Mass extinction of lizards and snakes at the Cretaceous–Paleogene boundary (original) (raw)
Abstract
The Cretaceous–Paleogene (K-Pg) boundary is marked by a major mass extinction, yet this event is thought to have had little effect on the diversity of lizards and snakes (Squamata). A revision of fossil squamates from the Maastrichtian and Paleocene of North America shows that lizards and snakes suffered a devastating mass extinction coinciding with the Chicxulub asteroid impact. Species-level extinction was 83%, and the K-Pg event resulted in the elimination of many lizard groups and a dramatic decrease in morphological disparity. Survival was associated with small body size and perhaps large geographic range. The recovery was prolonged; diversity did not approach Cretaceous levels until 10 My after the extinction, and resulted in a dramatic change in faunal composition. The squamate fossil record shows that the end-Cretaceous mass extinction was far more severe than previously believed, and underscores the role played by mass extinctions in driving diversification.
Keywords: evolution, adaptive radiation
Today, lizards and snakes (Squamata) are represented by more than 9,000 living species, which exploit an extraordinary range of ecological niches and habitats; they include insectivores, carnivores, herbivores, and omnivores, as well as terrestrial, arboreal, fossorial, and aquatic forms (1, 2). The history of this radiation extends deep into the Mesozoic. After the appearance of crown squamates in the Jurassic (3), lizards and snakes underwent a Cretaceous radiation (4, 5), and by the late Cretaceous most major groups had appeared, including iguanians, geckos, skinks, anguids, and platynotans (3, 4), as well as many snake lineages (5). However, with the exception of the marine mosasaurs, all major squamate lineages are thought to have survived the end of the Cretaceous. Consequently, the Cretaceous–Paleogene (K-Pg) extinction that ended the Mesozoic is considered to have had little effect on squamate evolution (4, 6, 7).
The K-Pg extinction represents one of the most severe mass extinctions in the history of life (8). Its causes remain a matter of debate (7, 9–12), but suggest that the extinction resulted from the Chicxulub asteroid impact (9–12), when debris shot into the atmosphere by the impact would have darkened the skies, causing a shutdown of photosynthesis and subsequent collapse of the food chain (10–13). Terrestrial ecosystems were particularly hard hit. Notably, the nonavian dinosaurs became extinct at this time (9), but the K-Pg event also resulted in severe extinctions among mammals (14), birds (15), insects (16), and plants (17). Given this, it would be remarkable had squamates not been affected.
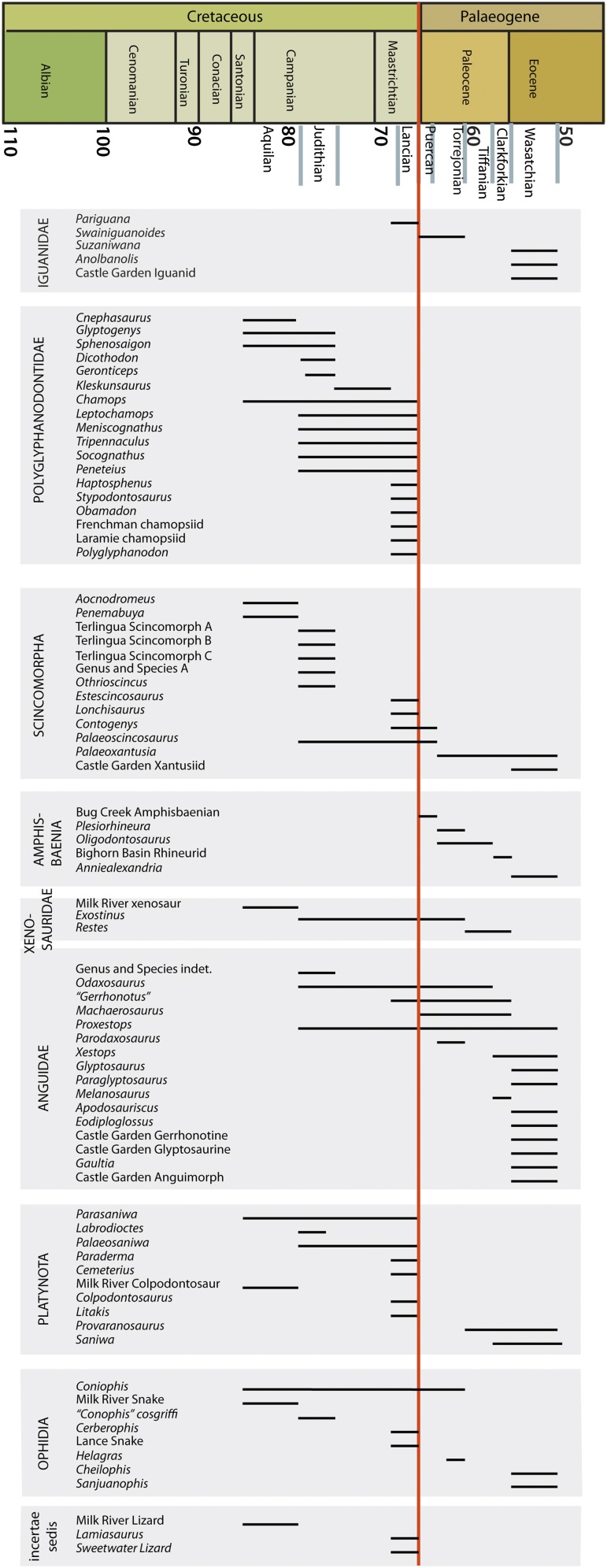
In this light, we restudied the squamate fossil record across the K-Pg boundary, focusing on North America. Globally, few fossils are available to address this problem, because (i) squamates are generally small and lightly built, limiting their preservation potential, and (ii) few terrestrial sequences span the K-Pg boundary (17). Western North America is unique, however, in having a rich record of lizards and snakes from both the late Maastrichtian (5, 18–22) and the early Paleocene (23–27), making this the only place in the world where the problem can be studied. Critically, a chronostratigraphic framework (SI Appendix) makes it possible not only to examine patterns of turnover, but also to constrain the timing of extinction.
The only previous study of this problem focused on the Maastrichtian–Paleocene transition in eastern Montana. High turnover was observed for fossil vertebrates across the K-Pg boundary; among squamates, 3 of 11 Maastrichtian species (27%) persisted into the Paleocene (14, 28). However, the limited focus of that study makes it difficult to discern whether this high turnover was a regional or local event; furthermore, the rarity of Paleocene vertebrate fossils raises the possibility that survivors have been overlooked, and that turnover is exaggerated by sampling artifacts (14). Consequently, the concept of a mass extinction of squamates has received little attention.
Here we combine data from the literature (19–27) and museum collections to create a detailed picture of squamate diversity and disparity in the Maastrichtian and Paleocene of North America. This study includes localities from New Mexico to Alberta (Dataset S1) and includes all known species, as well as previously unrecognized Maastrichtian species (SI Appendix).
Systematic Paleontology
A total of 27 lizards and three snakes occur in the late Maastrichtian of North America, including 21 previously recognized forms (19–22) and nine heretofore unreported species (Fig. 1). Thus, this assemblage ranks as one of the most diverse fossil squamate assemblages yet discovered. These species represent a wide range of squamate lineages, many of which are now extinct (Fig. 2).
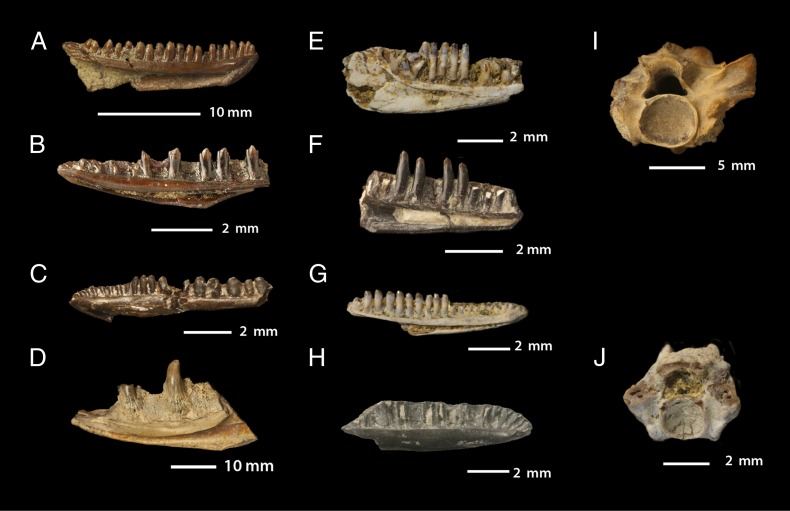
Fig. 1.
Maastrichtian lizards and snakes. (A_–_H) Lizard dentaries; (I and J) snake vertebrae. (A) Socognathus brachyodon, n. sp., YPM-PU 16724 Lance Formation, western WY. (B) Obamadon gracilis, n. gen et sp., UCMP 128873, Hell Creek Formation, MT. (C) Laramie polyglyphanodont, UCM 42164, Laramie Formation, CO. (D) Cemeterius monstrosus n. gen. et sp., USNM 25870, Hell Creek Formation, MT. (E) Pariguana lancensis n. gen et sp., AMNH 22208; Lance Formation, eastern WY. (F) Lamiasaurus ferox n. gen et sp., UW 25116A, Lance Formation, southern WY. (G) Lonchisaurus trichurus n. gen et sp., AMNH 15446, Lance Formation, eastern WY. (H) Sweetwater lizard, UW 25116B, Lance Formation, southern WT. (I) Cerberophis robustus n. gen et sp., UCMP 130696, Hell Creek Formation, MT. (J) Lance alethinophidian, Lance Formation, WY.
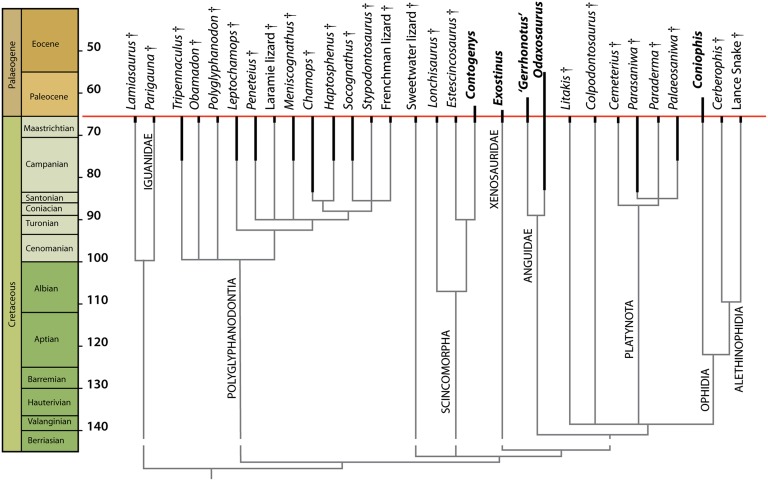
Fig. 2.
Systematics of North American squamate assemblage. Adams consensus with alethinophidian placement following a recent study (5). Complete results are provided in SI Appendix. Species crossing the K-Pg boundary are shown in bold type.
The fauna is dominated by the extinct Polyglyphanodontia. Previously allied with the Teiidae (20–22), these lizards are now recognized as a distinct clade (3, 29) that may lie outside of Scleroglossa entirely (3). Polyglyphanodontians are characterized by a V-shaped dentary symphysis, a long splenial that slots into the subdental shelf, subapical tooth implantation, and tricuspid teeth. Twelve species are present (Fig. 2). The seven previously recognized species are the polyglyphanodontid Polyglyphanodon sternbergi (30) and the chamopsiids Chamops segnis (18), Leptochamops denticulatus (19), Meniscognathus altmani (20), Haptosphenus placodon (20), Stypodontosaurus melletes (21), and Peneteius aquilonius (22). Here two previously identified species are placed in Polyglyphanodontia. One, previously referred to Iguanidae (21), is here identified as the polyglyphanodontian Tripennaculus n. sp. (SI Appendix). A second, unnamed lizard from the Frenchman Formation of Saskatchewan (21) is placed in Chamopsiidae. In addition, three previously unreported species are recognized here. The first of these species is a chamopsiid, Socognathus brachyodon (Fig. 1_A_), referred to Socognathus (21) on the basis of the low yet robust mandible, massive symphysis, and reduced accessory cusps. It differs from Socognathus unicuspis in having closely packed, bulbous teeth (SI Appendix). The second is Obamadon gracilis (Fig. 1_B_), a small polyglyphanodontian distinguished by tall, slender teeth with large central cusps separated from small accessory cusps by lingual grooves (SI Appendix). The third species is an unnamed chamopsiid from Colorado (Fig. 1_C_), characterized by enlarged back teeth (SI Appendix).
Three scincomorphs are present. Contogenys sloani (31) has previously been identified as a stem xantusiid (32) but here is placed in the Globauridae, a group of Cretaceous stem scincids (3). Estescincosaurus cooki (20) represents a scincoid, but its position within the clade cannot be resolved. Lonchisaurus trichurus (Fig. 1_G_) is a previously unrecognized species distinguished by a long straight dentary, numerous closely packed teeth, and weakly curved crowns with broad, bluntly pointed apices. Its affinities within Scincomorpha remain uncertain.
Anguimorphs rival polyglyphanodontians in diversity (Fig. 2). Exostinus lancensis (19) lies on the stem of the extant Xenosaurus (20, 33). Anguidae include Odaxosaurus piger (19), a basal glyptosaurine, and Gerrhonotus spp. (20), a stem gerrhonotine. Litakis gilmorei (20), and Colpodontosaurus cracens (20) are anguimorphs of uncertain affinities, neither of which can be placed in any extant clade.
Four varanoid-like platynotans are present: Palaeosaniwa sp (20), Parasaniwa wyomingensis (19), Paraderma bogerti (20), and one heretofore unreported species, Cemeterius monstrosus (Fig. 1_D_). Cemeterius is a large platynotan distinguished by a massive jaw and short, robust, lingually expanded teeth lacking serrations (SI Appendix). Palaeosaniwa and Paraderma are typically interpreted as crown varanoids (20, 34), but our analysis places these species and Cemeterius on the varanoid stem near Parasaniwa (Fig. 2). Palaeosaniwa is the largest lizard in the assemblage, and also the largest terrestrial lizard known from the Cretaceous; with an estimated snout-vent length (SVL) of 82 cm, it has a predicted mass of 6 kg (Dataset S1).
A single iguanid is present in the assemblage. Until now, there has been no definitive evidence of Iguanidae from the Cretaceous of North America; previous reports of Cretaceous iguanids (21) instead represent polyglyphanodontians. Here we report the oldest iguana known from North America, Pariguana lancensis (Fig. 1_E_). Pariguana shares with iguanians a lip beneath the Meckelian fossa formed by an upfolded ventral margin of the dentary. Posterior extension of the dentary angular process and the anterolateral extension of the coronoid onto the dentary indicate that Pariguana is more closely related to crown Iguanidae than are the Asian stem iguanids. Pariguana resembles Paleocene iguanids (23) in having teeth with a broad central cusp flanked by minute accessory cusps, but is distinguished by constriction of the Meckelian fossa ahead of the anterior inferior alveolar foramen (SI Appendix). The existence of Pariguana shows that dispersal of iguanids from Asia to North America occurred in the Cretaceous, not in the Paleocene as previously thought (3).
Two previously unreported lizards of uncertain affinities are also present. Lamiasaurus ferox (Fig. 1_F_) is distinguished by tall, curved crowns with constricted apices and lingual ridges. Present analysis places it as a stem iguanian on the basis of the short splenial. A second, unnamed species (Fig. 1_H_) comes from southern Wyoming; it represents a nonanguimorph autarchoglossan.
Three snakes are known: the stem snake Coniophis precedens (18) and two alethinophidians (5). Cerberophis robustus (Fig. 1_I_) is a large basal alethinophidian (5) previously referred to Boidae (28). Cerberophis is distinguished by broad vertebrae with hypertrophied synapophyses, and enlarged prezygapophyses with accessory ridges anteriorly (SI Appendix). A vertebral width of 17 mm implies an SVL >1.7 m and a mass >2.9 kg (Dataset S1), making Cerberophis large enough to potentially prey on any of the Cretaceous mammals, as well as on small dinosaurs and hatchlings of larger dinosaurs. The third species is an unnamed small alethinophidian (5) (Fig. 1_J_).
Patterns of Extinction
The fauna shows extremely high turnover across the K-Pg boundary. Of the 30 species found in the late Maastrichtian, only five—Exostinus, “Gerrhonotus,” Odaxosaurus, Contogenys, and _Coniophis_—occur in the Paleocene. Thus, survival and extinction rates are 17% and 83%, respectively. The survival rate is lower than previously estimated (14), because sample sizes are larger, because taxonomic revision has increased the diversity of the Maastrichtian victims, and because the mistaken report of Contogenys from the Hell Creek increased estimates of survival.
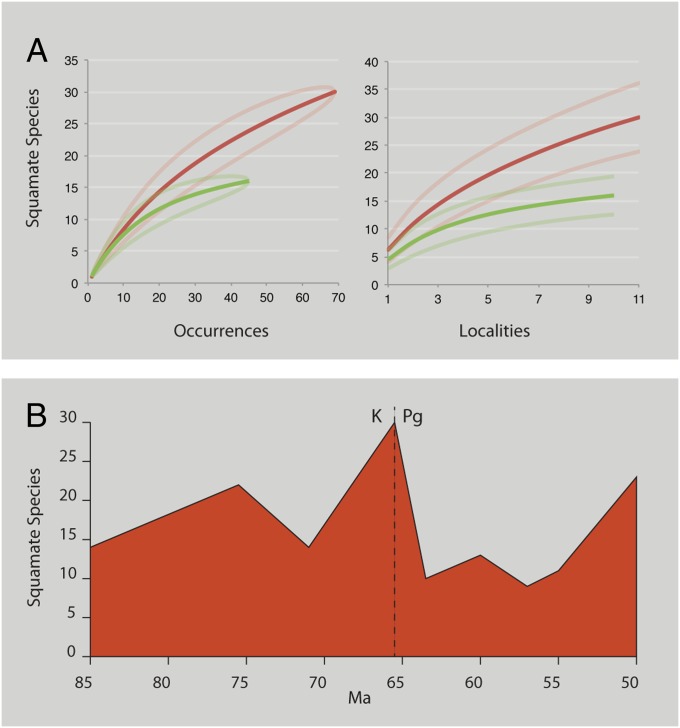
It has been argued that high turnover at the K-Pg boundary is in part an artifact caused by poor sampling in the Paleocene (14). To test this hypothesis, we computed species accumulation curves using rarefaction of occurrence data and sample-based (Mao-Tau) (35) rarefaction (Fig. 3). These sampling curves show that the Paleocene is not undersampled relative to the Maastrichtian. Surprisingly, despite the rarity of Paleocene fossils, the fossil record is actually less complete in the Maastrichtian, because (as discussed below) most victims of the extinction have restricted geographic ranges, making them difficult to find. Insofar as sampling artifacts distort the picture, they may make the extinction appear less severe than it actually was.
Fig. 3.
Comparisons of late Maastrichtian and Paleocene diversity. (A) Late Maastrichtian (Lancian) and early Paleocene (Puercan-Torrejonian). (B) Diversity of lizard genera by land vertebrate age (using range-through assumption), Aquilan to Wasatchian.
The extinction resulted in the loss of the most diverse clade of Late Cretaceous lizards, the Polyglyphanodontia, which represents some 40% of Maastrichtian diversity. Many other lineages, including the lineages represented by Palaeosaniwa, Litakis, Colpodontosaurus, Lamiasaurus, and Cerberophis, disappeared as well. Although we do not use a Linnean taxonomy, these lineages are arguably sufficiently phylogenetically and morphologically distinct to warrant family rank. Thus, the absence of extinction among terrestrial lizard “families” (7) is a taxonomic artifact. In a traditional, Linnean framework (19–21), virtually all families appear to cross the K-Pg boundary (7), because lineages that became extinct were either lumped into modern families or simply excluded from analysis because they could not be classified.
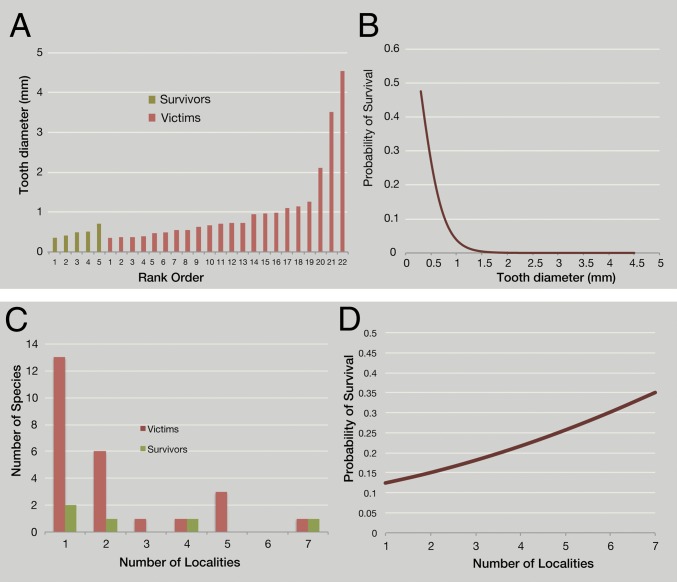
Survival at the K-Pg boundary is highly nonrandom. Small size has been identified as a determinant of survival (36), yet size selectivity is evident even among the squamates. The most striking pattern is the extinction of all large lizards and snakes. The Maastrichtian fauna includes a number of larger forms, including Palaeosaniwa and Cerberophis, but large squamates are conspicuously absent from the Paleocene. The largest known early Paleocene lizard is Provaranosaurus acutus. Comparisons with varanids suggest an SVL of 305 mm and a mass of 415 g (Dataset S1), compared with an estimated SVL of 850 mm and mass of 6 kg for the largest Maastrichtian lizard, Palaeosaniwa. The largest early Paleocene snake is Helagras prisciformis, with an estimated SVL >950 mm and a mass >520 g, compared with >1,700 mm and 2.9 kg for the largest Maastrichtian snake, Cerberophis.
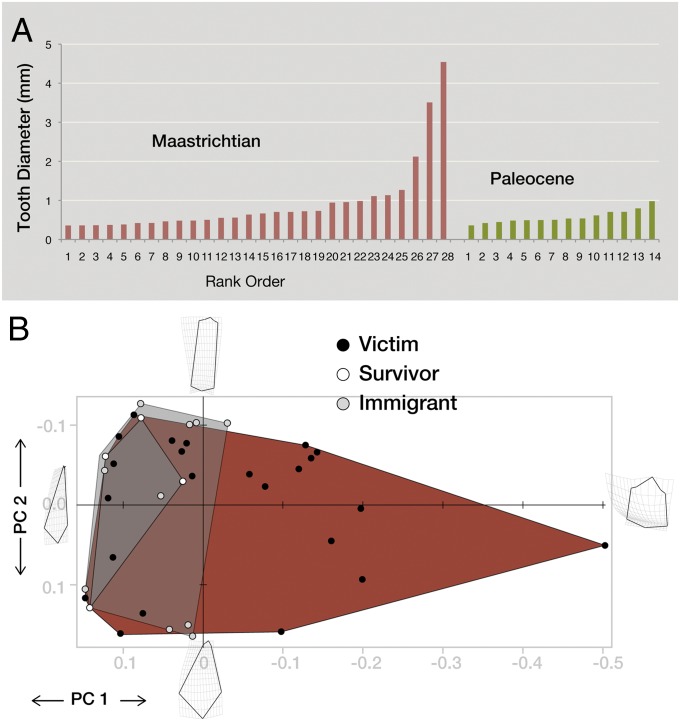
Because tooth diameter is correlated with SVL (Dataset S1), we used tooth diameter to examine the relationship between survival and body size. Average tooth diameter is significantly smaller for the survivors (mean, 0.494 mm) than for victims (mean, 1.07 mm) (P < 0.05, t test). We used logistic regression to quantify how survival probability is affected by size. This approach allowed us to use a continuous independent variable (e.g., tooth diameter) to predict a categorical dependent variable (e.g., survival vs. extinction), and to describe that response with a curve. The relationship between survival and tooth diameter is described by the logistic equation logit(survival) = 0.9881 − 4.1350 × (tooth diameter) (model χ2 = 4.384; df = 1; P = 0.0363), demonstrating how the odds of survival drop precipitously with increasing size (Fig. 4).
Fig. 4.
Selective survivorship of squamates at the K-Pg boundary. (A) Cenograms (size data for species in rank order), with tooth diameter as a proxy for size, for K-Pg survivors and victims. (B) Logistic regression, showing decreasing survival probability at larger sizes. (C) Number of localities occupied by survivors and victims. (D) Logistic regression showing increasing survival probability with increasing geographic range (using number of localities as a proxy for range size).
Analysis of selectivity is complicated by the nonindependence of data points, however. For example, the large stem varanoids are related and thus may share features other than body size, such as foraging strategy, breeding biology, or vulnerability to cold. Selection against one of these traits would eliminate these large-bodied forms, creating the appearance of size selectivity. Thus, we used phylogenetically independent contrasts (PIC) analysis to examine the role of phylogeny (SI Appendix). Contrasts of survivorship against contrasts for tooth diameter continue to show a negative correlation between survival and size, although the correlation is not significant (P = 0.37, two-tailed t test).
The processes behind this size selectivity are not known, but in the absence of photosynthesis, invertebrates feeding on dead plant and animal matter might have been the only available food, favoring the survival of small insectivores (13). In addition, small animals might have been able to seek shelter against the heating pulse caused by re-entering ejecta (11) or heavy frosts caused by impact winter (11).
Size selectivity may help explain why nonavian dinosaurs became extinct, suggesting that it was nonavian dinosaurs’ failure to evolve a diverse fauna of small-bodied species, rather than a decrease in the diversity of large-bodied forms, that ultimately sealed their fate. A number of small, nonavian dinosaurs are now known from the Late Cretaceous, including alvarezsaurids (37) and microraptorine dromaeosaurids (38), and taphonomic biases almost certainly obscure the true diversity of small dinosaurs (38, 39). However, the fact remains that during the late Maastrichtian, small dinosaurs were vastly outnumbered by other small vertebrates, including a minimum of 30 squamates, 18 birds (15), and 50 mammal species (40). Strikingly, birds—the only dinosaurs to survive— were the only dinosaurs with a high diversity of small-bodied (<5 kg) forms (15). In this context, a discussion of a decline in large dinosaur diversity in the Maastrichtian (9) is perhaps beside the point. A high diversity of large herbivores and carnivores in the latest Maastrichtian would have been unlikely to change the fate of the nonavian dinosaurs, because no animals occupying these niches survived. Instead, the rarity of small dinosaurs—resulting perhaps from being outcompeted by squamates and mammals for these niches —led to their downfall.
Survival also may be influenced by biogeography (41). Survivors occur in more localities (mean, 3 localities) than victims (mean, 2.16 localities), as found for marine invertebrates (41); the difference is not statistically significant, however, by the Mann–Whitney U test. The relationship between survival and range is described by the logistic equation logit(survival) = −2.1768 + 0.0228 (occurrences) (Fig. 4). Again, the relationship is positive, but not significant. PIC analysis, plotting contrasts of survivorship against range size, shows a weakly positive but not significant association between survival and range size (P = 0.57, two-tailed t test).
The effects of the K-Pg extinction on squamates also can be shown in terms of morphological disparity. Using tooth diameter as a proxy for body size, it is evident that the early Paleocene fauna has a dramatically reduced range of sizes compared with the Maastrichtian fauna (Fig. 5_A_). Tooth shape disparity, analyzed using principle component analysis and measured in terms of sum of ranges, product of ranges, sum of variances, or product of variances (SI Appendix), also drops across the K-Pg boundary as forms with specialized ziphodont, brachyodont, and tricuspid teeth disappear (Fig. 5_B_). The disparity is not significantly lower than expected, however, given the small number of survivors; thus, we cannot reject the hypothesis that the loss of disparity resulted from extinction that is random with respect to tooth shape, rather than from selection against particular morphotypes (SI Appendix).
Fig. 5.
Disparity of late Maastrichtian and early Paleocene squamates. (A) Cenograms showing Maastrichtian and Paleocene size disparity. (B) Comparison of shape disparity in teeth of Maastrichtian and Paleocene squamates, showing results of a principle components analysis of 2D landmark data. The Maastrichtian fauna is circumscribed by the red hull, with survivors bounded by a dark-gray hull and survivors plus immigrants bounded by a light-gray hull.
Critically, stratigraphic data make it possible to constrain the tempo of turnover, which is necessary to test competing hypotheses about the causes of the extinction. The vast majority of the species that became extinct can be shown to persist high in stratigraphic section, and many have a last occurrence less than 300,000 y before the Chicxulub impact (SI Appendix). Although the precise age of last occurrence cannot be estimated in all cases, we emphasize that the assemblage described here is as close as we can come to providing a picture of the fauna just before the Chicxulub impact. In particular, because many genera persisted for 10 My or longer (Fig. 6), and because vertebrate faunas showed little turnover in the late Maastrichtian (42, 43) it is unlikely that a significant number of these species became extinct in the short intervals separating these localities from the K-Pg boundary.
Fig. 6.
Cretaceous–Paleogene faunal succession. The Cretaceous fauna is dominated by Polyglyphanodontidae, whereas the Paleogene fauna is dominated by Anguidae and Iguanidae.
It has been argued that environmental stresses in the latest Maastrichtian, including climate change and sea level change, contributed to the end-Cretaceous mass extinction by causing a decline in diversity leading up to the K-Pg boundary (9). Discussions of diversity have tended to focus on dinosaurs, but whether they experienced a minor decline during the Maastrichtian (9) or did not (10, 42, 43), the persistence of an exceptionally diverse lizard and snake fauna just before the K-Pg extinction argues against the idea that late Maastrichtian terrestrial ecosystems were stressed before the Chicxulub impact. The available evidence suggests instead that extinctions among the squamates were abrupt and coincided with the K-Pg boundary, supporting the hypothesis that the Chicxulub asteroid impact was the sole cause of the end-Cretaceous mass extinction.
Recovery and Radiation
Extinction at the K-Pg boundary was followed by recovery in the Paleocene and Eocene. A number of new lizard lineages occur in the basal Paleocene, notably the stem varanoid Provaranosaurus, xantusiids, and amphisbaenians (27). These may represent opportunistic invaders that colonized the area in the aftermath to exploit niches left vacant by the extinction, as seen among mammals (10, 44). Despite this, early Paleocene diversity is considerably lower than late Maastrichtian diversity (Fig. 3). Subsequently, ecological release provided by the extinction allowed the survivors to stage an adaptive radiation, paralleling the adaptive radiations staged by mammals (6, 45, 46), birds (46, 47), and fish (48). The community that emerges in the early Eocene is dominated by groups that are either minor components of the Cretaceous fauna or unknown from the Cretaceous, particularly the Anguidae, Iguanidae, Xantusiidae, Macrostomata, and Amphisbaenia (Fig. 6). However, diversity does not approach Cretaceous levels until the early Eocene, 10 My later, when diversity rapidly increases (Fig. 3_B_) with the onset of late Paleocene–early Eocene global warming (49). Thus, lizards recovered more slowly than mammals, which rebounded to Maastrichtian diversity levels within 1.5 My of the K-T extinction (44), and exceeded Cretaceous diversity within 5 My of the extinction (45) as they radiated to occupy niches left open by dinosaurs. Unlike mammals, however, squamates appear to have simply reoccupied the niches they occupied before the extinction. This reoccupation of niches was also delayed; by the middle Paleocene, lizards had yet to recover the range of body sizes and morphotypes found in the Maastrichtian (Fig. 5).
The end-Cretaceous extinction of squamates was likely a global phenomenon. As in North America, the Late Cretaceous of Asia is dominated by archaic forms (50, 51), including Polyglyphanodontia and stem members of Iguanidae, Acrodonta, Scincidae, Gekkota, and Varanidae (3), all of which are absent from the Paleogene. Poor stratigraphic constraint makes it impossible to address the timing of these extinctions, but the dramatic turnover of the Asian fauna from the Cretaceous to the Paleogene (51) is consistent with a worldwide extinction of squamates at the end of the Cretaceous. The disappearance of the giant marine Mosasauridae from the world’s oceans (4, 6, 7) underscores the reach of this extinction. Our analysis focuses on terrestrial forms, but including mosasaurs would increase estimates of extinction rates and especially of disparity loss. Finally, the appearance of lacertids in the Paleocene of Europe (52), derived acrodonts in the Early Eocene of Asia (53), boid snakes in the Paleocene of South America (54), and caenophidian snakes in the Early Eocene of India (55) document the diversification of these lineages in the aftermath of the K-Pg extinction, suggesting that the Paleogene radiation of squamates was global as well.
Discussion and Conclusions
The squamate fossil record shows that the Chicxulub asteroid impact coincides with severe extinction at the species level, the loss of many branches of the squamate tree, and reduced morphological disparity. Although the fossil record (4) and the molecular clock (56) agree that many squamate clades originated in the Cretaceous, perhaps as part of the “Cretaceous Terrestrial Revolution” (57), these patterns are not inconsistent with mass extinction, given that clades can survive the loss of the majority of their members. The K-Pg extinction ultimately led to the diversification of the lizards and snakes that dominate the fauna today, as the ancestors of modern groups literally emerged from the ashes of the asteroid impact. Thus, the squamate fossil record provides a striking example of how mass extinction works as a form of creative destruction, and shows how the origins of the modern biota can be understood only in light of catastrophes occurring in deep time.
Materials and Methods
To investigate the relationships of the Cretaceous lizards, we conducted a morphological phylogenetic analysis using a new squamate matrix (3) and then added 27 Maastrichtian lizards and Coniophis, along with 12 new characters, for a total of 219 species and 622 characters (Dataset S2). Analyses were conducted using PAUP* 4.0 b10 to implement 10 runs of the parsimony ratchet (200 iterations, 25% reweighting) to find a series of shortest trees. Heuristic search was then used to generate 100,000 trees to estimate the strict and Adams consensus (SI Appendix). Relationships of other snakes follow a recent study (5).
To investigate sampling effects, sample-based rarefaction (SI Appendix) with EstimateS was used to compare the quality of the Maastrichtian and Paleocene fossil records. Selectivity with respect to body size and range size were investigated using logistic regression. PIC analysis was conducted using Mesquite. Mass estimates for lizards and snakes were conducted by regressing jaw and vertebral length against SVL, and then using regression equations to estimate mass (Dataset S1). Morphological disparity was studied using tooth diameter to examine size disparity, and landmark analysis was conducted using TPSDig and TPSRelW to examine shape disparity (SI Appendix).
Supplementary Material
Corrected Supporting Information
Acknowledgments
We thank Matt Friedman for discussions, Dave Fastovsky and Steve Brusatte for constructive reviews; and the curators and staff of the American Museum of Natural History, Smithsonian Institution, University of California Museum of Paleontology, University of Wyoming, and Yale Peabody Museum of Natural History, and the staff of Digimorph at University of Texas at Austin for specimen access. This research was funded in part by National Science Foundation Grant DEB-0132227 (to J.A.G.). N.R.L. was funded by the Yale Institute for Biospheric Studies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Pianka ER, Vitt LJ. Lizards: Windows to the Evolution of Diversity. Berkeley, CA: Univ of California Press; 2003. p. 346. [Google Scholar]
- 2.Greene HW. Snakes: The Evolution of Mystery in Nature. Berkeley, CA: Univ of California Press; 1997. p. 351. [Google Scholar]
- 3.Gauthier J, Kearney M, Maisano JA, Rieppel O, Behlke A. Assembling the squamate tree of life: Perspectives from the phenotype and the fossil record. Bull Yale Peabody Mus. 2012;53:3–105. [Google Scholar]
- 4.Evans SE. At the feet of the dinosaurs: The early history and radiation of lizards. Biol Rev Camb Philos Soc. 2003;78(4):513–551. doi: 10.1017/s1464793103006134. [DOI] [PubMed] [Google Scholar]
- 5.Longrich NR, Bhullar B-AS, Gauthier JA. A transitional snake from the Late Cretaceous period of North America. Nature. 2012;488(7410):205–208. doi: 10.1038/nature11227. [DOI] [PubMed] [Google Scholar]
- 6.Novacek M. 100 million years of land vertebrate evolution: The Cretaceous–Early Tertiary transition. Ann Missouri Bot Gard. 1999;86:230–258. [Google Scholar]
- 7.Macleod N, et al. The Cretaceous–Tertiary biotic transition. J Geol Soc London. 1997;154:265–292. [Google Scholar]
- 8.Raup DM, Sepkoski JJ., Jr Mass extinctions in the marine fossil record. Science. 1982;215(4539):1501–1503. doi: 10.1126/science.215.4539.1501. [DOI] [PubMed] [Google Scholar]
- 9.Archibald JD, Fastovsky DE. In: The Dinosauria. Weishampel DB, Dodson P, Osmolska H, editors. Berkeley, CA: Univ of California Press; 2004. pp. 672–684. [Google Scholar]
- 10.Fastovsky DE, Sheehan PM. The extinction of the dinosaurs in North America. GSA Today. 2005;15(3):4–10. [Google Scholar]
- 11.Schulte P, et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous–Paleogene boundary. Science. 2010;327(5970):1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez LW, Alvarez W, Asaro F, Michel HV. Extraterrestrial cause for the Cretaceous–Tertiary extinction. Science. 1980;208(4448):1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan PM, Fastovsky DE. Major extinctions of land-dwelling vertebrates at the Cretaceous–Tertiary boundary, Eastern Montana. Geology. 1992;20:556. [Google Scholar]
- 14.Archibald JD, Bryant LJ. 1990. Global Catastrophes in Earth History: An Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality, Geological Society of America Special Paper, eds Sharpton VL, Ward PD (Geological Society of America, Boulder, CO), Vol 247, pp 549–562.
- 15.Longrich NR, Tokaryk TT, Field DJ. Mass extinction of birds at the Cretaceous–Paleogene (K-Pg) boundary. Proc Natl Acad Sci USA. 2011;108(37):15253–15257. doi: 10.1073/pnas.1110395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labandeira CC, Johnson KR, Wilf P. Impact of the terminal Cretaceous event on plant–insect associations. Proc Natl Acad Sci USA. 2002;99(4):2061–2066. doi: 10.1073/pnas.042492999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols DJ, Johnson KR. Plants and the K-T Boundary. Cambridge, UK: Cambridge Univ Press; 2008. p. 280. [Google Scholar]
- 18.Marsh OC. Notice of new reptiles from the Laramie Formation. Am J Sci. 1892;43:449–453. [Google Scholar]
- 19.Gilmore CW. Fossil lizards of North America. Memoirs Natl Acad Sci. 1928;22:1–201. [Google Scholar]
- 20.Estes R. Fossil vertebrates from the Late Cretaceous Lance Formation, eastern Wyoming. Univ California Pub Geol Sci. 1964;49:1–180. [Google Scholar]
- 21.Gao K-Q, Fox RC. Taxonomy and evoluton of Late Cretaceous lizards (Reptilia: Squamata) from Western Canada. Bull Carnegie Mus Nat Hist. 1996;33:1–107. [Google Scholar]
- 22.Estes R. Relationships of two lizards (Sauria, Teiidae) Breviora. 1969;317:1–8. [Google Scholar]
- 23.Sullivan RM. Fossil lizards from Swain Quarry, “Fort Union Formation,” middle Paleocene (Torrejonian) J Vertebr Paleontol. 1982;56(4):996–1010. [Google Scholar]
- 24.Sullivan RM, Lucas SG. Annotated list lower vertebrates from the Paleocene Nacimiento Formation. J Herpetol. 1986;20(2):202–209. [Google Scholar]
- 25.Sullivan RM. Paleocene Caudata and Squamata from Gidley and Silberling quarries, Montana. J Vertebr Paleontol. 1991;11:293. [Google Scholar]
- 26.Sullivan RM, Lucas SG. Palaeoscincosaurus middletoni, new genus and species (Squamata? Scincidae) from the Early Paleocene (Puercan) Denver Formation, Colorado. J Vertebr Paleontol. 1996;16:666. [Google Scholar]
- 27.Gilmore CW. Paleocene faunas of the Polecat Bench Formation, Park County, Wyoming, part II: Lizards. Proc Am Philos Soc. 1942;85:159–167. [Google Scholar]
- 28.Bryant LJ. Non-dinosaurian lower vertebrates across the Cretaceous–Tertiary boundary in northeastern Montana. Univ Calif Publ Geol Sci. 1989;134:1–107. [Google Scholar]
- 29.Nydam RL, Caldwell MW, Fanti F. Borioteiioidean lizard skulls from Kleskun Hill (Wapiti Formation; upper Campanian), west-central Alberta, Canada. J Vertebr Paleontol. 2010;30:1090–1099. [Google Scholar]
- 30.Gilmore CW. New fossil lizards from the Upper Cretaceous of Utah. Smithsonian Misc Collect. 1940;99:1–3. [Google Scholar]
- 31.Estes R. A scincoid lizard from the Cretaceous and Paleocene of Montana. Breviora. 1969;331:1–9. [Google Scholar]
- 32.Nydam RL, Fitzpatrick BM. The occurrence of Contogenys-like lizards in the Late Cretaceous and Early Tertiary of the western interior of the USA. J Vertebr Paleontol. 2009;29(3):677–701. [Google Scholar]
- 33.Bhullar B-AS. The power and utility of morphological characters in systematics: A fully resolved phylogeny of Xenosaurus and its fossil relatives (Squamata: Anguimorpha). Bull Museum Compar Zool. 2011;160(3):65–182. [Google Scholar]
- 34.Conrad J. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull Am Mus Nat Hist. 2008;310:1–182. [Google Scholar]
- 35.Colwell RK, Mao CX, Chang J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology. 2004;85(10):2717–2727. [Google Scholar]
- 36.Archibald JD. Dinosaur Extinction and the End of an Era: What the Fossils Say. New York: Columbia Univ Press; 1996. p. 237. [Google Scholar]
- 37.Longrich NR, Currie PJ. Albertonykus borealis, a new alvarezsaur (Dinosauria: Theropoda) from the Early Maastrichtian of Alberta, Canada: Implications for the systematics and ecology of the Alvarezsauridae. Cretac Res. 2009;30:239–252. [Google Scholar]
- 38.Longrich NR, Currie PJ. A microraptorine (Dinosauria-Dromaeosauridae) from the Late Cretaceous of North America. Proc Natl Acad Sci USA. 2009;106(13):5002–5007. doi: 10.1073/pnas.0811664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown CM, Evans DC, Campione NE, O'Brien LJ. Evidence for taphonomic size bias in the Dinosaur Park Formation (Campanian, Alberta), a model Mesozoic terrestrial alluvial-paralic system. Palaeogeogr Palaeoclimatol Palaeoecol. 2012 doi: 10.1016/j.palaeo.2012.06.027. [DOI] [Google Scholar]
- 40.Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. Mammals from the Age of Dinosaurs: Origins, Evolution and Structure. New York: Columbia Univ Press; 2004. p. 648. [Google Scholar]
- 41.Jablonski D. Colloquium paper: Extinction and the spatial dynamics of biodiversity. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11528–11535. doi: 10.1073/pnas.0801919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson DA, Schaefer T, Johnson KR, Nichols DJ, Hunter JP. Vertebrate biostratigraphy of the Hell Creek Formation in southwestern North Dakota and northwestern South Dakota. Special Paper Geol Soc Am. 2002;361:145–167. [Google Scholar]
- 43.Lillegraven JA, Eberle JJ. Vertebrate faunal changes through Lancian and Puercan time in southern Wyoming. J Paleontol. 1999;73:691–710. [Google Scholar]
- 44.Clemens WA. Evolution of the mammalian fauna across the Cretaceous–Tertiary boundary in northeastern Montana and other areas of the western interior. Special Paper Geol Soc Am. 2002;361:217–245. [Google Scholar]
- 45.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48(1):107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 46.Feduccia A. Explosive evolution in tertiary birds and mammals. Science. 1995;267(5198):637–638. doi: 10.1126/science.267.5198.637. [DOI] [PubMed] [Google Scholar]
- 47.Ericson PGP, et al. Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol Lett. 2006;2(4):543–547. doi: 10.1098/rsbl.2006.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman M. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc R Soc B Biol Sci. 2010;277(1688):1675–1683. doi: 10.1098/rspb.2009.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westerhold T, Röhl U, Donner B, McCarren HK, Zachos J. A complete high-resolution Paleocene benthic stable isotope record for the central Pacific (ODP site 1209) Paleoceanography. 2011;26:2216. [Google Scholar]
- 50.Gao K, Hou L. Systematics and taxonomic diversity of squamates from the Upper Cretaceous Djadochta Formation, Bayan Mandahu, Gobi Desert, People’s Republic of China. Can J Earth Sci. 1996;33:578–598. [Google Scholar]
- 51.Alifanov VR. Macrocephalosaurs and the early evolution of lizards of Central Asia. Tr Paleontol Inst. 2000;272:1–126. [Google Scholar]
- 52.Augé M. Evolution of Paleogene lizards in Europe. Mem Mus Natl Hist Nat. 2005;192:1–369. [Google Scholar]
- 53.Averianov A, Danilov I. Agamid lizards (Reptilia, Sauria, Agamidae) from the Early Eocene of Kyrgyzstan. Neues Jahrb Geol Palaontol, Monatsh. 1996;12:739–750. [Google Scholar]
- 54.Head JJ, et al. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature. 2009;457(7230):715–717. doi: 10.1038/nature07671. [DOI] [PubMed] [Google Scholar]
- 55.Rage J-C, et al. A diverse snake fauna from the early Eocene of Vastan Lignite Mine, Gujarat, India. Acta Palaeontol Pol. 2008;53(3):391–403. [Google Scholar]
- 56.Hedges SB, Vidal N. In: The Timetree of Life. Hedges SB, Kumar S, editors. New York: Oxford Univ Press; 2009. pp. 383–389. [Google Scholar]
- 57.Lloyd GT, et al. Dinosaurs and the Cretaceous Terrestrial Revolution. Proc R Soc B Biol Sci. 2008;275(1650):2483–2490. doi: 10.1098/rspb.2008.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Corrected Supporting Information