Neuropsychiatric Conditions among Patients with Dyskeratosis Congenita: A Link with Telomere Biology? (original) (raw)
. Author manuscript; available in PMC: 2013 May 1.
Published in final edited form as: Psychosomatics. 2012 Mar 27;53(3):230–235. doi: 10.1016/j.psym.2011.09.003
Abstract
Background
Dyskeratosis congenita (DC), an inherited bone marrow failure syndrome (IBMFS), is caused by defects in telomere biology which result in very short germline telomeres. Telomeres, long nucleotide repeats and a protein complex at chromosome ends, are essential for chromosomal stability. Several association studies suggest that short telomeres are associated with certain psychiatric disorders, including mood disorders and schizophrenia. There are two cases in the literature of schizophrenia and DC occurring as co-morbid conditions. We noted that many patients with DC in our cohort had neuropsychiatric conditions.
Methods
Subjects were participants in NCI’s IBMFS prospective cohort study. Psychiatric evaluation was incorporated into our clinical assessment in January 2009. Fourteen DC or DC-like patients, including six children, were evaluated in this study through in person interview by either a psychiatrist specialized in psychosomatic medicine or a child and adolescent psychiatrist.
Results
Three of the six pediatric subjects, and five of the eight adults had a neuropsychiatric condition such as a mood, anxiety, or adjustment disorder, intellectual disability, attention deficit hyperactivity disorder, or pervasive developmental disorders. The lifetime occurrence of any of these disorders in our study was 83% in pediatric subjects and 88% in adults. Notably, the literature reports neuropsychiatric conditions in 25% and 38% in chronically ill children and adults, respectively.
Conclusion
This pilot study suggests that patients with DC may have higher rates of neuropsychiatric conditions than the general population or other chronically ill individuals. This potential link between very short telomeres and neuropsychiatric conditions warrants further study.
Keywords: Dyskeratosis congenita, telomere, mood disorder, neuropsychiatric disorders
INTRODUCTION
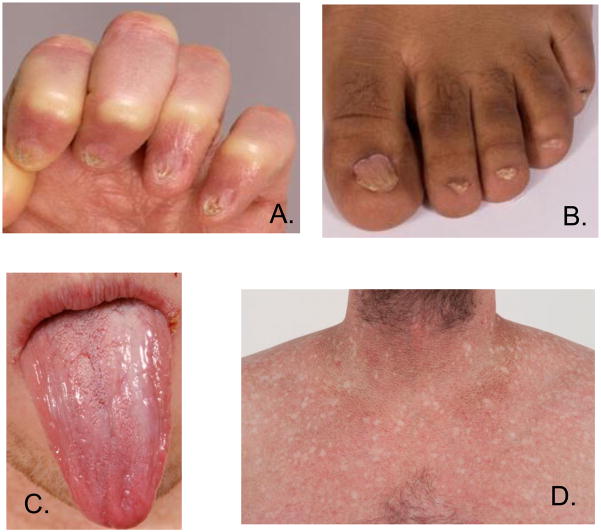
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome (IBMFS) caused by defects in telomere biology1,2. It is characterized by the classic triad of dysplastic nails, lacy reticular skin pigmentation, and oral leukoplakia (Figure 1). However, the triad may develop at variable rates. DC is associated with very high rates of bone marrow failure (BMF), which affects approximately half of all patients by age 50 years3. This can be associated with myelodysplastic syndrome and, potentially, evolution to acute myelogenous leukemia (AML). Patients with DC have an estimated 11-fold increased risk of cancer compared with the general population. These cancers include AML, squamous cell head and neck or anogenital cancers4. Patients with DC are also at high risk of numerous other medical problems, including pulmonary fibrosis, liver disease, and stenosis of the esophagus, urethra, and/or lacrimal ducts, as well as avascular necrosis of the hips or shoulders. Specific treatment for DC-related complications is primarily supportive and requires a multi-disciplinary, personalized approach. Hematopoeitic stem cell transplantation is the only curative therapy for BMF but is limited by significant hepatic, pulmonary, and other side effects5,6. Oral androgens can be effective in improving blood counts in patients with DC1,2. However, side effects such as liver toxicity, virilization, or mood swings can limit their tolerability.
Figure 1. The diagnostic triad of dyskeratosis congenita.
1.
A.) dysplastic fingernails, B.) dysplastic toenails, C.) oral leukoplakia, D.) abnormal skin pigmentation.
The prevalence of DC in the general population is unknown. Approximately 300 cases have been identified in a registry study from the U.K.2 and more than 70 individuals with DC have been evaluated in the National Cancer Institute’s IBMFS study3. Advances in understanding of the clinical consequences of aberrations in telomere biology7 and the development of a diagnostic test for DC have led to increased recognition of this complex disorder8.
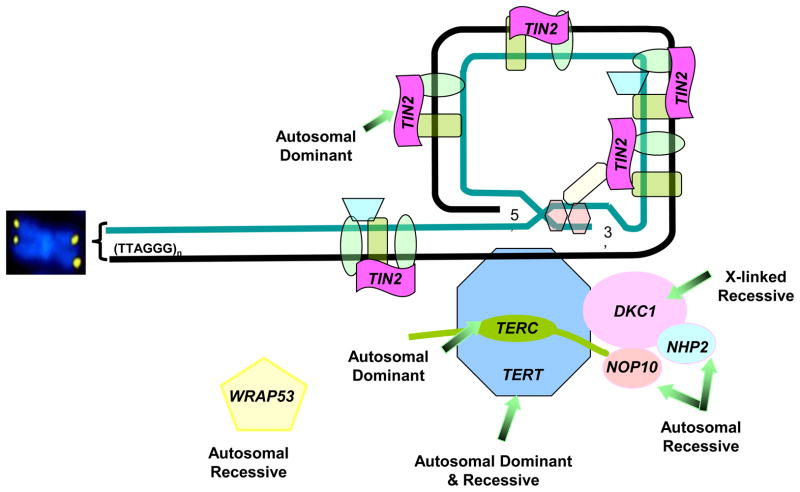
Telomeres are long nucleotide repeats and a protein complex at chromosome ends that are essential for chromosomal stability7,9. They shorten with each cell division due to the inability of DNA polymerases to replicate the 3’ end of linear DNA. When telomeres reach a critical length, cellular senescence or apoptosis are triggered. Thus, telomeres are a marker of cellular replicative capacity and aging. In approximately 60% of patients, germline mutations in one of seven different telomere biology genes cause DC: DKC1 (X-linked recessive), TERC (autosomal dominant), TERT (autosomal dominant and recessive), TINF2 (autosomal dominant), and the autosomal recessive genes NHP2,NOP10, and _WRAP53 (_TCAB1) 8,10,11 (Figure 2) . Telomeres are exceedingly short in patients with DC8,10.
Figure 2. Telomere structure and genes important in dyskeratosis congenita (DC).
Telomeres consist of long (TTAGGG)n nucleotide repeats and a protein structure, termed shelterin, at chromosome ends. The telomerase enzyme complex consists of TERT, TERC, DKC1, NOP10, NHP2, and GAR1 (not shown). WRAP53 codes for TCAB1 which transports telomerase. TINF2 codes for TIN2, a component of the shelterin telomere protection complex. The inheritance pattern of mutations in these genes is noted. Approximately 60% of patients with classic DC will have a germline mutation in one of these genes1.
Abbreviations: TINF2, TRF1-interacting nuclear factor 2; TERT, telomerase; TERC, telomerase RNA component; DKC1, dyskerin; NOP10, NOLA3, nucleolar protein family A, member 3; NHP2, NOLA2, nucleolar protein family A, member 2; WRAP53, telomerase cajal body associated protein 1 (TCAB1)
Epidemiologic studies have identified associations between shorter germline (e.g., blood or buccal cell DNA) telomere length and psychiatric disorders such as major depressive disorder12,13, bipolar disorder13, schizophrenia14, and post-traumatic stress disorder (PTSD) in adulthood following childhood trauma15. Shorter germline telomeres are also noted in adult caregivers of chronically ill children and subjects with other psychosocial stressors16, and in chronically institutionalized children from Romania17. In addition, one study found reduced levels of lymphocyte telomerase, the reverse transcriptase that extends the telomeric nucleotide repeats, in individuals with schizophrenia18. However, a study of telomere length in cerebellar neurons did not find an association between telomere length and serious psychiatric illness19.
There are limited data on the relationship between DC and neuropsychiatric conditions. Developmental delay is present in two clinically severe forms of DC: Hoyeraal-Hreidarsson syndrome20,1, which includes cerebellar hypoplasia and immunodeficiency, and Revesz Syndrome21,1, which includes bilateral exudative retinopathy and intracranial calcifications. Schizophrenia has been reported as a co-morbid condition in two adults with DC22,23, but to our knowledge there have been no other reports of neuropsychiatric conditions associated with DC. We noted that several subjects in our prospective cohort study of DC had significant neuropsychiatric symptoms. This descriptive report presents the results of clinical psychiatric evaluation in a consecutive subset of our patients.
METHODS
Patients with DC and their families were followed at the National Cancer Institute (NCI) as part of the IRB-approved study entitled “Etiologic Investigation of Cancer Susceptibility in Inherited Bone Marrow Failure Syndromes (IBMFS)” (http://marrowfailure.cancer.gov, protocol number 02-C-0052, NCT00027274)3 which opened to patient accrual in 2002. This protocol includes detailed medical record review, physical examination, laboratory evaluations, telomere length measurement by flow cytometry with fluorescence in situ hybridization of leukocyte subsets8, genetic counseling, and genetic testing. Patients were classified as having DC if they had at least two of the three features of the diagnostic triad, or one of the triad plus either hypoplastic bone marrow or at least two other somatic features, such as esophageal stenosis, epiphora, dental abnormalities, liver disease, or pulmonary fibrosis24. All patients with DC had very short (<1st percentile for age) telomeres in leukocyte subsets. “DC-like” patients represent a less clinically significant variant of DC but still have very short telomeres1. Patients were classified as “DC-like” if they had bone marrow failure, very short telomeres, and features suggestive of DC, but only one or no mucocutaneous findings.
Over time, and as the number of participants with DC increased, we noted that the frequency of neuropsychiatric conditions in DC seemed higher than expected based on our experience with patients who have different IBMFS. Therefore, routine psychiatric diagnostic evaluation was incorporated into clinical evaluations of patients with DC during their visit at the NIH Clinical Center (CC) starting in January 2009. Adult subjects met with a psychiatrist specializing in psychosomatic medicine and pediatric subjects and their parents were seen separately and together by a child and adolescent psychiatrist for a clinical diagnostic interview during the course of their stay at the CC. The psychiatrist reviewed current and past psychiatric symptoms, and diagnoses, if present, were made according to DSM-IV TR criteria25. Diagnoses were recorded in the subject’s chart and the research database along with demographic and medical information, and genotype when known.
RESULTS
A total of ten individuals with classic DC and four DC-like subjects were evaluated at the NIH CC over 28 months between January 2009 and April 2011 (Table 1). There were six females and eight males, with a median age of 18.5 years (range 9 to 50 years). Six subjects were in the pediatric age group (less than 18 years old). One of the subjects with DC met clinical criteria for the Hoyeraal-Hreidarsson Syndrome.
Table 1.
Characteristics of subjects with dyskeratosis congenita or DC-like phenotypes.
| Number of Subjects | Median Age in years, (range) | Males: Females | Causative Gene | ||||
|---|---|---|---|---|---|---|---|
| DKC1 | TERC | TERT | Unknown# | ||||
| DC* | Adults | 7 | 19 (18–50) | 5:2 | 3 | 2 | 2 |
| Children | 3 | 15 (9–16) | 2:1 | 2 | 1 | ||
| DC-like | Adults | 1 | 31 | 0:1 | 1 | ||
| Children | 3 | 16 (13–17) | 0:3 | 3 | |||
| Overall | 14 | 18.5 (9–50) | 8:6 | 3 | 2 | 2 | 7 |
In total, three pediatric subjects (50%) and five adults (63%) met current DSM-IV TR criteria for at least one neuropsychiatric condition (Table 2). Primary psychiatric disorders (mood, anxiety, psychotic, and adjustment disorders) were present in 2 (33%) pediatric and 4 (50%) adult subjects; neurocognitive disorders (attention deficit hyperactivity disorder [ADHD], intellectual disability [“Mental Retardation” in DSM-IV nomenclature], learning disabilities, and pervasive developmental disorders) were present in 2 (33%) pediatric and 2 (25%) adult subjects. Overall, the presence of any neuropsychiatric diagnosis in pediatric and adult subjects increased to 83% and 88%, respectively, when past diagnoses were included (Table 2). Only one pediatric (17%) and one adult (13%) subject had never met criteria for any DSM-IV diagnosis, and both of these patients had the classic DC triad. One pediatric patient had three diagnoses, including ADHD, history of learning disorders (reading disorder and disorder of written expression), and mood disorder not otherwise specified (NOS). Four adult patients with classic DC had more than one disorder. Specifically, one patient with DC had pervasive developmental disorder NOS and intellectual disability; one had intellectual disability and major depressive disorder; one with current adjustment disorder and previous panic disorder and major depressive disorder; another had a current anxiety disorder NOS and a previous psychotic disorder NOS.
Table 2.
Current and Lifetime Diagnoses of DSM-IV TR Diagnoses in DC and DC-like Subjects
| Current Diagnoses | Ever Diagnosed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children | Adults | All subjects (n=14) | Children | Adults | All subjects (n=14) | |||||
| DC (n=3) | DC-like (n=3) | DC (n=7) | DC-like (n=1) | DC (n=3) | DC-like (n=3) | DC (n=7) | DC-like (n=1) | |||
| Any Primary Psychiatric Disorder | 2 | 0 | 3 | 1 | 6 (43%) | 2 | 1 | 5 | 1 | 9 (64%) |
| Mood disorders | 2 | 1 | 3 | 2 | 1 | 3 | 6 | |||
| Anxiety disorders | 1 | 1 | 2 | 2 | 1 | 3 | ||||
| Psychotic disorder | 0 | 1 | 1 | |||||||
| Adjustment disorder | 1 | 1 | 2 | 2 | ||||||
| Any Neurocognitive Disorder | 1 | 1 | 2 | 0 | 4 (29%) | 1 | 2 | 2 | 0 | 5 (36%) |
| PDD | 1 | 1 | 1 | 1 | ||||||
| Learning disorders | 1 | 1 | 2 | |||||||
| ADHD | 1 | 1 | 2 | 1 | 1 | 2 | ||||
| Intellectual Disability | 2 | 2 | 2 | 2 | ||||||
| Any DSM-IV TR Diagnosis | 2 | 1 | 5 | 1 | 8 (57%) | 2 | 3 | 6 | 1 | 12 (86%) |
| No DSM-IV TR Diagnosis | 1 | 2 | 3 | 0 | 6 (43%) | 1 | 0 | 1 | 0 | 2 (14%) |
Mood disorders occurred most frequently, affecting three of the six (50%) pediatric subjects (two with major depression, one with possible bipolar disorder) and three of the eight (38%) adult subjects (all with major depression). Anxiety disorders (including social phobia, panic disorder, generalized anxiety disorder, and possible obsessive compulsive disorder, 38%), adjustment disorders (25%), and intellectual disability (25%) were also common in adult subjects. Learning disorders (reading disorder, disorder of written expression, and developmental speech disorder, 33%) and ADHD (33%) were present in pediatric subjects.
DISCUSSION
This study provides pilot data suggesting that DC and DC-like patients may have a high occurrence of neuropsychiatric disorders requiring clinical attention. In our study, the proportion of pediatric (50%) and adult (75%) subjects having experienced a primary psychiatric disorder during their lifetime was much higher than the 25% commonly reported for chronically ill children26 and 38% found in medically ill adults27. Patients with DC in our study experienced a range of primary psychiatric disorders but mood disorders were the most common. Neurodevelopmental disorders were also very common in this sample, with half of pediatric subjects and a quarter of adult subjects carrying these diagnoses.
The strengths of our study include detailed clinical, laboratory, and genetic analyses of all patients reported. The small sample size is a limitation of the study and makes analysis of prevalence rates relative to the general population and other medically ill patients difficult, but the rarity of the disorder limited accrual of a larger sample. Another limitation is the use of clinical interview, rather than structured diagnostic tools, to assess for neuropsychiatric disorders. Our study population may also have been weighted toward more impaired individuals, as it was limited to the subset of patients with DC and their family members who were motivated to travel to the NIH CC for an in-person assessment of their DC.
Our data suggest that routine screening for neuropsychiatric conditions and referral to specialty mental health services as indicated may be an important component of comprehensive clinical care for patients with DC. In addition, the relative frequency of intellectual disability, pervasive developmental disorders, and learning disorders in our patient population suggests a need for early neuropsychological assessment of pediatric patients with suspected DC in order to help guide academic and therapeutic interventions. More generally, a psychosomatic psychiatrist caring for a patient with DC can also collaborate with other members of the patient’s medical team to help the patient enhance their adherence to secondary and tertiary prevention recommendations (such as tobacco or sun avoidance, cancer screening, bone marrow evaluations, and health maintenance recommendations) and help patients cope with the psychosocial disruptions that accompany a chronic illness such as DC. Monitoring for neuropsychiatric sequelae of DC complications or treatments, such as side effects of androgen therapy, is also an important component of DC-related clinical care.
Patients with DC may be a key population in which to study potential links between telomere biology and brain disorders. A future study with larger sample sizes and validated diagnostic measures would help to clarify whether rates of psychiatric and/or developmental disorders in this population do indeed exceed those in other chronically ill patients and in healthy controls. Studies in this population could shed light on whether short telomeres predispose patients to develop certain neuropsychiatric conditions, whether telomere shortening is a downstream consequence of the physical effects of psychiatric symptoms and stress, and/or whether neuropsychiatric conditions and telomere shortening are both expressions of another common biological insult. Genotype-phenotype correlations between genes mutated in DC and neuropsychiatric disorders may also yield important information on the contribution of these genes to neurodevelopment.
In summary, this case series provides pilot data suggesting a high occurrence of neuropsychiatric conditions among patients with DC. Our findings suggest that careful screening for psychiatric symptoms and developmental disorders may be indicated among patients with DC. Further study with this population also has the potential to yield significant insights into the pathobiological connections between telomere biology and development of neuropsychiatric conditions.
Acknowledgments
We thank the patients and families who have generously contributed to our understanding of dyskeratosis congenita and telomere biology disorders. Lisa Leathwood, RN, Westat, Inc (NIH contracts N02-CP-11019, N02-CP-65504 and N02-CP-65501) provided outstanding study support. This work was funded by the intramural research programs of the National Cancer Institute and the National Institute of Mental Health, National Institutes of Health.
Footnotes
DISCLOSURES
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Savage SA, Bertuch AA. The Genetics and Clinical Manifestations of Telomere Biology Disorders. Genet Med. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walne AJ, Dokal I. Advances in the Understanding of Dyskeratosis Congenita. Br J Haematol. 2009;145:164–172. doi: 10.1111/j.1365-2141.2009.07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, Carr AG, Greene MH, Rosenberg PS. Malignancies and Survival Patterns in the National Cancer Institute Inherited Bone Marrow Failure Syndromes Cohort Study. Br J Haematol. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in Dyskeratosis Congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz AC, Orchard PJ, Baker KS, Giller RH, Savage SA, Alter BP, Tolar J. Disease-Specific Hematopoietic Cell Transplantation: Nonmyeloablative Conditioning Regimen for Dyskeratosis Congenita. Bone Marrow Transplant. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Fuente J, Dokal I. Dyskeratosis Congenita: Advances in the Understanding of the Telomerase Defect and the Role of Stem Cell Transplantation. Pediatr Transplant. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 7.Aubert G, Lansdorp PM. Telomeres and Aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 8.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very Short Telomere Length by Flow Fluorescence in Situ Hybridization Identifies Patients With Dyskeratosis Congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palm W, de Lange T. How Shelterin Protects Mammalian Telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 10.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very Short Telomeres in the Peripheral Blood of Patients With X-Linked and Autosomal Dyskeratosis Congenita. Blood Cells Mol Dis. 2001;27:353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 11.Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of Telomerase Trafficking by TCAB1 Mutation Causes Dyskeratosis Congenita. Genes Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere Length of Patients With Major Depression Is Shortened but Independent From Therapy and Severity of the Disease. Depress Anxiety. 2010;27:1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 13.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere Shortening and Mood Disorders: Preliminary Support for a Chronic Stress Model of Accelerated Aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Yu WY, Chang HW, Lin CH, Cho CL. Short Telomeres in Patients With Chronic Schizophrenia Who Show a Poor Response to Treatment. J Psychiatry Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC. Childhood Trauma Associated With Short Leukocyte Telomere Length in Posttraumatic Stress Disorder. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated Telomere Shortening in Response to Life Stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, Fox NA, Zeanah CH, Nelson CA. Telomere Length and Early Severe Social Deprivation: Linking Early Adversity and Cellular Aging. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porton B, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Kao HT. Telomerase Levels in Schizophrenia: a Preliminary Study. Schizophr Res. 2008;106:242–247. doi: 10.1016/j.schres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Cheng L, Craig DW, Redman M, Liu C. Cerebellar Telomere Length and Psychiatric Disorders. Behav Genet. 2010;40:250–254. doi: 10.1007/s10519-010-9338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, Blouin P, Segura JF, Cezard JP, Peuchmaur M, Vulliamy T, Dokal I, Verloes A. Further Delineation of the Congenital Form of X-Linked Dyskeratosis Congenita (Hoyeraal-Hreidarsson Syndrome) Eur J Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- 21.Revesz T, Fletcher S, al Gazali LI, DeBuse P. Bilateral Retinopathy, Aplastic Anaemia, and Central Nervous System Abnormalities: a New Syndrome? J Med Genet. 1992;29:673–675. doi: 10.1136/jmg.29.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milgrom H, Stoll HL, Jr, Crissey JT. Dyskeratosis Congenita: A Case With New Features. Arch Dermatol. 1964;89:345–349. doi: 10.1001/archderm.1964.01590270031007. [DOI] [PubMed] [Google Scholar]
- 23.Mahiques L, Febrer I, Vilata JJ, Fortea JM. A Case of Dyskeratosis Congenita Associated With Schizophrenia and Two Malignancies. J Eur Acad Dermatol Venereol. 2006;20:1159–1161. doi: 10.1111/j.1468-3083.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- 24.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in Dyskeratosis Congenita: Their Impact on Telomere Length and the Diversity of Clinical Presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2000. Fourth. [Google Scholar]
- 26.Snell C, DeMaso DR. Adaptation and Coping in Chronic Childhood Physical Illness. 2010. pp. 21–31. [Google Scholar]
- 27.Hansen MS, Fink P, Frydenberg M, Oxhoj ML. Use of Health Services, Mental Illness, and Self-Rated Disability and Health in Medical Inpatients. Psychosom Med. 2002;64:668–675. doi: 10.1097/01.psy.0000024104.87632.94. [DOI] [PubMed] [Google Scholar]