Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers (original) (raw)
Abstract
Ashwagandha (Withania somnifera) (WS), a “rasayana” drug, is recommended for balavardhan and mamsavardhan. The study was intended to evaluate dose-related tolerability, safety, and activity of WS formulation in normal individuals. The design was prospective, open-labeled, variable doses in volunteers. Eighteen apparently healthy volunteers (12M:6F, age:18-30 years, and BMI: 19-30) were enrolled. After baseline investigations, they received WS capsules (Rx) (aqueous extract, 8:1) daily in two divided doses with increase in daily dosage every 10 days for 30 days (750 mg/day ×10 days, 1 000 mg/day × 10 days, 1 250 mg/day × 10 days). Volunteers were assessed for symptoms/signs, vital functions, hematological and biochemical organ function tests. Muscle activity was measured by hand grip strength, quadriceps strength, and back extensor force. Exercise tolerance was determined using cycle ergometry. Lean body weight and fat% were computed from skin fold thickness measurement. Adverse events were recorded, as volunteered by the subjects. Repeated measures ANOVA, McNemar's test, and paired t test were employed. All but one volunteer tolerated WS without any adverse event. One volunteer showed increased appetite, libido, and hallucinogenic effects with vertigo at the lowest dose and was withdrawn from study. In six subjects, improvement in quality of sleep was found. Organ function tests were in normal range before and after the intervention. Reduction in total- and LDL- cholesterol and increase of strength in muscle activity was significant. Total body fat percentage showed a reduction trend. WS, in escalated dose, was tolerated well. The formulation appeared safe and strengthened muscle activity. In view of its traditional Rasayana use, further studies are planned to evaluate potential of this drug in patients of sarcopenia.
Keywords: Ayurvedic plant drug, exercise tolerance, muscle activity, muscle strength, Rasayana drug, Withania somnifera
INTRODUCTION
Ashwagandha (Withania somnifera) (WS) has been described in Ayurvedic literature as a Rasayana drug and is ascribed with the properties of _balya_[1] and mamsavivardhan.[2] In traditional clinical practice, WS root powder preparations are most commonly prescribed for enhancing the general strength. Our previous study of WS in peri- and post-menopausal women showed improvement in the quality of life, fatigability, and weakness.[3] Primary objective of this exploratory study, under Reverse Pharmacology,[4] was to evaluate effects of incremental doses of WS on physiological, biochemical functions and vital signs in normal volunteers and assess its safety and tolerability. Secondary objective was to evaluate effects of WS on body fat%, lean body weight, muscle strength, and exercise tolerance.
MATERIALS AND METHODS
After approval of the institutional Ethics Committee for Research on Human Subjects (ECRHS) and written informed consent from each volunteer, 18 volunteers (12M and 6F) were enrolled for the study within 14 days of the screening for physical examination, routine biochemical tests including organ function tests, X-ray chest, and electrocardiogram. Volunteers of either sex in the age group of 18 to 30 years, BMI within 19 to 30 kg/m2, with no history of any medication/health promoting supplements/exercise program in the preceding 1 month were included. Subjects who received/donated blood in the three months preceding screening were excluded.
WS extract was available as gelatine capsules in the sizes of 250 and 500 mg in a plastic container with plastic lid containing silica gel. Each container had 30 capsules. The capsules were stored in at room temperatures (24-32°C) and protected from light.
Each volunteer received three doses increased every 10 days. Thus, 750 mg/day (equivalent to 6 g of crude pulverized roots of WS) was administered orally for first 10 days, 1000mg/day (equivalent to 8 g) for next 10 days, and 1250mg/day (equivalent to 10 g) for last 10 days. The dosing schedule was as follows: 250 mg in the morning and 500 mg at night from Day1 to 10; 500 mg in the morning and 500 mg at night from Day 11 to Day 20; and 500 mg in the morning and 750 mg at night from Day 21 to Day 30.
The activity was assessed for exercise tolerance (by Cycle Ergometer), muscle strength (by Hand grip force, Quadriceps force, and Back extensor force), and body fat percentage and lean body weight (by measuring skin fold thickness at four areas such as Biceps, Triceps, Subscapular, and Suprailiac with a Skin Fold Caliper in millimeters).
Tolerability was assessed by careful recording of adverse events and judging their causal relationship to the test drug. Complete hemogram, liver function tests, renal function tests, fasting sugar, lipid profile, and ECG were performed at baseline, 11th, 21st, and 31st day to assess clinical safety profile.
Data were expressed as mean ± S.D. Repeated measures ANOVA was used to compare between different time points. P<0.05 was considered significant. Safety and tolerability data were analyzed using McNemar's test and data on muscle strength, fat%, and exercise tolerability was analyzed using paired t test to compare before and after the exercises. For Ayurvedic parameters, descriptive statistics was used.
RESULTS
Mean age of the 18 volunteers was 24.33 ± 2.14 years (Range: 20-27 years). Mean height was 165.94 ± 7.43 cm (Range: 155-175 cm). Baseline mean weight was 66.65 ± 8.79 kg (Range: 56-91 kg), and mean BMI was 24.28 ± 2.70 kg/m2. All volunteers were non-smokers, non-tobacco chewers, and non-alcoholic.
Majority of the volunteers did not show any noteworthy baseline symptoms. No abnormality was detected on clinical examination during the study period. No clinically significant change was found in pulse, temperature, systolic and diastolic blood pressure. Average mean body weight and BMI of the volunteers did not show significant change throughout the study.
Hematological parameters such as total white blood cells count, differential count, red blood cells count, hemoglobin, platelets count, and ESR remained within normal range in all the visits for all the volunteers.
No significant change was observed in Serum Bilirubin, Proteins, Albumin, Alanine Transaminase, Aspartate Transaminase, and Alkaline Phosphatase at all the visits in each of the volunteers and it remained within normal range.
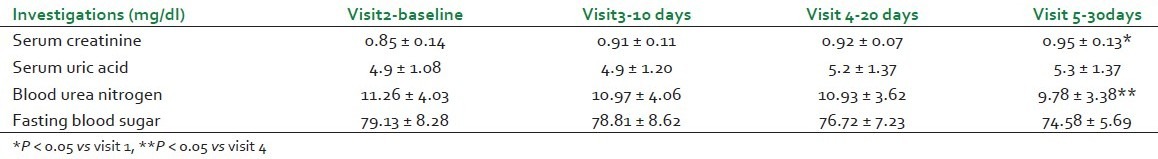
No significant change in serum uric acid and fasting blood sugar was seen throughout the study. However, average serum creatinine had increased and blood urea nitrogen had decreased at visit 5 as compared with visit 1 [Table 1].
Table 1.
Renal function tests, S. Uric acid, and fasting blood sugar
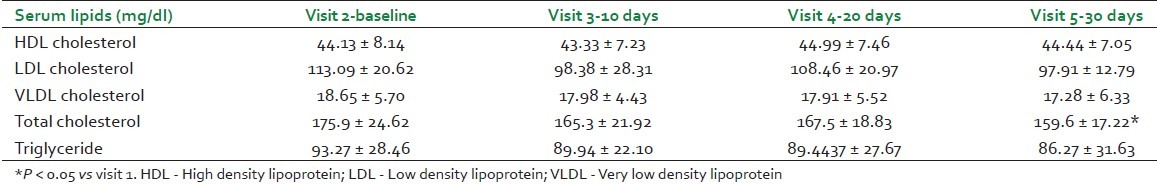
Significant decrease was seen in total cholesterol at visit 5 as compared to visit 1, and decreasing trend in each follow-up visit was observed in triglycerides. However, no significant change in serum HDL, LDL, and VLDL cholesterol was seen [Table 2].
Table 2.
Lipid profile
All the three tests for the assessment of muscle strength viz. hand grip force, quadriceps force, and back extensor force showed an increase from visits 2 to 5. The increase in quadriceps force and back extensor force was gradual and statistically significant [Table 3].
Table 3.
Assessment of physical parameters
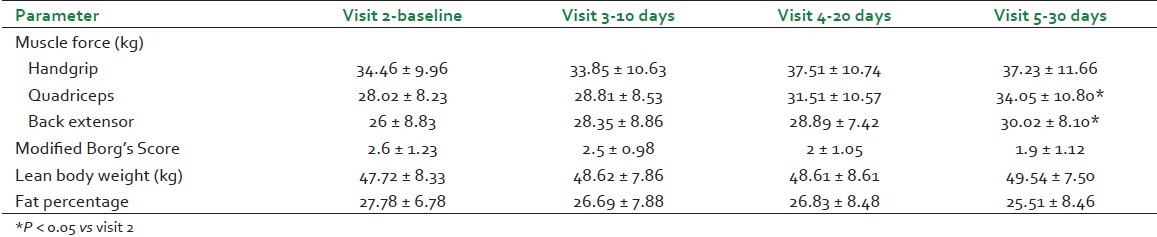
Cycle ergometer exercise assessment for heart rate, respiratory rate, maximum heart rate intensity percentage, energy expended post-exercise, distance covered in six minutes, speed reached in six minutes, and performance power percentage remained within normal range; however, the modified Borg's score[5] indicative of breathlessness index has shown gradual improvement [Table 3].
Assessment of lean body weight has shown gradual increase in every visit, whereas fat percentage has shown gradual decrease from visit 2 to visit 5 [Table 3].
DISCUSSION
This volunteer study has essentially ensured the hematological and biochemical organ function safety of WS in higher doses from 6 to 10 g of crude pulverized roots of WS administered in aqueous extract form. Although the doses were given in gradual incremental schedule, each dose was administered for 10 days and no immediate intolerance or any adverse effects were observed in vital functions such as body temperature, pulse rate, respiratory rate, systolic and diastolic blood pressure. No significant change was reported in volunteers’ appetite, bowel and bladder habits. The duration of sleep although did not exhibit significant change, the quality of sleep reportedly improved in 6 volunteers. In Ayurvedic literature[6] and traditional practice, “_Needrajanan_” (sleep induction) is one of the important clinical indications and products of WS for sleep are available in Indian market.
All but one volunteer tolerated the increased doses of WS. The volunteer who reported diverse clinical symptoms on third day of drug administration was withdrawn from the drug on sixth day and all the symptoms disappeared within a couple of days. The symptoms manifested were unusual increase in appetite, libido, and hallucinogenic effects with vertigo. WS is known for its CNS active properties.[7] Although clinical/experimental studies have demonstrated anxiolytic,[8,9] antidepressant,[10,11] and neuroprotective activity[12–14] of WS extracts or active molecules, the hallucinogenic activity reported in this volunteer needs further investigation. WS although is stated in Ayurvedic literature for enhancing the libido,[15] the unusually heightened sexual desire within 3 days of drug administration is difficult to explain. Interestingly, as per Ayurvedic protocol, this volunteer has a Kapha pradhan Prakruti, and Shukrasar, Rasasar, and Asthisar physical status. This finding may illustrate significance in understanding the constitution of an individual before administering Ayurvedic drug.
Assessment of activity for muscle strength, exercise tolerance, fat percentage, and lean body weight was undertaken in view of its traditional medical use. Stepwise increment in groups of muscle force at three different locations such as upper extremity, lower extremity, and trunk along with gradual increase in serum creatinine levels within normal range suggest muscle mass promotive activity.[16] The increase in creatinine is less likely due to adversity of renal function since blood urea nitrogen is concomitantly decreased. These findings support the classical description and traditional practices of WS being mentioned and used as mamsawardhak. In past, a volunteer study[17] has shown improvement in hand grip strength with WS. The loss of muscle mass, strength, and muscle performance in postmenopausal and aging population need attention. Our team has been studying sarcopenia at menopausal transition and beyond.[18]
The decreasing trend in fat% and simultaneous increase in lean body weight compliment the findings of muscle promotive activity. It was noteworthy that total body weight and BMI did not change significantly; further interesting was to note the significant reduction in total cholesterol and gradual decreasing trend in triglycerides. Lipid-lowering activity of WS has been reported earlier in hypercholesterolemic subjects within 30 days.[19] Another interesting finding was gradual improvement in modified Borg's score of breathlessness observed post-cycle ergometer exercise. Recently, improvement is reported in the physical performance and strength parameters in healthy volunteers receiving WS aqueous extract 500 mg/day for 8 weeks.[20] All these findings objectively demonstrate the balya property of WS.
CONCLUSIONS
The volunteer study demonstrates that WS, when given in the form of aqueous extract in capsules with gradual escalating doses from 750 to 1250mg/day, was well tolerated. The formulation was found to be safe on hematological and biochemical organ function tests. This study has also demonstrated muscle strengthening, lipid lowering, and improved quality of sleep in view of its traditional use as balya. Further studies are planned to evaluate potential of this drug in patients of sarcopenia.
Footnotes
Source of Support: Indian Council for Medical Research (ICMR)
Conflict of Interest: None declared.
REFERENCES
- 1.Shastri B. In: Guduchyadi varg. 9th ed. Bhavprakash-Vidyotini HV, editor. Varanasi: Chowkhamba Sanskrit Sansthan; 1999. pp. 393–4. [Google Scholar]
- 2.Tripathi I. In: Vaatvyadhiprakaran, Sootra 90. 2nd ed. Chakrpanidatt VC, Vaidyprabha HV, editors. Varanasi: Chowkhamba Sanskrit Sansthan; 1994. pp. 132–58. [Google Scholar]
- 3.Pandey S. Proceedings of ICMR Symposium. Mumbai: Kasturba Health Society; 2008. Changes in Body Composition: Metabolic Syndrome and Sarcopenia in Menopause; pp. 112–32. [Google Scholar]
- 4.Vaidya AD. Reverse Pharmacology a paradigm shift for new drug discovery based on Ayurvedic epistemology. In: Muralidharan TS, Raghava V, editors. Ayurveda in Transition. 1st ed. Kottakkal, Kerala: Arya Vaidya Sala; 2010. pp. 27–38. [Google Scholar]
- 5.Burdon JG, Juniper EF, Killian KJ, Hargrave FE, Campbell EJ. The perception of breathlessness in asthma. Am Rev Respir Dis. 1982;126:825–8. doi: 10.1164/arrd.1982.126.5.825. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya BG. In: Kantakaryaadi varg. 2nd ed. Nighantu A, editor. Varanasi: Chowkhamba Bharati Acadamy; 1999. pp. 134–41. [Google Scholar]
- 7.Vaidya AD. The status and scope of Indian medicinal plants acting on central nervous system. Indian J Pharmacol. 1997;29:340–3. [Google Scholar]
- 8.Andrade C, Aswath A, Chaturvedi SK, Srinivasa M, Raguram R. A double blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295–301. [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta GL, Rana AC. Effect of Withania somnifera dunal in ethanol-induced anxiolysis and withdrawal anxiety in rats. Indian J Exp Biol. 2008;46:470–5. [PubMed] [Google Scholar]
- 10.Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7:463–9. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 11.Gupta GL, Rana AC. Protective effect of Withania somnifera dunal root extract against protracted social isolation induced behavior in rats. Indian J Physiol Pharmacol. 2007;51:345–53. [PubMed] [Google Scholar]
- 12.Ahmad M, Saleem S, Ahmad A, Ansari A, Yousuf S, Hoda MN, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–47. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Seal CJ, Howes MJ, Kite GC, Okello EJ. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and β-amyloid(1-42)-induced cytotoxicity in differentiated PC12 cells. Phytother Res. 2010;24:1567–74. doi: 10.1002/ptr.3261. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar M, Sharma D, Salvi M. Neuroprotective effects of Withania somnifera dunal: A possible mechanism. Neurochem Res. 2009;34:1975–83. doi: 10.1007/s11064-009-9987-7. [DOI] [PubMed] [Google Scholar]
- 15.Sharma G. Guduchyadivarga. In: Sharma P, editor. Dhanvantari Nighantu. 1st ed. Varanasi: Chaukhambha Oriental; 1982. p. 64. [Google Scholar]
- 16.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–54. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataraghavan S, Seshadri C, Sundaresan TP, Revathi R, Rajagopalan V, Janaki K. The comparative effect of milk fortified with Ashwagandha and punarnava in children – a double-blind study. J Res Ayur Sid. 1980;1:370–85. [Google Scholar]
- 18.Vaidya R, Pathak N, Raut A, Patkar D, Vaidya A. Sarcopenia at menopause: Is it phase specific? In: Singh M, editor. Osteoporosis. Mumbai: Indian Menopause Society; Forthcoming. [Google Scholar]
- 19.Andallu B, Radhika B. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol. 2000;38:607–9. [PubMed] [Google Scholar]
- 20.Sandhu JS, Shah B, Shenoy S, Chauhan S, Lavekar GS, Padhi MM. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res. 2010;1:144–9. doi: 10.4103/0974-7788.72485. [DOI] [PMC free article] [PubMed] [Google Scholar]