K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 8.
Published in final edited form as: Science. 2011 Feb 11;331(6018):768–772. doi: 10.1126/science.1198785
Abstract
Endocrine tumors such as aldosterone-producing adrenal adenomas (APAs), a cause of severe hypertension, feature constitutive hormone production and unrestrained cell proliferation; the mechanisms linking these events are unknown. We identify two recurrent somatic mutations in and near the selectivity filter of the potassium (K+) channel KCNJ5 that are present in 8 of 22 human APAs studied. Both produce increased sodium (Na+) conductance and cell depolarization, which in adrenal glomerulosa cells produces calcium (Ca2+) entry, the signal for aldosterone production and cell proliferation. Similarly, we identify an inherited KCNJ5 mutation that produces increased Na+ conductance in a Mendelian form of severe aldosteronism and massive bilateral adrenal hyperplasia. These findings explain pathogenesis in a subset of patients with severe hypertension and implicate loss of K+ channel selectivity in constitutive cell proliferation and hormone production.
Aldosterone, a steroid hormone synthesized by the adrenal glomerulosa, is normally produced in two conditions, intravascular volume depletion and hyperkalemia (high plasma K+ level) (1). Volume depletion activates the renin-angiotensin system, producing the hormone angiotensin II (AII), which signals via its G protein–coupled receptor (GPCR) in glomerulosa cells. The resting membrane potential is set by K+ channel activity (2); both AII signaling and hyperkalemia cause membrane depolarization and activation of voltage-gated Ca2+ channels. Increased intracellular Ca2+ provides the normal signal for aldosterone production, and sustained increases lead to glomerulosa cell proliferation (1, 3–5); AII also causes increased inositol 1,4,5-trisphosphate (IP3) and transient Ca2+ release from intracellular stores. Aldosterone signaling in the kidney increases electrogenic Na+ reabsorption, defending intravascular volume, and also increases K+ secretion.
In primary aldosteronism, the adrenal gland constitutively produces aldosterone in the absence of AII or hyperkalemia, resulting in hypertension and variable hypokalemia (low plasma K+ level). Primary aldosteronism is found in ~10% of patients referred for evaluation of hypertension. A third or more of these have aldosterone-producing adenoma (APA, also known as Conn’s syndrome) of the adrenal cortex (6); of the remainder, a small fraction have mutations that cause constitutive expression of aldosterone synthase (7), and the rest are classified as idiopathic.
APAs are typically solitary, well circumscribed, and diagnosed between ages 30 and 70 (8). They come to medical attention due to new or worsening hypertension, often with hypokalemia. Aldosterone is elevated while renin levels are suppressed (reflected in a high aldosterone:renin ratio), and a characteristic adrenal mass is seen on computed tomography (CT). Adrenal vein sampling demonstrates predominant aldosterone secretion from the gland harboring the tumor. APAs virtually always remain benign, without local invasion or distant metastasis (9). Surgical removal ameliorates or cures hypertension in the large majority of patients (10). The mechanisms responsible for neoplasia and cell-autonomous aldosterone production are unknown.
We studied 22 patients with APA (table S1) (11). All came to medical attention with hypertension and variable hypokalemia. All had high aldosterone:renin ratios and unilateral adrenal cortical mass on CT. At surgery, adrenocortical tumors of mean diameter 2.8 cm were removed, and pathology in all cases confirmed adrenocortical adenoma.
Genotyping of tumors on Illumina 1M-Duo chips demonstrated two gross classes of tumors: those with zero or few chromosome arms with loss of heterozygosity (LOH) (11 with none, 3 with 1 to 4 LOH events) and those with many large LOH segments (8 with 11 to 19 LOH segments) (table S1 and fig. S1). Subjects with low LOH tumors tended to be younger with smaller tumors.
We performed whole exome capture and Illumina sequencing on four APA-blood pairs from unrelated subjects with no LOH segments. Each tumor sample was assessed by histology to be free of normal adrenal cells; some admixture with blood and stromal cells is inevitable, and we accordingly sequenced samples to high depth of coverage to enable detection of somatic mutations. The mean coverage of each targeted base was 183-fold for blood DNA and 158-fold for tumor DNA, and 97% of all targeted bases in tumor samples were read at least eight times (table S2). We identified high-probability somatic mutations in each tumor (P = 10−4 to 10−56 of chance occurrence) (fig. S2), and confirmed each by direct Sanger sequencing (11). Twelve of 13 putative somatic mutations were confirmed by Sanger sequencing versus 0 of 28 with 10−4 < P < 10−3 (Table 1, table S3, and fig. S2). The results identified a small number of somatic mutations in each tumor, with a mean of 2.3 protein-altering and 0.8 silent mutations (Table 1 and table S3). Among bases covered ≥40-fold (86% of all targeted bases), this represents 0.15 somatic mutations per megabase of exome sequence, a low value compared with a number of malignant tumors (12, 13).
Table 1.
Protein-changing somatic mutations in aldosterone-producing adenomas.
| Tumor | Chr | Position | Base change | Gene | Effect on protein | No. of reads from tumor | No. of reads from blood | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. allele | Non-ref. allele | % of all reads | Ref. allele | Non-ref. allele | |||||||
| APA9 | 14 | 99,813,560 | C>G | YY1 | T372R | 115 | 69 | 37.5% | 184 | 0 | 1.3 × 10−24 |
| 9 | 114,858,771 | C>G | ZFP37 | V7L | 47 | 23 | 32.9% | 77 | 0 | 4.0 × 10−9 | |
| APA12 | 11 | 86,341,084 | C>A | FZD4 | C121F | 491 | 139 | 22.1% | 871 | 0 | 1.6 × 10−55 |
| 11 | 128,286,829 | G>A | KCNJ5 | G151R | 120 | 59 | 33.0% | 290 | 0 | 1.9 × 10−28 | |
| 12 | 56,159,261 | G>A | ARHGAP9 | R66C | 149 | 65 | 30.4% | 282 | 1 | 1.1 × 10−25 | |
| APA15 | 11 | 128,286,881 | T>G | KCNJ5 | L168R | 159 | 65 | 29.0% | 456 | 0 | 3.5 × 10−35 |
| X | 53,239,430 | C>T | KDM5C | V1341M | 30 | 30 | 50.0% | 54 | 0 | 7.6 × 10−11 | |
| APA22 | 21 | 43,054,087 | G>A | PDE9A | Exon 13 splice donor GT>AT | 90 | 31 | 25.6% | 123 | 0 | 6.8 × 10−10 |
| 2 | 140,918,376 | T>G | LRP1B | R3429S | 60 | 14 | 18.9% | 80 | 0 | 1.7 × 10−5 |
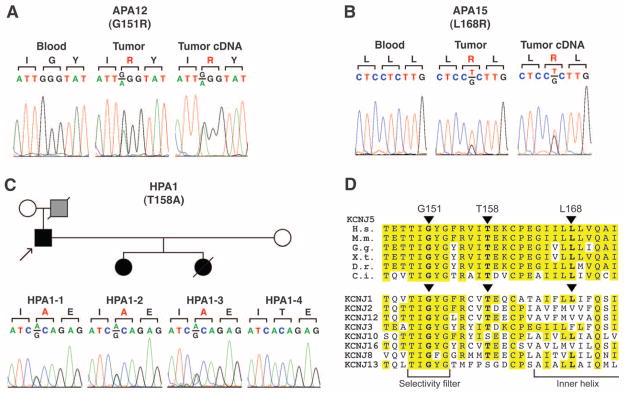
Considering the small number of somatic protein-altering mutations, it was remarkable that one gene, KCNJ5 (Kir3.4), was mutated in two tumors (Table 1, Fig. 1, and figs. S3 and S4). KCNJ5 encodes an inwardly rectifying K+ channel (14). One mutation was G151R, which was present in 33% of tumor reads and none in blood. The other was L168R, present in 29% of tumor reads and none in blood. Each was confirmed as a somatic mutation by Sanger sequencing. Both the wild-type (WT) and mutant KCNJ5 transcripts were detected in APA cDNA (Fig. 1 and fig. S4). The G151R and L168R mutations are absent in the dbSNP, 1000 Genomes, and Catalogue of Somatic Mutations in Cancer (COSMIC) databases. Sequencing 900 KCNJ5 alleles from unrelated subjects revealed neither mutation and only two missense variants, R39H and M210I, both in cytoplasmic domains. Staining of normal human adrenal gland with antibodies to KCNJ5 demonstrated selective staining of zona glomerulosa cells (fig. S5), consistent with tumors arising from mutation in these cells.
Fig. 1.
Mutations in KCNJ5 in aldosterone-producing adenoma and inherited aldosteronism. (A) Sequences of blood and tumor genomic DNA and tumor cDNA of KCNJ5 codons 150 to 152 in APA12. (B) Sequences of KCNJ5 codons 167 to 169 in APA15. (C) KCNJ5 mutation in kindred HPA1. At top, kindred structure is shown; affected members are shown as filled symbols; gray symbol represents a subject who died at age 36 with severe hypertension, suspected to be affected. KCNJ5 sequences of codons 157 to 159 are shown. Reverse strand traces for (A) to (C) are shown in fig. S4. (D) Conservation of G151, T158, and L168 in orthologs and paralogs. These positions are conserved among chordate orthologs that last shared a common ancestor 750 million years ago. H.s., Homo sapiens; M.m., Mus musculus; G.g., Gallus gallus; X.t., Xenopus tropicalis; D.r., Danio rerio; C.i., Ciona intestinalis. Shown below are the sequences of selected human inward rectifier K+ channels, demonstrating high conservation among diverse members of this family.
Sequencing of KCNJ5 in the other 18 APA-blood pairs identified six additional somatic mutations. Remarkably, all were either the G151R or L168R mutation. In sum, there were two G151R mutations and six L168R mutations among the 22 tumors (table S1 and fig. S4). The mutations were expressed in tumor cDNA in the six samples studied (fig. S4). Mutant allele frequencies in tumor DNA and cDNA are consistent with mutations being heterozygous in tumor cells. All KCNJ5 mutations were in the low LOH group, including 7 of the 11 tumors with no LOH segments.
Even using an inflated estimate of one somatic mutation per million base pairs in these tumors, the probability of seeing either of two somatic mutations recur by chance in 6 of 20 other tumors is <10−30, strongly implicating these two mutations in the pathogenesis of APA. The recurrence of the identical mutations strongly implies a genetic gain-of-function mechanism.
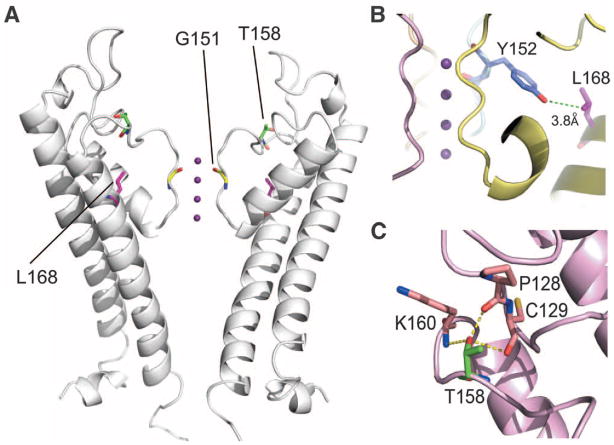
The crystal structures of a number of K+ channels have been determined, and the general features are highly conserved (15); the closest to KCNJ5 is chicken KCNJ12, another inward rectifier (16). The wild-type amino acids, G151 and L168, lie at highly conserved positions, which we mapped onto the KCNJ12 structure. G151 is the first glycine of the GYG motif of the K+ channel selectivity filter (Figs. 1D and 2A); glycine at this position is found in virtually every K+ channel in the biological world (17, 18). The main chain carbonyl groups of G151 face the pore in the channel tetramer (Fig. 2A), and their distances from one another approximate the distances of oxygen atoms in the hydration shell surrounding K+ ions, stripping water from the ion (15, 16). L168 is also conserved among KCNJ5 orthologs and inward rectifiers (Fig. 1D). L168 lies in the second transmembrane domain (inner helix) of KCNJ5; its side chain abuts the highly conserved tyrosine side chain of the GYG motif (Fig. 2B).
Fig. 2.
Location of human mutations in KCNJ5 mapped onto the crystal structure of chicken K+ channel KCNJ12 (16). (A) Location of mutations. The extracellular and transmembrane domains of two subunits from the channel tetramer are shown with K+ ions (purple) traversing the selectivity filter; human KCNJ5 and chicken KCNJ12 are 89% identical in the pore helix and selectivity filter. G151 lies in the selectivity filter at a position conserved among virtually all K+ channels. Its main chain carbonyl group faces the channel pore. T158 lies just above the selectivity filter, and L168 is in the second transmembrane domain (inner helix) with its side chain projecting toward the selectivity filter. (B) View of the side chains of L168 and the highly conserved Y152 of the selectivity filter, showing their close proximity. (C) View of T158, which makes hydrogen bonds with conserved positions P128 and C129.
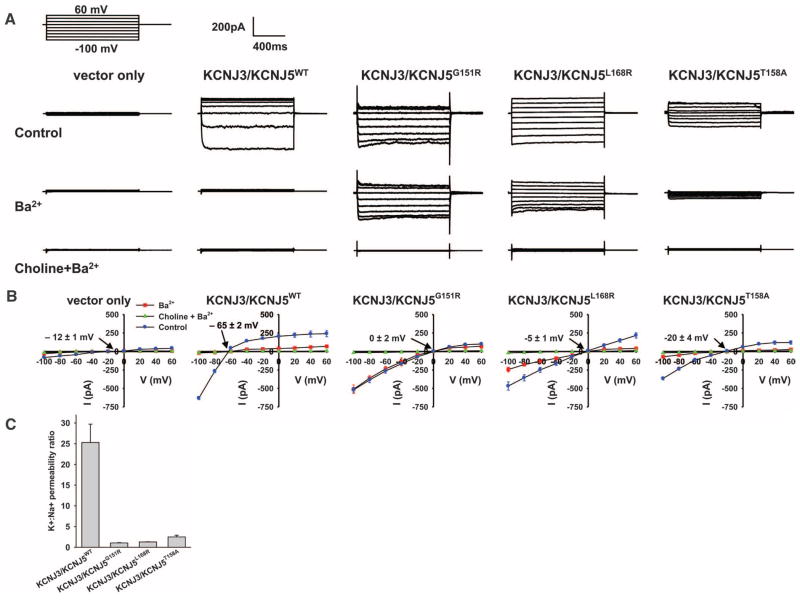
Previous studies have shown that mutations in and near K+ channel selectivity filters can alter channel selectivity to produce nonselective cation channels (17, 19). KCNJ5 exists both as homotetramers and heterotetramers with KCNJ3 (KCNJ3 is inactive as a homotetramer) (20); heterotetramers are more active than homotetramers, and activity can be increased by activation of GPCRs such as dopamine D2 (14, 21). Both KCNJ5 and KCNJ3 are expressed in human adrenal cortex (table S4). We expressed wild-type or mutant KCNJ5 with KCNJ3 in 293T cells (Fig. 3 and fig. S6) and measured currents at voltages from −100 mV to +60 mV using perforated whole-cell recording with physiologic solutions including 140 mM K+ inside and 5 mM K+, 140 mM Na+ outside the cell (11). KCNJ3/KCNJ5WT induced a robust inwardly rectifying Ba2+-sensitive current that hyperpolarized the membrane with a reversal potential of −65 ± 2 mV (Fig. 3). Co-expression with the dopamine D2 receptor and addition of dopamine increased current by ~50% (fig. S7, A and B). These are all characteristic features of KCNJ3/KCNJ5 heterotetramers (14). The K+:Na+ permeability ratio, estimated from the Goldman equation, was 25.3 ± 4.4:1. In contrast, KCNJ3/KCNJ5G151R channels produced currents that showed loss of inhibition by Ba2+ and membrane depolarization with a shift of the reversal potential to 0 ± 2 mV. This depolarization is attributable to increased Na+ conductance: whereas substitution of choline for Na+ had no effect on the WT channel, elimination of Na+ markedly inhibited KCNJ3/KCNJ5G151R currents either with (Fig. 3) or without (fig. S7, C and D) Ba2+. The calculated K+:Na+ permeability ratio is diminished to 1.0 ± 0.1:1, consistent with loss of channel selectivity. This loss of ion selectivity is similar to effects seen with other selectivity filter mutations (17). KCNJ3/KCNJ5L168R channels behave similarly, producing a reversal potential of −5 ± 1 mV and a K+:Na+ permeability ratio of 1.3 ± 0.1:1 (Fig. 3). Similar results were obtained with KCNJ5 homotetramers (fig. S8). In glomerulosa cells, membrane depolarization activates voltage-gated Ca2+ channels, increasing intracellular Ca2+, thereby increasing aldosterone production (Fig. 4) (1). Similarly, chronic Ca2+ stimulation promotes increased proliferation in glomerulosa (3–5) and other cell types (22, 23), which can account for clonal expansion of cells harboring these somatic mutations and adenoma formation.
Fig. 3.
KCNJ5 mutations result in loss of channel selectivity and membrane depolarization. (A) Representative whole-cell recordings of 293T cells transfected with empty vector or KCNJ3 plus WT or mutant KCNJ5. The pipette holding potential was 0 mV before clamping, and the cell was clamped from −100mV to +60 mV, with 20 mV increments. Top row: extracellular solution contained 140 mM NaCl, 5 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, 10 mM HEPES, pH 7.4; intracellular solution contained 140 mM KCl, 4 mM MgCl2, 1 mM CaCl2, 1 mM EGTA, 5 mM HEPES, pH 7.4. Middle row: 1mM BaCl2 was added. Bottom row: 140 mM choline chloride was substituted for extracellular NaCl. (B) Current-voltage relationships from cells expressing indicated constructs (n = 3 to 7 for each construct). Reversal potentials in control conditions are indicated. WT channel shows a highly negative reversal potential and is inhibited by Ba2+ but not substitution of choline for Na+ (see also fig. S7). Mutant channels show less negative reversal potentials; currents are inhibited by elimination of Na+ but show variable inhibition by Ba2+. (C) K+:Na+ permeability ratios calculated from the reversal potentials (11) show loss of ion selectivity of the mutant channels. Data in (B) and (C) are shown as mean ± SEM. Reversal potentials and K+:Na+ permeability ratios are significantly different between wild-type and mutant channels (P < 0.01 by Student’s t test).
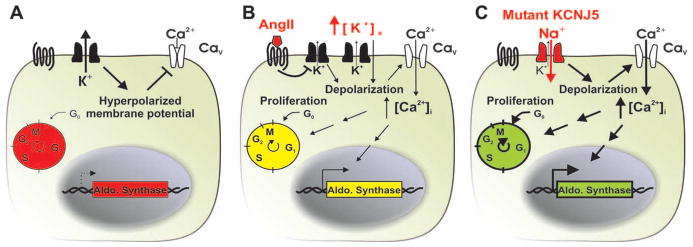
Fig. 4.
Proposed mechanism underlying aldosterone-producing adenoma and Mendelian aldosteronism. (A) Adrenal glomerulosa cells have a high resting K+ conductance, which produces a highly negative membrane potential (2). (B) Membrane depolarization by either elevation of extracellular K+ or closure of K+ channels by angiotensin II activates voltage-gated Ca2+ channels, increasing intracellular Ca2+ levels (1). This provides signals for increased expression of enzymes required for aldosterone biosynthesis, such as aldosterone synthase, and for increased cell proliferation. (C) Channels containing KCNJ5 with G151R, T158A, or L168R mutations conduct Na+, resulting in Na+ entry, chronic depolarization, constitutive aldosterone production, and cell proliferation.
These inferences from somatic mutations in tumors suggest that inherited mutations in KCNJ5 with similar effect could cause a Mendelian form of primary aldosteronism with bilateral adrenal hyperplasia, because in this case every adrenal cell would harbor the mutation. We recently described just such a syndrome of unknown cause in a father and his two daughters who were all diagnosed between ages 4 and 7 with severe hypertension, aldosteronism, and massive adrenal hyperplasia (table S5) (24). All three individuals had a radical intervention, bilateral adrenalectomy in childhood. Pathology demonstrated massive hyperplasia of the adrenal cortex (paired adrenal weights up to 81 g; normal <12 g). The sequence of KCNJ5 identified a heterozygous T158A mutation that cosegregated with the disease (Fig. 1C and fig. S4I). This variant is absent in the dbSNP and 1000 Genomes databases and in 900 control alleles. This threonine is conserved among KCNJ5 orthologs and other inward rectifiers (Fig. 1D) and lies in the loop between the selectivity filter and the second transmembrane domain; its hydroxyl group hydrogen bonds with conserved residues in the loop between the first transmembrane domain and the pore helix, constraining the structure (Fig. 2C). The T158A mutation eliminates these hydrogen bonds. Similar to the other mutations, KCNJ3/KCNJ5T158A channels showed reduced selectivity (K+:Na+ permeability ratio of 2.5 ± 0.4:1) and membrane depolarization, with a reversal potential of −20 ± 4 mV (Fig. 3B). Similar results were seen in homotetramers (fig. S8).
These findings implicate inherited and acquired mutations in KCNJ5 in aldosteronism associated with cell autonomous proliferation. The very small number of somatic mutations observed, the young age of many APA subjects with KCNJ5 mutations (four of eight under age 35), and Mendelian transmission of the inherited syndrome are consistent with the KCNJ5 mutations being sufficient for both constitutive aldosterone secretion and cell proliferation. The increased Na+ conductance and membrane depolarization resulting from these mutations implicate activation of voltage-gated Ca2+channels in the pathophysiologic mechanism (Fig. 4) (1, 3–5).
The effects of these mutations in and near the selectivity filter to reduce channel selectivity are consistent with previous in vitro studies (17, 19). In addition, mutation of the homologous glycine to serine in KCNJ6 in the weaver mouse also produces a Na+-conducting channel, leading to selective loss of neurons in cerebellum and substantia nigra (25). Glomerulosa cells have constitutively open “leak” K+ channels and a high Na+/K+ adenosine triphosphatase activity (26); such differences may contribute to different fates in these cell types.
Because mutations in and near K+ channel selectivity filters can alter ion selectivity (19), the restricted spectrum of mutations found in APAs is noteworthy. One possible explanation is that mutant channels must cause sufficient Na+ permeability for tumor development but not so great as to cause cell death (23, 25); these requirements may restrict the mutational spectrum. The lower relative Na+ permeability observed with the inherited T158A mutation is consistent with allelic variation in effect. It will be of interest to determine the prevalence and spectrum of KCNJ5 mutations in other cohorts of patients with APAs and with unexplained aldosteronism.
These findings also raise the question of the normal role of KCNJ5 in glomerulosa cells. In rodent and cow, members of the “leak” K+ channel family (KCNK2, KCNK3, and KCNK9) appear to set the resting potential (27, 28). Dopamine, an inhibitor of aldosterone release, increases activity of K+ channels containing KCNJ5 (21), suggesting that KCNJ5 may normally inhibit aldosterone production. We sequenced KCNK2, KCNK3, and KCNK9 in the tumor cohort and found no mutations, consistent with KCNJ5 having a privileged role in producing APA.
Lastly, these findings demonstrate a role for ion channel mutations in neoplasia. The distinct mechanism of these KCNJ5 mutations may be related to the benign nature of these tumors. It will be of interest to determine whether other endocrine neoplasias have related mutations that account for concomitant cell proliferation and hormone release. Mutations in other K+ channel genes have been identified in various human cancers, but their importance has been uncertain. These include mutations altering conserved residues in or near voltage-regulating segments in KCNB2, KCNC2, and KCNQ5 from glioblastoma, breast cancer, and colorectal cancers (12, 13, 29). Investigation of the functional consequences of these mutations will be of interest.
Supplementary Material
supplementary
Acknowledgments
We thank the patients whose participation made this study possible and the staff of the Yale West Campus Genomics Center and the Endocrine Surgical Laboratory, Clinical Research Centre, University Hospital, Uppsala. Supported in part by the Fondation Leducq Transatlantic Network in Hypertension, National Institutes of Health (NIH) grant DK54983, the Yale Center for Human Genetics and Genomics, Yale NIH O’Brien Center for Kidney Research, and the Yale NIH Clinical Translational Science Award, and by the Swedish Cancer Society, the Swedish Research Council, and the Lions Cancer Fund, Uppsala. U.I.S. is a fellow of the Deutsche Forschungsgemeinschaft; B.Z. is an investigator of the Yale Medical Scientist Training Program; T.C. is a Doris Duke–Damon Runyon Clinical Investigator; R.P.L. is a paid scientific advisor to Merck and is an investigator of the Howard Hughes Medical Institute.
Footnotes
References and Notes
- 1.Spät A, Hunyady L. Physiol Rev. 2004;84:489. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- 2.Spät A. Mol Cell Endocrinol. 2004;217:23. doi: 10.1016/j.mce.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 3.McEwan PE, Lindop GB, Kenyon CJ. Am J Physiol. 1996;271:E192. doi: 10.1152/ajpendo.1996.271.1.E192. [DOI] [PubMed] [Google Scholar]
- 4.Pawlikowski M, Gruszka A, Mucha S, Melen-Mucha G. Endocr Regul. 2001;35:139. [PubMed] [Google Scholar]
- 5.Tanabe A, et al. J Endocrinol Invest. 1998;21:668. doi: 10.1007/BF03350796. [DOI] [PubMed] [Google Scholar]
- 6.Rossi GP, et al. PAPY Study Investigators. J Am Coll Cardiol. 2006;48:2293. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 7.Lifton RP, et al. Nature. 1992;355:262. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 8. chap 24 Saunders; Philadelphia: 2009. [Google Scholar]
- 9.Ghose RP, Hall PM, Bravo EL. Ann Intern Med. 1999;131:105. doi: 10.7326/0003-4819-131-2-199907200-00005. [DOI] [PubMed] [Google Scholar]
- 10.Calvo-Romero JM, Ramos-Salado JL. Postgrad Med J. 2000;76:160. doi: 10.1136/pmj.76.893.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Materials and methods are available as supporting material on Science Online.
- 12.Wood LD, et al. Science. 2007;318:1108. [Google Scholar]
- 13.Sjöblom T, et al. Science. 2006;314:268. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 14.Krapivinsky G, et al. Nature. 1995;374:135. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 15.Doyle DA, et al. Science. 1998;280:69. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 16.Tao X, Avalos JL, Chen J, MacKinnon R. Science. 2009;326:1668. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heginbotham L, Lu Z, Abramson T, MacKinnon R. Biophys J. 1994;66:1061. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux B. Annu Rev Biophys Biomol Struct. 2005;34:153. doi: 10.1146/annurev.biophys.34.040204.144655. [DOI] [PubMed] [Google Scholar]
- 19.Dibb KM, et al. J Biol Chem. 2003;278:49537. doi: 10.1074/jbc.M307723200. [DOI] [PubMed] [Google Scholar]
- 20.Corey S, Clapham DE. J Biol Chem. 1998;273:27499. doi: 10.1074/jbc.273.42.27499. [DOI] [PubMed] [Google Scholar]
- 21.Gregerson KA, et al. Endocrinology. 2001;142:2820. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- 22.Kahl CR, Means AR. Endocr Rev. 2003;24:719. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- 23.Roderick HL, Cook SJ. Nat Rev Cancer. 2008;8:361. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 24.Geller DS, et al. J Clin Endocrinol Metab. 2008;93:3117. doi: 10.1210/jc.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro B, et al. Science. 1996;272:1950. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- 26.Hajnóczky G, et al. Endocrinology. 1992;130:1637. doi: 10.1210/endo.130.3.1311245. [DOI] [PubMed] [Google Scholar]
- 27.Enyeart JJ, Xu L, Danthi S, Enyeart JA. J Biol Chem. 2002;277:49186. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 28.Czirják G, Enyedi P. Mol Endocrinol. 2002;16:621. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- 29.Parsons DW, et al. Science. 2008;321:1807. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary