Adiponectin Dysregulation and Insulin Resistance in Type 1 Diabetes (original) (raw)
Abstract
Context:
Type 1 diabetes (T1D) is associated with insulin resistance despite elevated levels of the insulin-sensitizing protein adiponectin. Whether the expected positive correlation between adiponectin and insulin sensitivity is preserved in a T1D population is unknown.
Objective:
We measured the correlation between total and high-molecular-weight (HMW) adiponectin and insulin sensitivity in T1D patients and nondiabetic controls and identified determinants of adiponectin levels in patients with T1D.
Design and Participants:
Fasting total and HMW adiponectin were measured in 86 subjects from the Coronary Artery Calcification in T1D (CACTI) cohort (39 T1D, 47 nondiabetic; age 45 ± 8 yr; 55% female). The association of adiponectin levels with insulin sensitivity was analyzed.
Setting:
The study was conducted at an academic research institute.
Methods:
Fasting total and HMW adiponectin were measured by RIA and ELISA, respectively. Insulin sensitivity was measured by a hyperinsulinemic-euglycemic clamp. Multivariate linear regression was used to identify determinants of adiponectin levels.
Results:
Adiponectin levels positively correlated with insulin sensitivity in both subject groups (total adiponectin, r = 0.33 P < 0.05 for T1D, r = 0.29 P < 0.05 controls), but insulin sensitivity was lower in T1D subjects at any given level of total or HMW adiponectin. Adiponectin levels were independently associated with age, gender, and trunk fat, but these variables did not account for increased adiponectin in patients with T1D.
Conclusion:
Adiponectin levels are positively correlated with insulin sensitivity in T1D patients. However, T1D patients have decreased insulin sensitivity compared with controls at every level of adiponectin, suggesting an important adaptive change of adiponectin set point.
Type 1 diabetes (T1D) is primarily a disease of insulin deficiency but is also characterized by insulin resistance (1, 2). In nonautoimmune, insulin-resistant states including obesity and type 2 diabetes, circulating levels of the fat-derived protein adiponectin are decreased compared with controls (3, 4), and insulin resistance correlates closely to decreased adiponectin (5). In contrast, T1D (6) and other autoimmune disorders (7, 8) are associated with increased adiponectin. The relationship between circulating adiponectin levels and insulin action in insulin-resistant patients with T1D remains poorly understood.
Adiponectin, an adipocyte-secreted protein, is an important determinant of whole-body insulin sensitivity (9) and has protective cardiovascular effects (10). Adiponectin is found in the circulation in three distinct multimeric forms: low molecular weight, medium molecular weight, and high molecular weight (HMW) (11). HMW adiponectin, the presumed active form, is most closely associated with insulin sensitivity (12). To our knowledge, the association of HMW adiponectin to insulin sensitivity in T1D has not been described.
In the present study, we examined the relationship between adiponectin and insulin sensitivity in subjects with and without T1D to determine whether the expected relationship between these measures is altered in patients with T1D. Furthermore, we sought to identify determinants of adiponectin in subjects with T1D and also non-diabetes mellitus (non-DM) controls.
Materials and Methods
Study population
The present analysis includes subjects from the Coronary Artery Calcification in Type 1 Diabetes (CACTI) hyperinsulinemic-euglycemic clamp substudy (2). This substudy cohort consisted of subjects recruited from the previously described CACTI study (13) who completed a hyperinsulinemic-euglycemic clamp. Subjects were adults with T1D or nondiabetic controls and had no history of cardiovascular disease. Subjects in the substudy were representative of the full CACTI cohort (2). One subject was excluded from the present analysis due to elevated creatinine. We present data for the remaining 86 subjects (39 T1D and 47 nondiabetic) in the CACTI clamp substudy. All participants provided informed consent, and the study was approved by the Colorado Combined Institutional Review Board.
Body composition
Dual x-ray absorptiometry scans were performed for body composition (percentage body fat, percentage trunk fat) and determination of fat free mass. Abdominal computed tomography scans for calculation of abdominal visceral and sc fat areas were performed within 1 yr of the clamp study. Anthropometric measures included height, weight, and waist circumference.
Continuous glucose monitoring
All subjects with T1D underwent masked continuous glucose monitoring (MiniMed Gold System; Medtronic, Minneapolis, MN) for the 3 d before the clamp. Continuous glucose monitoring measures included overall glycemic control, hypoglycemia, hyperglycemia, and glycemic variability as previously described (14).
Hyperinsulinemic-euglycemic clamp (HEC) visit
A detailed description of the HEC protocol used has been published previously (2). A three-stage HEC was performed using the method of DeFronzo et al. (15). Briefly, a primed continuous infusion of insulin was administered at a rate of 4 mU/m2 · min for 1.5 h, 8 mU/m2 · min for 1.5 h, and then 40 mU/m2 · min for the final 1.5 h. An infusion of 20% dextrose was titrated throughout the procedure to maintain blood glucose approximately 90 mg/dl. Arterialized blood was sampled every 5 min for bedside determination of glucose concentration (Analox, Lunenberg, MA). Arterialized blood samples for hormone and substrate measurements were taken at baseline and during the last 10 min of each stage. A steady state was achieved during the last 30 min of the high insulin infusion stage and mean glucose infusion rate [GIR; milligram per kilogram fat free mass (FFM) per minute] during this time was used as the measure of insulin sensitivity.
Measurements
Fasting plasma samples for adiponectin measurements were obtained at the time of the clamp visit. Total adiponectin levels were measured by RIA (LINCO Research, Inc., St. Charles, MO) with an intraassay precision of 1.78–3.59% coefficient of variation (CV), and interassay precision of 6.90–9.25% CV. The HMW adiponectin was measured by ELISA (Millipore, Billerica, MA); intraassay precision was 2.5–4.7% CV and interassay precision was 5.8–6.9% CV.
Statistical analyses
Student's t tests were used to compare the means of continuous variables between the T1D and non-DM groups. Factors correlated with total and HMW adiponectin were identified using Pearson correlation coefficients. Linear regression models were run to identify independent predictors of adiponectin levels for both total and HMW adiponectin. The independent association of adiponectin levels with insulin sensitivity was explored using forward selection multivariate regression analysis. SAS version 9.2 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. A P < 0.05 was considered statistically significant.
Results
Characteristics of the study participants are presented in Table 1, stratified by diabetes status and gender. T1D and non-DM groups were similar for gender distribution, age, blood pressure, body mass index (BMI), and adiposity (waist circumference, percentage body fat, percentage trunk fat, and visceral fat area). Expected diabetes-group differences were observed in glycosylated hemoglobin, fasting insulin, and fasting glucose. Lipid panels showed lower total cholesterol, triglycerides, and low-density lipoprotein cholesterol, and higher low-density lipoprotein cholesterol in the T1D group compared with the non-DM group. As previously reported (2), subjects with T1D were significantly more insulin resistant than subjects without diabetes, requiring lower GIR of 5.4 ± 3.7 mg/kg FFM per minute in men and 6.2 ± 3.4 mg/kg FFM per minute in women with T1D, compared with 10.0 ± 5.2 mg/kg FFM per minute and 15.6 ± 5.0 mg/kg FFM per minute in their non-DM counterparts. T1D patients had higher mean total and HMW adiponectin (microliters per milliliter) than nondiabetic controls (12.3 ± 5.8 vs. 9.6 ± 5.5, P = 0.03; and 6.5 ± 4.6 vs. 4.4 ± 3.5, P = 0.02, respectively) despite having lower insulin sensitivity. Stratified by gender, both total and HMW adiponectin levels were higher in men with T1D compared with non-DM control men (11.3 ± 5.6 vs. 7.3 ± 4.4 and 4.6 ± 2.9 vs. 2.7 ± 1.4 μg/ml, respectively, P < 0.05 for both), although values were not significantly different in the women. The magnitude of the differences observed in total adiponectin was larger than that in HMW adiponectin, consistent with increases in all adiponectin multimers in the group with T1D. However, most (62%) of the increase in total adiponectin was attributed to an increase in the HMW adiponectin isoform.
Table 1.
Characteristics of study participants by diabetes status and gender
| T1D (n = 39) | Non-DM (n = 47) | |||
|---|---|---|---|---|
| Men (n = 18) | Women (n = 21) | Men (n = 20) | Women (n = 27) | |
| Age (yr) | 47 ± 10 | 44 ± 9 | 47 ± 6 | 45 ± 8 |
| Diabetes duration (yr) | 23 ± 8 | 22 ± 8 | N/A | N/A |
| Insulin dose (units/kg · d) | 0.57 ± 0.13 | 0.57 ± 0.19 | N/A | N/A |
| HbA1c (%) | 7.5 ± 0.9a | 7.5 ± 0.9a | 5.4 ± 0.3 | 5.5 ± 0.3 |
| Fasting insulin (μU/ml) | 28.0 ± 16.0a | 36.2 ± 35.3a | 10.4 ± 5.0b | 7.4 ± 2.2 |
| Fasting glucose (mg/dl) | 122.7 ± 54.5 | 109.2 ± 19.5a | 99.1 ± 8.7b | 92.9 ± 6.1 |
| Systolic blood pressure (mm Hg) | 116 ± 11 | 111 ± 10 | 120 ± 9b | 109 ± 11 |
| Diastolic blood pressure (mm Hg) | 79 ± 6b | 73 ± 8 | 80 ± 7b | 73 ± 7 |
| BMI (kg/m2) | 28.3 ± 4.2 | 25.8 ± 4.3 | 27.2 ± 3.6 | 25.2 ± 4.3 |
| Waist circumference (cm) | 94.7 ± 9.3c | 82.4 ± 11.7 | 95.6 ± 10.6c | 79.5 ± 8.6 |
| Body fat (%) | 24.3 ± 6.2c | 32.5 ± 6.7 | 24.2 ± 3.2c | 33.6 ± 6.6 |
| Trunk fat (%) | 25.7 ± 7.5 | 30.4 ± 8.6 | 27.4 ± 4.4b | 31.2 ± 7.6 |
| Visceral fat area (cm3) | 56.5 (32.9–73.0) | 37.5 (25.0–41.4) | 60.1 (45.2–87.9)c | 35.0 (25.0–44.7) |
| Total cholesterol (mg/dl) | 143 ± 32d | 135 ± 33d | 171 ± 25 | 171 ± 32 |
| Triglycerides (mg/dl) | 70 ± 23d | 69 ± 42d | 126 ± 73 | 94 ± 38 |
| LDL cholesterol (mg/dl) | 68 ± 24a | 66 ± 25a | 101 ± 25 | 93 ± 27 |
| HDL cholesterol (mg/dl) | 61 ± 31d | 56 ± 13 | 45 ± 9c | 60 ± 15 |
| Serum creatinine (mg/dl) | 1.1 ± 0.2b | 0.9 ± 0.1a | 1.2 ± 0.2b | 1.0 ± 0.1 |
| Albumin excretion rate (mg/min) | 6.0 (4.0–9.4) | 3.9 (3.1–6.0) | 4.0 (3.4–5.7) | 3.3 (3.0–5.2) |
| GIR (mg/kg FFM per min) | 5.4 ± 3.7d | 6.2 ± 3.4a | 10.0 ± 5.2c | 15.6 ± 5.0 |
| Total adiponectin (μg/ml) | 11.3 ± 5.6d | 13.4 ± 6.1 | 7.3 ± 4.4b | 11.9 ± 6.0 |
| Total adiponectin (geometric mean, μg/ml) | 10.2 ± 1.8d | 12.2 ± 1.6 | 6.2 ± 1.8b | 10.4 ± 1.7 |
| HMW adiponectin (μg/ml) | 4.6 ± 2.9_b, d_ | 7.5 ± 4.7 | 2.7 ± 1.4c | 5.8 ± 4.0 |
| HMW adiponectin (geometric mean, μg/ml) | 4.0 ± 2.4 | 5.9 ± 2.2 | 2.2 ± 2.0b | 4.4 ± 2.3 |
To identify possible determinants of total and HMW adiponectin levels, associations between these measures and variables thought likely to influence adiponectin levels were measured within each group. Total adiponectin levels were positively associated with older age (r = 0.37, P < 0.05) and greater diabetes duration (r = 0.42, P < 0.05) and negatively associated with daily weight-adjusted insulin dose (r = −0.50, P < 0.05) in subjects with T1D. Expected negative correlations were found between total adiponectin and adiposity measures including BMI, percentage trunk fat, and visceral fat area. The associations with HMW adiponectin were similar to those observed with total adiponectin. No associations were observed between adiponectin and renal function measures including serum creatinine and albumin excretion rate.
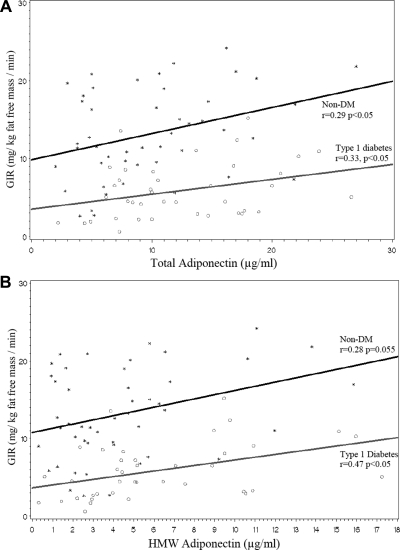
The correlation between GIR and both total adiponectin (r = 0.33, P < 0.05 for patients with T1D; r = 0.29, P < 0.05 for controls) and HMW adiponectin (r = 0.47, P < 0.05 for patients with T1D; r = 0.28, P = 0.055 for controls) was similar for the two groups (Fig. 1). However, at any given level of total or HMW adiponectin, patients with T1D had decreased insulin sensitivity (lower GIR) compared with nondiabetic controls (P < 0.0001 for the difference in GIR by diabetes status when adjusted for either total or HMW adiponectin). In contrast, insulin-stimulated free fatty acid suppression was not associated with either total or HMW adiponectin (data not shown).
Fig. 1.
Relationship between total (A) and HMW (B) adiponectin and GIR in subjects with T1D and non-DM controls. Scatterplot and predicted regression lines show the relationship between adiponectin and GIR. A presents total adiponectin and B presents HMW adiponectin. Data for subjects with T1D is presented by open circles and gray lines. Data for non-DM control subjects is presented by star symbols and black lines. P < 0.0001 indicates the difference in GIR by diabetes status when adjusted for either total or HMW adiponectin.
The relationships between total and HMW adiponectin and glycemic control were explored in subjects with T1D using data obtained during continuous glucose monitoring. Neither total nor HMW adiponectin levels were correlated with any included measure of glycemic control or glycemic variability.
In the forward stepwise regression analyses, T1D status, older age, female gender, and percentage trunk fat were independently associated with both total and HMW adiponectin. However, T1D status remained a strong independent positive determinant of both adiponectin measures and was associated with 2.6 μg/ml higher mean total adiponectin (R2 = 0.21, P = 0.03) and 1.6 μg/ml higher mean HMW adiponectin levels (R2 = 0.41, P = 0.02).
Discussion
We measured the correlation between total and HMW adiponectin and insulin sensitivity in subjects with T1D and non-DM controls. Consistent with previous reports (6, 16), mean total and HMW adiponectin were higher in patients with T1D compared with non-DM controls. We found that group differences in adiponectin levels are largely but not entirely due to increased HMW adiponectin in T1D patients. As observed in populations without T1D, total and HMW adiponectin levels were positively associated with insulin sensitivity within each group. Thus, among patients with T1D, higher adiponectin was associated with higher insulin sensitivity. However, despite elevated mean adiponectin levels, patients with T1D had decreased insulin sensitivity compared with nondiabetic controls. In fact, insulin sensitivity was lower for subjects with T1D compared with non-DM controls at any given level of total or HMW adiponectin, suggesting an increased set point or dysregulation of adiponectin function in the subjects with T1D. Our findings are consistent with a relative adiponectin resistance among subjects with T1D and support the hypothesis that factors unrelated to adiponectin contribute to decreased insulin sensitivity in this population.
Although the expected correlations between adiponectin and age, gender, and adiposity were found, these variables did not explain the observed increased levels of adiponectin in the T1D population. Adiponectin has previously been reported to be inversely associated with renal function in patients with T1D (17). However, we did not observe this association in our cohort of patients with normal renal function, and renal function did not explain the observed increased levels of adiponectin in the T1D group. Further mechanistic studies are required to elucidate the causative factors of increased total and HMW adiponectin in subjects with T1D.
Recent studies have reported a positive association between adiponectin and cardiovascular as well as all-cause mortality in patients with T1D (18, 19) unexplained by renal dysfunction, catabolism, inflammation, or preexisting cardiovascular disease. Our finding of an expected positive association between adiponectin and insulin sensitivity despite decreased insulin sensitivity in patients with T1D at any given level of adiponectin suggests a complex association of total and HMW adiponectin with insulin sensitivity and health outcomes in people with T1D.
Our findings are limited by a relatively small sample size, although the CACTI study of 86 subjects is large for a clamp study, especially given the complexity of such studies in subjects with T1D. In addition, our data are cross-sectional and we are not able to determine causation based on our study design.
In summary, we find that both total and HMW adiponectin are increased in patients with T1D compared with nondiabetic controls, and this observation is not explained by group differences in age, gender, or fat distribution. Among patients with T1D, adiponectin levels are positively correlated to insulin sensitivity, similar to the relationship among adults without diabetes. However, insulin sensitivity is lower for patients with T1D at any given level of total or HMW adiponectin suggesting a change in total and HMW adiponectin set point in these patients.
Acknowledgments
This study was performed at the Clinical Translational Research Center at the University of Colorado Denver, supported by National Institutes of Health Grant M01 RR000051, and at the Barbara Davis Center for Childhood Diabetes (Aurora, CO). Author contributions included the following: R.I.P. researched data and wrote the manuscript. J.K.S.-B. researched data and contributed to discussion and edited the manuscript. C.E. researched data and reviewed the manuscript. I.S. and B.B. researched data and contributed to discussion and reviewed the manuscript. M.R. contributed to discussion and edited the manuscript. D.M.M. researched data and contributed to discussion and reviewed the manuscript. The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the views of the National Institutes of Health or other funders.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute Grants R01 HL61753 and R01 HL079611, and the Diabetes Endocrinology Research Center Clinical Investigation Core Grant P30 DK57516. Additional support was provided by the National Institutes of Health, National Center for Research Resources Grant K23 RR022238 (to R.I.P.), American Diabetes Association Junior Faculty Award 1-10-JF-50 (to J.K.S.-B.), and Grant DK075360 (to D.M.M.).
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
Abbreviations:
BMI
Body mass index
CACTI
Coronary Artery Calcification in T1D
CV
coefficient of variation
DM
diabetes mellitus
FFM
fat free mass
GIR
glucose infusion rate
HEC
hyperinsulinemic-euglycemic clamp
HMW
high molecular weight
T1D
type 1 diabetes.
References
- 1.DeFronzo RA, Hendler R, Simonson D. 1982. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 31:795–801 [DOI] [PubMed] [Google Scholar]
- 2.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. 2011. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes 60:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. 1999. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83 [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. 2000. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599 [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. 2001. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 6.Imagawa A, Funahashi T, Nakamura T, Moriwaki M, Tanaka S, Nishizawa H, Sayama K, Uno S, Iwahashi H, Yamagata K, Miyagawa J, Matsuzawa Y. 2002. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care 25:1665–1666 [DOI] [PubMed] [Google Scholar]
- 7.Sada KE, Yamasaki Y, Maruyama M, Sugiyama H, Yamamura M, Maeshima Y, Makino H. 2006. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol 33:1545–1552 [PubMed] [Google Scholar]
- 8.Bossowski A, Sawicka B, Szalecki M, Koput A, Wysocka J, Zelazowska-Rutkowska B. 2010. Analysis of serum adiponectin, resistin and leptin levels in children and adolescents with autoimmune thyroid disorders. J Pediatr Endocrinol Metab 23:369–377 [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. 2001. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50:1126–1133 [DOI] [PubMed] [Google Scholar]
- 10.Hui X, Lam KS, Vanhoutte PM, Xu A. 2011. Adiponectin and cardiovascular health: an update [article online]. Br J Pharmacol. Available from http://www.ncbi.nlm.nih.gov/pubmed/21457225 [DOI] [PMC free article] [PubMed]
- 11.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. 2003. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278:40352–40363 [DOI] [PubMed] [Google Scholar]
- 12.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. 2004. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- 13.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M. 2003. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 52:2833–2839 [DOI] [PubMed] [Google Scholar]
- 14.Rodbard D. 2009. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther 11:551–565 [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. 1979. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 16.Leth H, Andersen KK, Frystyk J, Tarnow L, Rossing P, Parving HH, Flyvbjerg A. 2008. Elevated levels of high-molecular-weight adiponectin in type 1 diabetes. J Clin Endocrinol Metab 93:3186–3191 [DOI] [PubMed] [Google Scholar]
- 17.Schalkwijk CG, Chaturvedi N, Schram MT, Fuller JH, Stehouwer CD. 2006. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab 91:129–135 [DOI] [PubMed] [Google Scholar]
- 18.Jorsal A, Tarnow L, Frystyk J, Lajer M, Flyvbjerg A, Parving HH, Vionnet N, Rossing P. 2008. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int 74:649–654 [DOI] [PubMed] [Google Scholar]
- 19.Forsblom C, Thomas MC, Moran J, Saraheimo M, Thorn L, Waden J, Gordin D, Frystyk J, Flyvbjerg A, Groop PH. 2011. Serum adiponectin concentration is a positive predictor of all-cause and cardiovascular mortality in type 1 diabetes [article online]. J Intern Med. Available from http://www.ncbi.nlm.nih.gov/pubmed/21615808 [DOI] [PubMed]