Pressure Ulcers in Elderly Hip Fracture Patients Across the Continuum of Care (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 25.
Abstract
Objectives
The aim of this study was to identify care settings associated with increased pressure ulcer risk among elderly hip fracture patients in the post-fracture period.
Design
Prospective cohort study.
Setting
Nine hospitals that participate in the Baltimore Hip Studies network and 105 postacute facilities to which patients from these hospitals were discharged.
Participants
Hip fracture patients age ≥65 years who underwent surgery for hip fracture.
Measurements
A full-body skin examination was conducted at baseline (as soon as possible after hospital admission) and repeated on alternating days for 21 days. Patients were deemed to have an acquired pressure ulcer (APU) if they developed ≥1 new pressure ulcers stage 2 or higher following hospital admission.
Results
Among 658 study participants, the APU cumulative incidence at 32 days after initial hospital admission was 36.1% (standard error 2.5%). Compared to home, the adjusted APU incidence rate was highest during the initial acute hospital stay (relative rate [RR] 2.2, 95% confidence interval [CI] 1.3–3.7) and during re-admission to the acute hospital (RR 2.2, 95% CI 1.1–4.2). The relative rates in rehabilitation and nursing home settings were 1.4 (95% CI 0.8–2.3) and 1.3 (95% CI 0.8–2.1), respectively.
Conclusion
Approximately one-third of hip fracture patients developed an APU during the study period. The rate was highest in the acute setting, a finding that is significant in light of Medicare’s policy of not reimbursing hospitals for the treatment of hospital-acquired pressure ulcers. Hip fracture patients constitute an important group to target for pressure ulcer prevention in hospitals.
Keywords: pressure ulcers, hospitals, hip fracture
INTRODUCTION
Pressure ulcers are localized areas of injury that occur when skin and underlying tissue are compressed between a bony prominence and an external surface such as a mattress (1). Pressure ulcers in elderly patients can result in reduced quality of life, pain, longer hospital stays, higher health care costs, poor rehabilitation outcomes, and potentially serious complications (2–5). Pressure ulcer frequency has not declined in recent years (6), despite the adoption of national pressure ulcer prevention objectives (7,8) and clinical practice guidelines (9,10). National attention to this problem has increased since Medicare’s designation of pressure ulcers as a preventable complication of medical care and its announcement that it will no longer reimburse hospitals for the cost of treating hospital-acquired pressure ulcers (11).
Hip fracture patients are at high risk of pressure ulcers (12) by virtue of exposure to long periods of immobility before, during and after surgery. The care of hip fracture patients is characterized by short hospital stays and a rehabilitation phase in diverse postacute discharge settings (13–14). However, little is known about pressure ulcer risk as these patients move through different care settings. The aims of this study were to determine the incidence of pressure ulcers among elderly patients who have undergone surgery for hip fracture and to identify care settings associated with elevated pressure ulcer risk. We hypothesized that pressure ulcer incidence would be highest in the acute care setting, where patients are most likely to have pressure ulcer risk factors such as immobility, incontinence, and confusion.
METHODS
Design and Participants
We carried out a prospective cohort study between 2004 and 2007 in nine hospitals that participate in the Baltimore Hip Studies network (15) and in the 105 postacute facilities to which patients from these hospitals were discharged. To be eligible, patients had to be 65 years or older and undergo surgery for hip fracture (ICD-9 code 820) at one of the study hospitals. Patients were excluded if their hip fracture occurred during a hospital stay. Permission to contact patients for the study was obtained from attending physicians; only 1.5% of eligible patients were not enrolled because of the physician’s refusal. Written consent was obtained from patients with a Mini-Mental State Exam (MMSE) (16) score of 20 or greater. If the MMSE score was less than 20, verbal assent was obtained from patients and written consent from proxies. Proxy consent was obtained for patients who were unconscious or noncommunicative. The study was approved by the Institutional Review Boards of the University of Maryland Baltimore and of each of the participating hospitals.
Procedures
For each study participant, a full-body skin examination was performed at baseline (as soon as possible after hospital admission) by a specially trained research nurse and was repeated on alternating days for 21 days, for a total of 11 assessments. To ensure that all participants had the opportunity for at least 10 days of follow-up in a postacute setting, patients whose acute hospital stay was longer than 11 days were followed until there were 10 postacute follow-up days or until the total follow-up was 31 days, whichever came first. The follow-up skin examinations were carried out in the care setting where the patient resided at the time of the scheduled assessment.
Measures
The presence and severity of pressure ulcers were determined according to standard wound assessment practice (17). Patients were visually examined in flat supine and 45° inclined lateral-supine positions on right and left sides. Standard definitions (12) were used for pressure ulcer staging: stage 1 (alteration of intact skin with persistent redness), stage 2 (partial thickness dermal loss or serum-filled blister), and stages 3 and 4 (full thickness tissue loss without/with exposed bone, tendon, or muscle). Lesions in an area with active skin disease, wounds on the plantar surface of the forefoot and midfoot, and wounds on the leg between the malleolus and the popliteal fossa were not considered to be pressure ulcers. Patients were deemed to have an acquired pressure ulcer (APU) if they developed one or more new pressure ulcers stage 2 or higher following hospital admission.
Pressure ulcers observed at the baseline assessment were classified as pre-existing, possibly acquired, or definitely acquired, using criteria that have been previously described (18). According to these criteria, Stage 4 ulcers were always considered to be pre-existing. For stages 1, 2, and 3 (and unknown stage), the classification was based on a synthesis of information from up to four sources: the patient or patient’s family member, the hospital or facility chart, the transfer form from previous institution, and hospital staff members. A stage 1 pressure ulcer was always considered to be definitely acquired, unless there was at least one undisputed source that said it was pre-existing. For stage 2 and stage 3 pressure ulcers (and unknown stage), if there was consistent information from more than one source, that information was used to classify the pressure ulcer as pre-existing or definitely acquired; if there was conflicting information or no information, the pressure ulcer was classified as possibly acquired. For the primary analysis, possibly and definitely acquired pressure ulcers were considered to be APUs.
Each pressure ulcer observed during the study was photographed when first observed and at the last visit, and photos were reviewed to identify discrepancies that indicated need for additional training of research nurses. At the end of the study, photographs and data collection forms for every pressure ulcer stage 2 or higher identified during the study were reviewed to assure that the source of pressure, anatomic site, clinical history, and appearance were consistent with the research diagnosis.
Level and location of care were documented by the research nurses during the assessment visits. For days when there was no assessment visit, level and location of care were ascertained retrospectively at the next visit by consulting the patient, the caregiver, and the facility chart. Mutually exclusive care settings based on location and level of care were defined as follows: acute hospital (acute care provided in an acute hospital); rehabilitation (acute or skilled rehabilitation provided in an acute hospital or rehabilitation facility); nursing home; home; and readmission to acute hospital (return to the acute hospital setting, as defined above, after discharge from the initial acute setting).
Risk factor information was obtained by interview of patient or proxy at baseline, clinical observation at baseline, and chart review. Research nurses used the Subjective Global Assessment of Nutritional Status (19) to classify individuals as being at low, moderate, or high risk of nutrition-associated complications. The research nurse recorded the patient’s activity level and urinary/fecal incontinence status, based on observation and discussion with clinical staff. Weight and height were obtained from the medical chart or, when missing, from patient or proxy interview. History of chronic cognitive deficit was assessed from the medical chart; if missing in the chart, it was assumed to be absent. Cognitive status was measured at baseline with the MMSE (16); patients who were unconscious or noncommunicative received a score of 0. Arterial insufficiency was defined as absence of pedal pulses or ankle brachial index <1. Severity of illness was measured using the Rand Sickness at Admission Scale (hip fracture version) (20) and comorbidity by the Charlson Comorbidity Index (21), both of which use information in the medical chart. Albumin level was obtained from the medical chart, with normal defined as ≥3.0 g/dL (22) or missing.
In addition to these baseline risk factors, three variables were measured at each follow-up visit. Activity level and incontinence were assessed as described above. Acute mental status was assessed clinically by the research nurse and operationalized as the number of orientations (oriented to person, place, and/or time), ranging from 0 to 3.
Analysis
The cumulative incidence of APU was estimated by subtracting the Kaplan-Meier survivor function estimate (23) from one. The cumulative incidence estimates (and 95% confidence limits), by days since initial hospital admission, were displayed graphically for up to 32 days since initial hospital admission; this interval was chosen because the last study visit was performed 32 days following hospital admission.
A time-to-event analysis was performed, treating the first APU as the event of interest (regardless of whether other APUs were subsequently observed) and treating loss to follow-up as censoring, using a Poisson regression model with a log link function. We fit models using maximum quasi-likelihood with a robust variance estimator to allow for possible under- or overdispersion (24,25). Four different models were fit. Model 1 included only care setting (to estimate crude relative rates). Model 2 included care setting and baseline risk factors, Model 3 included care setting and baseline and time-dependent risk factors, and Model 4 included the same covariates plus number of days from initial hospital admission, a surrogate for unmeasured time-dependent risk factors that may confound the association between setting and incidence of pressure ulcers. Thus, the rate ratios in Model 4 are interpreted as comparisons of APU incidence rates among settings, statistically adjusted for differences in patient characteristics and for number of days from initial hospital admission. In all models, care setting was treated as a time-dependent variable; thus, we estimated the association between setting at a particular visit and APU at the same visit. Care setting was entered as a set of four indicator variables (initial acute hospital, rehabilitation, nursing home, and re-admission to acute hospital). The home setting was used as the reference category because we expected that pressure ulcer risk would be lowest in this group. Using the setting with the lowest incidence as the reference group resulted in all the estimated rate ratios being greater than 1 which facilitates interpretation of the study results. Admission hospital was included in all the models as a fixed effect. Sensitivity analysis using weighted estimating equations (WEE) (26) was performed to examine the effects of missing covariate data on study results. Analyses were performed by Dr. Hawkes with guidance from Drs. Shardell and Langenberg.
RESULTS
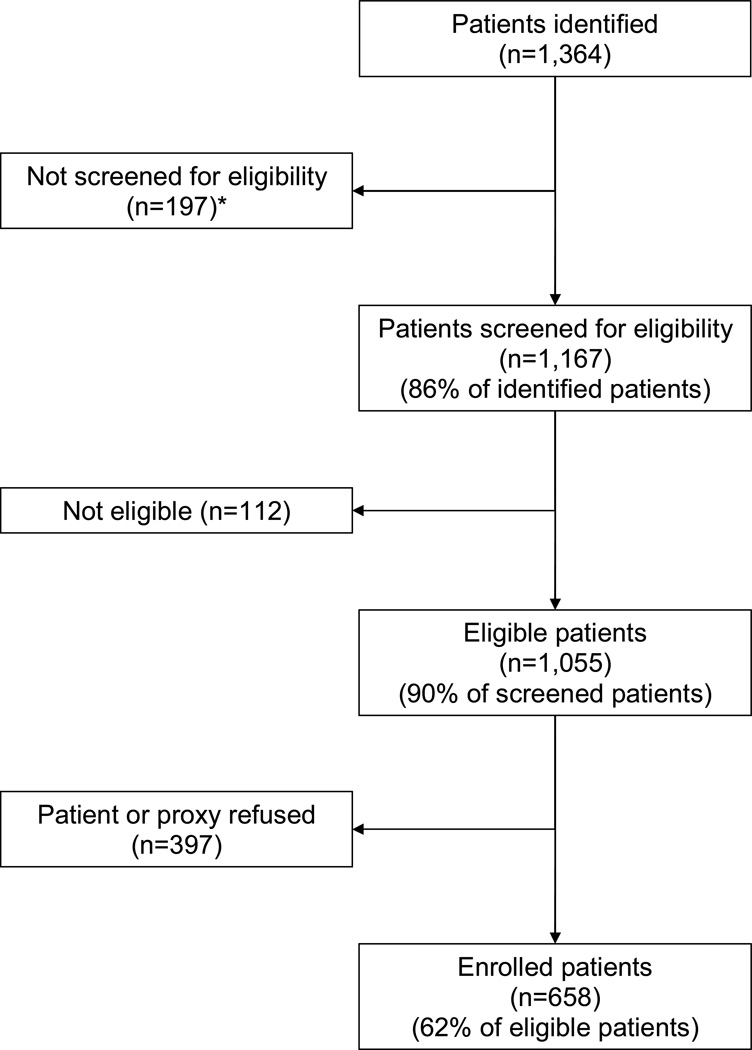
Details regarding the identification, screening, and enrollment of study participants are in Figure 1. The mean ages of enrolled (n=658) and eligible non-enrolled patients (n=397) were almost identical (83.2 [standard deviation (SD) 6.6] and 83.3 [SD 6.8] years, respectively); 23.1% of enrolled patients were men compared to 25.2% of eligible non-enrolled patients. Seventy-two patients (10.9%) were lost to follow-up; 28 died and 44 moved away or dropped out. The mean ages of patients who did and did not complete follow-up were almost identical (83.1 [SD 6.6] and 83.6 [SD 7.1] years, respectively); 23.2% of patients who completed the study were men compared to 22.2% of patients who did not. However, patients who did not complete the study had higher comorbidity and disease severity scores, had worse cognitive status, and were more likely to be bedbound and malnourished at baseline than patients who completed the study.
Figure 1.
Identification, Screening, and Enrollment of Study Participants
* 146 discharged from hospital before contact could be made, and 51 because patient, proxy, or physician could not be found or refused eligibility screening
Baseline characteristics of study participants are shown in Table 1. Among the 658 study participants, 208 developed one or more APUs. Patients with APUs had more severe illness, more comorbidity, poorer nutritional status, and poorer cognitive status at baseline than participants with no APUs (p<0.05). They were also more likely to have a history of chronic cognitive deficit, to be incontinent, and to be bedbound or chairbound (p<0.01). The mean interval between hospital admission and baseline assessment was 2.9 (SD 2.0, range 0–15) days; 11.7% of patients had the baseline assessment before the day of surgery (data not tabulated). The mean interval between initial hospital admission and the last follow-up visit (which is the sum of the time from admission to the baseline assessment plus the time from the baseline assessment to the last follow-up visit) was 22.2 (SD 3.9, range 1–32) days from initial hospital admission.
Table 1.
Baseline Characteristics of Study Participants, by APU Status
| Characteristics | PatientsWith NoAPU(N=450) | PatientsWith APU(N=208) | All Patients(N=658) | P Value* |
|---|---|---|---|---|
| N (%) or mean (SD) | ||||
| Mean age (yrs) | 83.1 (6.7) | 83.4 (6.5) | 83.2 (6.6) | .66 |
| Age ≥ 85 yrs | 208 (46.2%) | 98 (47.1%) | 306 (46.5%) | .83 |
| Male sex | 102 (22.7%) | 50 (24.0%) | 152 (23.1%) | .70 |
| White race | 441 (98.0%) | 204 (98.1%) | 645 (98.0%) | .95 |
| Community resident before admission | 316 (70.2%) | 133 (63.9%) | 449 (68.2%) | .11 |
| Trochanteric fracture | 202 (44.9%) | 93 (44.7%) | 295 (44.8%) | .97 |
| Partial or total arthroplasty | 152 (33.8%) | 82 (39.4%) | 234 (35.6%) | .16 |
| Mean Rand Sickness at Admission score | 11.7 (5.5) | 13.5 (6.9) | 12.3 (6.0) | <.001 |
| Mean Charlson Comorbidity Index | 1.2 (1.5) | 1.6 (1.4) | 1.3 (1.5) | .02 |
| Albumin < 3.0 g/dL | 138 (30.7%) | 69 (33.2%) | 207 (31.5%) | .52 |
| Mean MMSE score | 19.5 (10.5) | 16.0 (11.2) | 18.4 (10.9) | <.001 |
| History of chronic cognitive deficit | 112 (24.9%) | 83 (39.9%) | 195 (29.6%) | <.001 |
| Mean BMI (weight [kg]/height [m]2) | 24.0 (4.9) | 23.4 (5.4) | 23.8 (5.1) | .17 |
| High nutritional risk | 25 (5.6%) | 33 (16.3%) | 58 (9.0%) | <.001 |
| Incontinence | .004 | |||
| None | 331 (73.9%) | 127 (61.4%) | 458 (69.9%) | |
| Urinary only | 74 (16.5%) | 46 (22.2%) | 120 (18.3%) | |
| Fecal with or without urinary | 43 (9.6%) | 34 (16.4%) | 77 (11.8%) | |
| Activity level | <.001 | |||
| Walks | 79 (17.6%) | 19 (9.2%) | 98 (15.0%) | |
| Bedbound | 176 (39.3%) | 116 (56.0%) | 292 (44.6%) | |
| Chairbound | 193 (43.1%) | 72 (34.8%) | 265 (40.5%) | |
| Arterial insufficiency | 166 (36.9%) | 88 (42.3%) | 254 (38.6%) | .18 |
| Pre-existing pressure ulcers | 11 (2.4%) | 8 (3.9%) | 19 (2.9%) | .32 |
| Mean length of hospital stay (days) | 5.6 (2.8) | 6.6 (3.8) | 5.9 (3.2) | <.001 |
| Mean interval between initial hospital admission and baseline assessment (days) | 2.9 (2.0) | 2.8 (2.1) | 2.9 (2.0) | .56 |
| Baseline assessment before day of surgery | 37 (8.2%) | 40 (19.2%) | 77 (11.7%) | <.001 |
Among the 208 patients with at least one APU, 40 had more than one APU on the first day on which an APU was observed: 32 had two APUs, six had three APUs, and two had four APUs. Thus, a total of 258 APUs were observed on the first day on which at least one APU was observed. Nearly 90% of these APUs were stage 2; 10.9% were unstageable due to eschar, necrotic tissue, or dressing (Table 2). More than 47% were located on the sacrum or posterior iliac crest, and almost 20% on the heels.
Table 2.
Characteristics of APUs (N=258)*
| Characteristic | Number | % |
|---|---|---|
| Stage | ||
| 2 | 228 | 88.4 |
| 3 | 2 | 0.8 |
| Unstageable | 28 | 10.9 |
| Site | ||
| Sacrum, posterior iliac crest | 122 | 47.3 |
| Heels | 50 | 19.4 |
| Ischium | 31 | 12.0 |
| Spine, posterior ribs, scapula | 19 | 7.4 |
| Upper leg | 12 | 4.7 |
| Elbow | 7 | 2.7 |
| Tarsals, metatarsals, phalanges | 4 | 1.6 |
| Trochanter | 3 | 1.2 |
| Other | 10 | 3.9 |
Ninety-six patients (14.6%) developed one or more APUs during the initial acute hospital stay. Among 588 patients with 11 or more study visits, 18.2% had an APU at their last study visit (not tabulated).
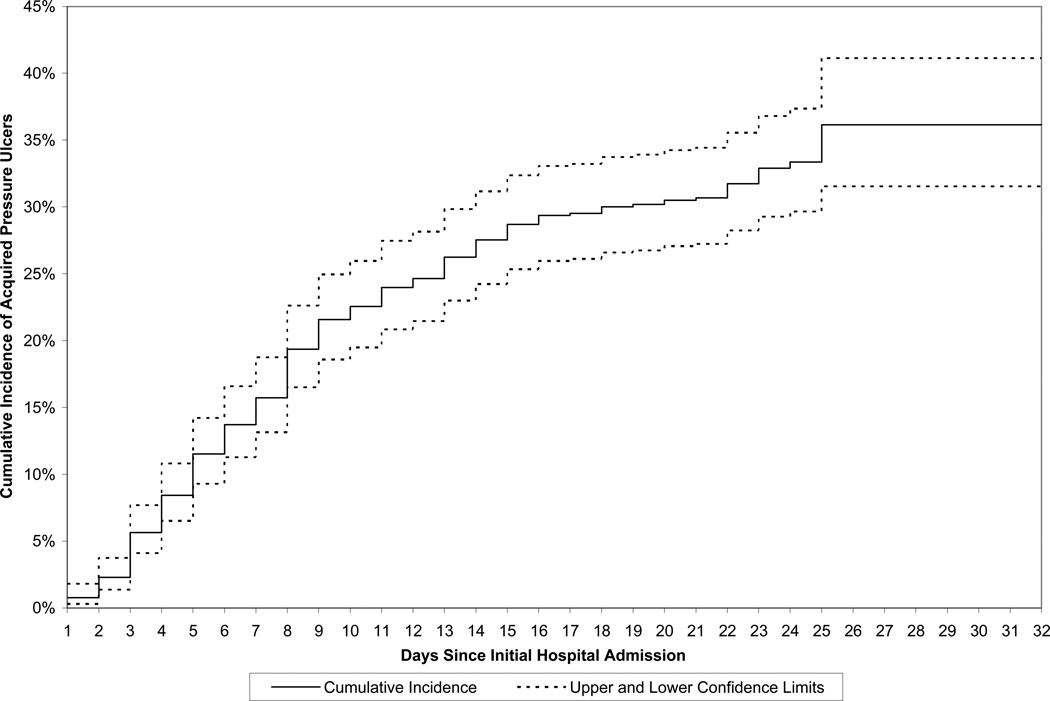
The cumulative incidence of first APU (with 95% confidence limits) is shown graphically in Figure 2. The cumulative incidence (standard error) at seven, 14, 21, and 32 days since initial hospital admission, was 15.7% (1.4%), 27.6% (1.8%), 30.7% (1.8%), and 36.1% (2.5%), respectively.
Figure 2.
Cumulative Incidence (and 95% Confidence Limits) of Acquired Pressure Ulcers, by Days Since Initial Hospital Admission
Forty-one patients (6.2% of all patients, 19.7% of patients with APUs) had a pressure ulcer at the baseline assessment that was classified as an APU according to the criteria described earlier; 31 (75.6%) had a definitely acquired pressure ulcer and the remainder had a possibly acquired pressure ulcer. Twenty-one of these 41 patients (51.2%) developed at least one other APU during follow-up while 20 developed no further APUs.
APU incidence rates were highest during the initial acute hospital stay and during re-admission to the acute hospital (Table 3, Model 1). Most of the estimated RRs were attenuated after adjustment for baseline and time-dependent risk factors, and days since initial hospital admission (Models 2 to 4). In the fully adjusted model (Model 4), the estimated incidence rates during the initial acute hospital stay and during re-admission to the acute hospital were both more than twice as high as at home (RR 2.2 and 2.2, 95% CI 1.3–3.7 and 1.1–4.2, respectively). Estimated APU incidence rates in rehabilitation and nursing home were 40% and 30% higher, respectively, than the rate at home.
Table 3.
APU Rates and Relative Rates, by Care Setting
| Care Setting | Numberof PatientswithAPUs | Person-Days atRisk | APU Rateper 100Person-Days(95% CI) | Relative Rate(95% CI),Model 1(n=658) | Relative Rate(95% CI),Model 2(n=619) | Relative Rate(95% CI),Model 3(n=614) | Relative Rate(95% CI),Model 4(n=614) |
|---|---|---|---|---|---|---|---|
| Acute hospital | 96 | 1984 | 4.8 (4.0–5.9) | 8.6 (5.6–13.3) | 6.2 (4.0–9.5) | 4.1 (2.5–6.7) | 2.2 (1.3–3.7) |
| Rehabilitation | 18 | 1457 | 1.2 (0.8–2.0) | 2.2 (1.4–3.6) | 2.2 (1.3–3.4) | 1.9 (1.1–3.1) | 1.4 (0.8–2.3) |
| Nursing home | 81 | 5405.5 | 1.5 (1.2–1.9) | 2.7 (1.7–4.1) | 2.1 (1.4–3.3) | 1.5 (0.9–2.4) | 1.3 (0.8–2.1) |
| Home | 6 | 1072 | 0.6 (0.2–1.2) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Re-admission to acute hospital | 7 | 236.5 | 3.0 (1.3–6.1) | 5.3 (3.0–9.3) | 4.0 (2.2–7.1) | 2.2 (1.1–4.2) | 2.2 (1.1–4.2) |
Inaccuracy in classifying pressure ulcers as being acquired versus pre-existing at the baseline assessment could result in biased RR estimates, especially for the initial acute setting. Therefore, we performed a sensitivity analysis in which pressure ulcers classified as possibly acquired at the baseline assessment were treated as though they were not acquired. The RRs for the initial acute setting from the fully adjusted model (Model 4) were almost identical in the primary analysis and the sensitivity analysis (2.2 [95% CI 1.3–3.7] and 2.1 [95% CI 1.2–3.5], respectively). Results of the WEE sensitivity analysis were similar to those of the unadjusted analyses, providing no evidence of bias introduced by missing data.
DISCUSSION
Approximately one-third of the hip fracture patients in this study experienced one or more new pressure ulcers during the 32 days following hospitalization for fracture. The rate at which pressure ulcers occurred was highest in the acute hospital setting, both during the initial stay and re-hospitalization. Adjusting for baseline and time-dependent pressure ulcer risk factors and days since initial hospital admission attenuated the relative rates of APU in the acute settings. However, even in the fully adjusted model, APU rates were more than twice as high in the acute setting as in the home setting. This suggests that setting-specific care-related factors may account for at least some of the higher risk in the acute care setting. A higher incidence in acute care could occur if recommended prevention guidelines (9–10) are not followed. Alternatively, it may be that recommended interventions are implemented but are not effective. In this regard, there is an urgent need to develop and rigorously evaluate innovative pressure ulcer prevention interventions.
In our study, 14.6% of patients experienced an APU while in the initial acute setting. Previous studies in which medical charts were used to identify pressure ulcers (27–33) have yielded lower estimates (weighted mean incidence 10.8%, range 6.8%–29.6%). There are only two studies published in the last 10 years in which pressure ulcers stage 2 and higher were identified by clinical examination of hip fracture patients, as in the current study. The incidence in these studies was 20% (n=121) (34) and 24% (n=45) (35), somewhat higher than in our study. These results may not be comparable as these studies were done in Europe and, in at least one of them (35), the mean hospital stay was much longer than in our study.
The incidence of APU was lowest in the home setting. This is not surprising given that, by the time most patients go home, they have recovered to some extent from their surgery and have regained some degree of mobility. However, even after adjusting in Model 4 for pressure ulcer risk factors and time since hospital admission, the home setting had the lowest APU rate. It is possible that the more personalized and intensive care that can be provided in the patient’s home results in more effective prevention of pressure ulcers than in institutional settings.
Previous studies of pressure ulcers in hip fracture patients have focused on a single type of facility, usually hospitals. A unique strength of this study is that we used a patient-centered design in which pressure ulcer risk was assessed as patients moved through different care settings over a three-week period. Focusing on the patient, rather than on the facility, is an essential first step to understanding potential gaps in care. Another strength is that we used intensive pressure ulcer detection methods by expert research nurses using an assessment protocol that has previously been shown to have high intrarater reliability and validity, and acceptable interrater reliability (36). A third strength is that the study included a large number of hospitals and postacute facilities and had a fairly large patient sample, particularly when compared to previous studies that used direct examination to identify pressure ulcers.
Selection bias is a possible study limitation given that 38% of eligible patients were not enrolled. Although enrolled patients were almost identical to non-enrolled patients with respect to age and sex, they may have differed on other factors that are related to pressure ulcer risk. Patients who were lost to follow-up had worse health status, compared to patients who completed the study, with respect to several baseline indicators, suggesting that pressure ulcer incidence may be even higher than estimated in this study. Since pressure ulcer stages were defined according to the NPUAP criteria that were in use at the time the study began (12), suspected deep tissue injury was not considered as a separate stage as is recommended in current staging criteria (1). The fact that a large number of pressure ulcer risk factors (37), including baseline characteristics, time-dependent factors, and time since initial hospital admission, were adjusted for in the multivariable analysis increases the validity of our study findings. Still, as in all observational studies, the possibility of residual confounding by unmeasured risk factors or protective factors cannot be excluded. Finally, there may have been errors in classifying pressure ulcers observed at baseline as being acquired versus pre-existing. However, a sensitivity analysis revealed that the results were almost unchanged even if all possibly acquired pressure ulcers observed at baseline were considered to be pre-existing.
The outcome of interest in this study was development of a first acquired pressure ulcer. Thus, once an APU was observed, the patient was no longer considered to be at risk. We decided to focus on the first APU because the impact on patient management of the transition from having no pressure ulcers to having a first pressure ulcer is greater than the impact of an increasing number of pressure ulcers beyond the first. This is because a first pressure ulcer triggers the need for interventions to treat the pressure ulcer and also to prevent the development of additional pressure ulcers. The associations between care setting and pressure ulcer incidence may have been different if the outcome had included subsequent pressure ulcers.
Hip fracture is a common, painful, and costly geriatric condition. There are more than 317,000 hospital admissions for hip fracture every year in the US, with an estimated aggregate cost of nearly $4 billion for hospital care alone (38). Almost all hip fractures occur in people age 70 and over (39) and for many patients, the hip fracture triggers mobility loss, dependence, and reduced quality of life (15). The results of the current study indicate that pressure ulcers are a common complication of hip fracture and that the highest incidence occurs in the hospital setting. In light of the recent introduction of Medicare’s non-reimbursement policy, our results suggest that hip fracture patients are an important group to target for the prevention of acquired pressure ulcers, especially in hospitals.
Results of this study also indicate that pressure ulcer risk persists, albeit diminished, after discharge from the hospital, with 18.2% of patients still having an APU at the end of the three-week study period. Although efforts are made to mobilize hip fracture patients as soon as possible after surgery, patients may spend much of their time seated in a chair where pressure relief is more difficult than on an appropriate mattress (40). Thus, although the acute hospital is the setting where pressure ulcer risk is highest, our findings should also increase awareness of the importance of pressure ulcer prevention among hip fracture patients in postacute settings and on maintaining the continuity of care across transitions between settings (14,41,42).
Questions remain about the chronicity of pressure ulcers that arise in the first few weeks following hip fracture and about the degree to which they influence long term outcomes. Studies are needed in which patients are followed for up to one year post-fracture to evaluate the impact of pressure ulcers on functional recovery, quality of life, and cost of care.
ACKNOWLEDGMENTS
Funding:
Supported by grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases (5R01 AR 47711); University of Maryland General Clinical Research Center Grant, General Clinical Research Centers Program, National Center for Research Resources (M01 RR 16500); and National Institute on Aging Claude D. Pepper Older Americans Independence Center (P30 AG028747); National Institute on Aging (T32-AG00262).
* Dr. Magaziner has grants from Merck and Co. and Novartis, Inc. He is also a consultant for Amgen and has given talks for Novartis.
Footnotes
Earlier versions of the results of this study were presented at the following meetings;
- Pressure ulcers and the transition between care settings. Center for Medicare & Medicaid Services Educational Series: Baltimore, 2006.
- Pressure ulcers and the transition between the hospital and nursing home. Quality Improvement Organization Skilled Nursing Facility Teleconference: Quality Partners of Rhode Island Nursing Home Quality Improvement Support Center, 2006.
- Pressure ulcers in elderly hip fracture patients across the continuum of care. Annual Pressure Ulcer Prevention Consensus Meeting, Idaho Pressure Ulcer Prevention Coalition: Boise ID, 2007.
- Baumgarten M, Hawkes WG, Langenberg P, Magaziner J, Margolis D, Orwig D, Palmer MH, Shardell MD, Sterling R. Pressure ulcers in elderly hip fracture patients across the continuum of care: final results. Gerontological Society of America meeting: San Francisco CA, 2007.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Authors’ Contributions:
Baumgarten: Study leadership, study concept, acquisition of funding, study design, oversight of data collection, data management and analysis, data interpretation, preparation of manuscript
Margolis: Study concept, study design, oversight of data collection, data interpretation, preparation of manuscript
Orwig: Study design, oversight of data collection, data management, data interpretation, preparation of manuscript
Shardell: Oversight of data collection, data management and analysis, data interpretation, preparation of manuscript
Hawkes: Study design, oversight of data collection, data management and analysis, data interpretation, preparation of manuscript
Langenberg: Study design, data analysis, data interpretation, preparation of manuscript
Palmer: Oversight of data collection, data interpretation, preparation of manuscript
Jones: Study design, oversight of data collection, data management and analysis, data interpretation, preparation of manuscript
McArdle: Oversight of data collection, data management and analysis, data interpretation, preparation of manuscript
Sterling: Study design, oversight of data collection, data interpretation, preparation of manuscript
Kinosian: Oversight of data collection, data interpretation, preparation of manuscript
Rich: Data collection, data management and analysis, data interpretation, preparation of manuscript
Sowinski: Oversight of data collection, data management, data interpretation, preparation of manuscript
Magaziner: Oversight of data collection, data interpretation, preparation of manuscript
Sponsor’s Role: None
REFERENCES
- 1.Black J, Baharestani M, Cuddigan J, et al. National Pressure Ulcer Advisory Panel's updated pressure ulcer staging system. Dermatol Nurs. 2007;19:343–349. [PubMed] [Google Scholar]
- 2.Roghmann MC, Siddiqui A, Plaisance K, et al. MRSA colonization and the risk of MRSA bacteraemia in hospitalized patients with chronic ulcers. J Hosp Infect. 2001;47:98–103. doi: 10.1053/jhin.2000.0903. [DOI] [PubMed] [Google Scholar]
- 3.Spilsbury K, Nelson A, Cullum N, et al. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs. 2007;57:494–504. doi: 10.1111/j.1365-2648.2006.04140.x. [DOI] [PubMed] [Google Scholar]
- 4.Allman RM, Goode PS, Burst N, et al. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care. 1999;12:22–30. [PubMed] [Google Scholar]
- 5.Kumar RN, Gupchup GV, Dodd MA, et al. Direct health care costs of 4 common skin ulcers in New Mexico Medicaid fee-for-service patients. Adv Skin Wound Care. 2004;17:143–149. doi: 10.1097/00129334-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Whittington KT, Briones R. National Prevalence and Incidence Study: 6-year sequential acute care data. Adv Skin Wound Care. 2004;17:490–494. doi: 10.1097/00129334-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. Washington DC: US Government Printing Office; 2000. Healthy People 2010: Understanding and improving health. [Google Scholar]
- 8.Lyder C, van Rijswijk L. Pressure ulcer prevention and care: preventing and managing pressure ulcers in long-term care: An overview of the revised federal regulation. Ostomy Wound Manage. 2005;(Suppl):2–6. [PubMed] [Google Scholar]
- 9.Panel for the Prediction and Prevention of Pressure Ulcers in Adults. Rockville MD: US Department of Health and Human Services; 1992. Pressure ulcers in adults: prediction and prevention. Clinical practice guideline, number 3. [Google Scholar]
- 10.Ratliff CR. WOCN's evidence-based pressure ulcer guideline. Adv Skin Wound Care. 2005;18:204–208. doi: 10.1097/00129334-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal MB. Nonpayment for performance? Medicare's new reimbursement rule. New Engl J Med. 2007;357:1573–1575. doi: 10.1056/NEJMp078184. [DOI] [PubMed] [Google Scholar]
- 12.National Pressure Ulcer Advisory Panel. Reston VA: National Pressure Ulcer Advisory Panel; 2001. Pressure ulcers in America: prevalence, incidence, and implications for the future. [DOI] [PubMed] [Google Scholar]
- 13.Gehlbach SH, Avrunin JS, Puleo E. Trends in hospital care for hip fractures. Osteoporos Int. 2007;18:585–591. doi: 10.1007/s00198-006-0281-0. [DOI] [PubMed] [Google Scholar]
- 14.Morris AH, Zuckerman JD. National Consensus Conference on Improving the Continuum of Care for Patients with Hip Fracture. J Bone Joint Surg Am. 2002;84A:670–674. doi: 10.2106/00004623-200204000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55:M498–M507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom N, Allman R, Alvarez O, et al. Treatment of pressure ulcers. Rockville MD: US Department of Health and Human Services; 1994. [Google Scholar]
- 18.Baumgarten M, Margolis D, Localio AR, et al. Pressure ulcers among elderly patients early in the hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61A:749–754. doi: 10.1093/gerona/61.7.749. [DOI] [PubMed] [Google Scholar]
- 19.Detsky AS, Smalley PS, Chang J. Is this patient malnourished? JAMA. 1994;271:54–58. doi: 10.1001/jama.271.1.54. [DOI] [PubMed] [Google Scholar]
- 20.Keeler EB, Kahn KL, Draper D, et al. Changes in sickness at admission following the introduction of the prospective payment system. JAMA. 1990;264:1962–1968. [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Detsky AS, Baker JP, O'Rourke K, et al. Predicting nutrition-associated complications for patients undergoing gastrointestinal surgery. J Parenter Enteral Nutr. 1987;11:440–446. doi: 10.1177/0148607187011005440. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 24.Breslow NE, Day NE, editors. The design and analysis of cohort studies. Lyon, France: International Agency for Research on Cancer; 1987. Statistical methods in cancer research, vol. 2. (IARC scientific publication no. 82). [PubMed] [Google Scholar]
- 25.McCullagh P, Nelder JA. Generalized linear models. 2nd edition. London: Chapman and Hall; 1989. [Google Scholar]
- 26.Robins JM, Rotnitzky A, Zhao LP. Estimation of regression coefficients when some regressors are not always observed. J Am Statist Assoc. 1994;89:846–866. [Google Scholar]
- 27.Soderqvist A, Ponzer S, Tidermark J. Cognitive function and pressure ulcers in hip fracture patients. Scand J Caring Sci. 2007;21:79–83. doi: 10.1111/j.1471-6712.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 28.Hommel A, Ulander K, Thorngren KG. Improvements in pain relief, handling time and pressure ulcers through internal audits of hip fracture patients. Scand J Caring Sci. 2003;17:78–83. doi: 10.1046/j.1471-6712.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 29.Peich S, Calderon-Margalit R. Reduction of nosocomial pressure ulcers in patients with hip fractures: A quality improvement program. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2004;17:75–80. doi: 10.1108/09526860410526682. [DOI] [PubMed] [Google Scholar]
- 30.Halm EA, Magaziner J, Hannan EL, et al. Frequency and impact of active clinical issues and new impairments on hospital discharge in patients with hip fracture. Arch Int Med. 2003;163:107–112. doi: 10.1001/archinte.163.1.107. [DOI] [PubMed] [Google Scholar]
- 31.Stotts NA, Deosaransingh K, Roll FJ, et al. Underutilization of pressure ulcer risk assessment in hip fracture patients. Adv Wound Care. 1998;11:32–38. [PubMed] [Google Scholar]
- 32.Baumgarten M, Margolis DJ, Berlin JA, et al. Risk factors for pressure ulcers among elderly hip fracture patients. Wound Rep Reg. 2003;11:96–103. doi: 10.1046/j.1524-475x.2003.11204.x. [DOI] [PubMed] [Google Scholar]
- 33.Rademakers LMF, Vainas T, van Zutphen SWAM, et al. Pressure ulcers and prolonged hospital stay in hip fracture patients affected by time-to-surgery. Eur J Trauma Emerg Surg. 2007;33:238–244. doi: 10.1007/s00068-007-6212-8. [DOI] [PubMed] [Google Scholar]
- 34.Houwing R, Rozendaal M, Wouters-Wesseling W, Buskens E, Keller P, Haalboom J. Pressure ulcer risk in hip fracture patients. Acta Orthop Scand. 2004;75:390–393. doi: 10.1080/00016470410001132-1. [DOI] [PubMed] [Google Scholar]
- 35.Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Reduced incidence of pressure ulcers in patients with hip fractures: A 2-year follow-up of quality indicators. Int J Qual Health Care. 2001;13:399–407. doi: 10.1093/intqhc/13.5.399. [DOI] [PubMed] [Google Scholar]
- 36.Localio AR, Margolis D, Kagan SH, et al. Use of photographs for the identification of pressure ulcers in elderly hospitalized patients: validity and reliability. Wound Rep Reg. 2006;14:506–513. doi: 10.1111/j.1743-6109.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 37.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.HCUP Nationwide Inpatient Sample Healthcare Cost and Utilization Project (HCUP) Rockville MD: Agency for Healthcare Research and Quality; 2005. [accessed April 7, 2008]. http://www.hcup-us.ahrq.gov/reports/factsandfigures/HAR_2005.pdf. [PubMed] [Google Scholar]
- 39.Benetos IS, Babis GC, Zoubos AB, et al. Factors affecting the risk of hip fractures. Injury. 2007;38:735–744. doi: 10.1016/j.injury.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Bliss M, Simini B. When are the seeds of postoperative pressure sores sown? Often during surgery. Br Med J. 1999;319:863–864. doi: 10.1136/bmj.319.7214.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaupre LA, Jones CA, Saunders LD, et al. Best practices for elderly hip fracture patients. A systematic overview of the evidence. J Gen Int Med. 2005;20:1019–1025. doi: 10.1111/j.1525-1497.2005.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coleman EA. Falling through the cracks: Challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51:549–555. doi: 10.1046/j.1532-5415.2003.51185.x. [DOI] [PubMed] [Google Scholar]