, et al. Encephalopathy and progression of human immunodeficiency virus disease in a cohort of children with perinatally acquired human immunodeficiency virus infection. Women and Infants Transmission Study Group. J Pediatr. 1998;132:808–12. doi: 10.1016/s0022-3476(98)70308-7. [DOI] [PubMed] [Google Scholar]
- 5.Brouwers P, Tudor-Williams G, DeCarli C, et al. Relation between stage of disease and neurobehavioral measures in children with symptomatic HIV disease. AIDS. 1995;9:713–20. doi: 10.1097/00002030-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Msellati P, Lepage P, Hitimana DG, Van Goethem C, Van de Perre P, Dabis F. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics. 1993;92:843–8. [PubMed] [Google Scholar]
- 7.Drotar D, Olness K, Wiznitzer M, et al. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. 1997;100:E5. doi: 10.1542/peds.100.1.e5. [DOI] [PubMed] [Google Scholar]
- 8.McGrath N, Fawzi WW, Bellinger D, et al. The timing of mother-to-child transmission of human immunodeficiency virus infection and the neurodevelopment of children in Tanzania. Pediatr Infect Dis J. 2006;25:47–52. doi: 10.1097/01.inf.0000195638.80578.e0. [DOI] [PubMed] [Google Scholar]
- 9.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–8. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Bagenda D, Nassali A, Kalyesubula I, et al. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics. 2006;117:729–40. doi: 10.1542/peds.2004-2699. [DOI] [PubMed] [Google Scholar]
- 12.Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Geneva: World Health Organization; 2006. Technical Reference Group on Paediatric HIV Care and Treatment. [Google Scholar]
- 13.Kamya MR, Gasasira AF, Achan J, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–66. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 14.Boivin MJ, Ruel TD, Boal HE, et al. HIV subtype A is associated with poorer neuropsychological performance compared to subtype D in ART-naive Ugandan children. AIDS. 2010;24:1163–70. doi: 10.1097/qad.0b013e3283389dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorsey G, Staedke S, Clark TD, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–9. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell BM, Bradley RH. Home observation for measurement of the environment. Little Rock, AR: University of Arkansas Press; 1979. [Google Scholar]
- 17.Bangirana P, John CC, Idro R, et al. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One. 2009;4:e7898. doi: 10.1371/journal.pone.0007898. doi:10.1371/journal.pone.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2009;52:636–42. doi: 10.1097/QAI.0b013e3181b32646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antiretroviral therapy for HIV infection in infants and children: Towards universal access. Recommendations for a public health approach: 2010 revision. World Health Organization; 2010. Technical Reference Group on Paediatric HIV Care and Treatment. [PubMed] [Google Scholar]
- 20.CDC Growth Charts: United States. Vital Health Statistics of the Center for Disease Control and Prevention / National Center for Health Statistics 314. 2000. Available at: www.cdc.gov/nchs/data/ad/ad314.pdf. Accessed 20 January 2012.
- 21.Boivin MJ, Giordani B. Improvements in cognitive performance for schoolchildren in Zaire, Africa, following an iron supplement and treatment for intestinal parasites. J Pediatr Psychol. 1993;18:249–64. doi: 10.1093/jpepsy/18.2.249. [DOI] [PubMed] [Google Scholar]
- 22.Tardieu M. HIV-1 and the developing central nervous system. Dev Med Child Neurol. 1998;40:843–6. [PubMed] [Google Scholar]
- 23.Okamoto S, Kang YJ, Brechtel CW, et al. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–6. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol. 2008;63:366–76. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- 25.Strain MC, Letendre S, Pillai SK, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol. 2005;79:1772–88. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillai SK, Pond SL, Liu Y, et al. Genetic attributes of cerebrospinal fluid-derived HIV-1 env. Brain. 2006;129:1872–3. doi: 10.1093/brain/awl136. [DOI] [PubMed] [Google Scholar]
- 27.Power C, McArthur JC, Johnson RT, et al. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–9. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunfee RL, Thomas ER, Gorry PR, et al. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci USA. 2006;103:15160–5. doi: 10.1073/pnas.0605513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacktor N, Nakasujja N, Skolasky RL, et al. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infect Dis. 2009;49:780–6. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford DB, Mitike MT, Mekonnen Y, et al. Neurological evaluation of untreated human immunodeficiency virus infected adults in Ethiopia. J Neurovirol. 2007;13:67–72. doi: 10.1080/13550280601169837. [DOI] [PubMed] [Google Scholar]
- 31.Ranga U, Shankarappa R, Siddappa NB, et al. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78:2586–90. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boivin MJ, Ruel TD, Boal HE, et al. HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS. 2010;24:1163–70. doi: 10.1097/qad.0b013e3283389dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchette N, Smith ML, Fernandes-Penney A, King S, Read S. Cognitive and motor development in children with vertically transmitted HIV infection. Brain Cogn. 2001;46:50–3. doi: 10.1016/s0278-2626(01)80032-4. [DOI] [PubMed] [Google Scholar]
- 34.Boivin MJ, Bangirana P, Byarugaba J, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360–6. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART) J Pediatr. 2005;146:402–7. doi: 10.1016/j.jpeds.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor–based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–93. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- 37.Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR., 3rd Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23:1893–901. doi: 10.1097/QAD.0b013e32832dc041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozyce ML, Lee SS, Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–70. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 39.Puthanakit T, Vonthanak S, Ananworanich J, et al. In: International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention. Rome, Italy: 2011. Randomized clinical trial of immediate versus deferred antiretroviral therapy initiation in children older than one year with moderate immunodeficiency: the PREDICT Study ( NCT00234091) TULBPE023. [Google Scholar]
- 40.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–65. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]