Proconiini Sharpshooters of Argentina, with Notes on Its Distribution, Host Plants, and Natural Enemies (original) (raw)
Abstract
The American tribe Proconiini (Hemiptera: Cicadellidae: Cicadellinae) is one of the largest groups of xylem-feeding insects and includes the majority of the known vectors of xylem-born phytopathogenic organisms. The significance of the pathogens that this group transmits gives them an important role as pests, mostly for citrus fruit, grapes, and almonds. Knowledge of these Hemiptera in Argentina is insufficient and fragmentary. Thus one of the aims of this paper is to summarize the available information of the Proconiini sharpshooters in Argentina. In addition, 14 species are mentioned for the first time in the country, and new distributional data are given for 18 species. Thirty-four new associations between sharpshooters and host plants are recorded. New records of egg parasitoids are given for Dechacona missionum, Molomea consolida, M. lineiceps, and Tapajosa similis.
Keywords : Auchenorrhyncha, Cicadellidae, Cicadellinae, biogeographic provinces, bionomics, parasitoids
Introduction
The Proconiini tribe (Hemiptera: Cicadellidae: Cicadellinae) is characterized by posterior legs at rest with knees not attaining posterior proepimeral margins, male pygofer and plates both usually with numerous evenly dispersed microsetae and antennal ledges usually protuberant in dorsal aspect (Young 1968). The tribe includes 422 species in 58 genera (McKamey 2007; Wilson et al. 2009) and is restricted to the New World, with only Homalodisca vitripennis having an extraAmerican distribution, after recent invasion of many islands in the Pacific Ocean (Pilkington et al. 2005). The sharpshooters are one of the largest groups of xylem-feeding insects and include the majority of the known vectors of xylem-born phytopathogenic organisms (Rakitov and Dietrich 2001; Redak et al. 2004).
The bacterium Xylella fastidiosa Wells (Xanthomonadales: Xanthomonadaceae) is a growing threat in the Neotropical region. It has been found in Mexico, Costa Rica, Venezuela, Paraguay, Brazil, and Argentina, and a clear association between the xylem-feeding habit of sharpshooters and their ability to transmit the bacterium has been observed (Hopkins 1989; Redak et al. 2004). Most South American countries are under high occurrence risk of this dangerous disease (Dellapé et al. 2011).
Xylella fastidiosa is the causal agent of diverse diseases: “Phony Peach Disease” (PPD), “Plum Leaf Scald” (PLS), “Pierce's Disease” (PD) of grapes, “Almond Leaf Scorch” (ALS), “Coffee Leaf Scorch” (CLS), and “Citrus Variegated Chlorosis” (CVC) (Gravena et al. 1998; Redak et al. 2004). The bacterium is a known threat in diverse regions of Argentina affecting almonds (ALS) in Catamarca and La Rioja provinces (Nome et al. 1992; Haelterman et al. 1996), as well as citrus orchards (CVC) in Misiones, Corrientes, and Entre Rios provinces (De Coll et al. 2000; Beltrán et al. 2004; Costa et al. 2009).
The information on faunistic aspects of Proconiini in Latin America is almost nonexistent, particularly in Argentina. In addition, most of the knowledge on proconiine vectors is derived from studies done in countries of the Nearctic region. Relatively few transmission studies have been carried out in the Neotropic, where the majority of sharpshooter species occur (Redak et al. 2004; Silva et al. 2007; Marucci et al. 2008).
In Argentina, the Proconiini tribe is mainly distributed in the northern region (Young 1968; Remes Lenicov et al. 1999; Virla et al. 2008), and there is almost no information regarding this economically important group. Only for few species is there available data, and most of them provide only distributional records and/or species association with commercial crops (Costilla et al. 1972; Remes Lenicov and Tesón 1985; Paradell 1995; Remes Lenicov et al. 1997, 1998, 1999, 2004; Virla et al. 2008).
To obtain a better understanding about this tribe in Argentina, this paper contributes new distributional records and/or host plants associations and parasitoids, and also summarizes the available data of the Proconiini sharpshooters in the country.
Materials and Methods
Three sources were used to achieve the objectives: (1) bibliographical data; (2) specimens housed in the most important entomological collections of Argentina: Instituto Miguel Lillo (IMLA), Museo de Ciencias Naturales de La Plata (MLP), Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN); and (3) research conducted by the working group.
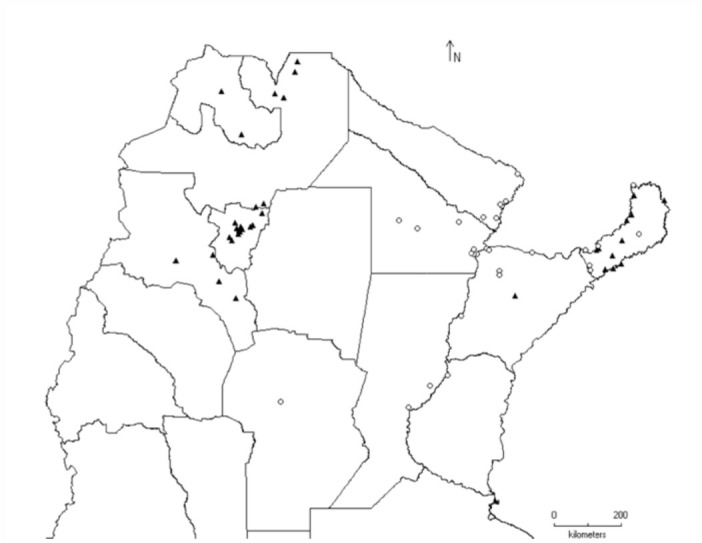
112 sites in 21 provinces of Argentina were surveyed between 22° S and 44° S (Figure 1). Most of the sites were sampled by sweeping on diverse crops, its surrounding vegetation, and both anthropically-modified environments and pristine ones. In four occasions, Malaise traps and yellow pan traps were used as well (in Buenos Aires, Córdoba, and Rio Negro provinces). The specimens collected were preserved in 70% ethanol, and voucher specimens were deposited in the IMLA and MLP collections.
Figure 1.
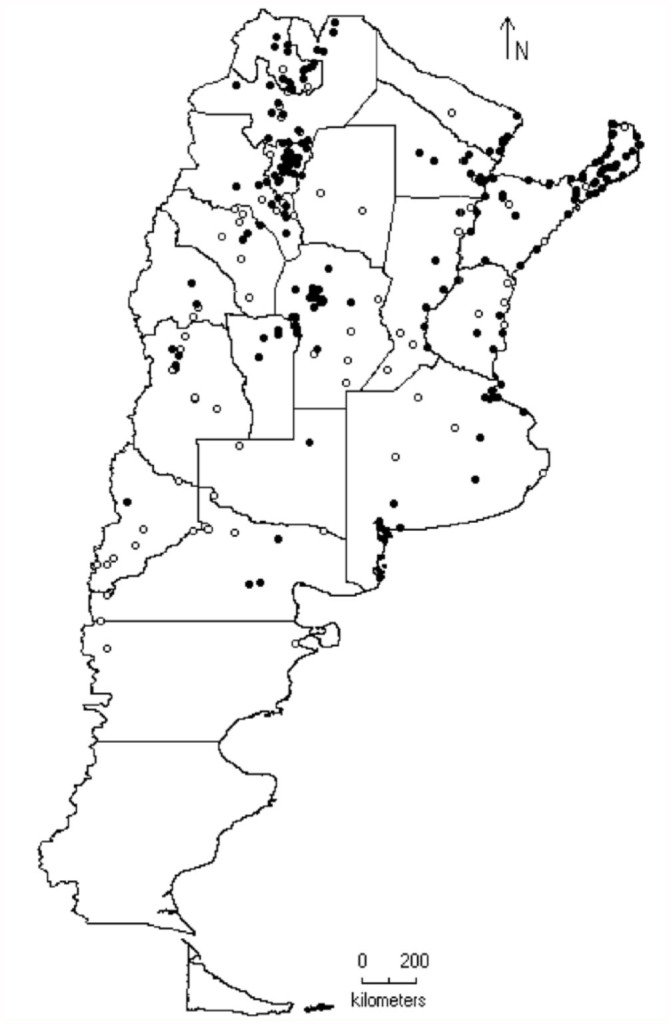
Distribution of the Proconiini sharpshooters in Argentina (black dots). White dots indicate sampled localities without occurrence of Proconiini species. High quality figures are available online.
Both male and female genitalia of the species were prepared for microscopic examination using Young's techniques (1968). The parts were stored in microvials with glycerin. The specimens were identified using descriptions provided by Schröeder (1959), Young (1968), Emmrich (1975, 1984), Remes Lenicov et al. (1999), and Marucci et al. (2002). Data on Anacuerna centrolinea (Melichar) were obtained from the collection of the Staatliches Museum Für Naturkunde Stuttgart, Germany (SMNS).
An extensive distribution list of all species studied was made using both our own data, bibliographic records, and data of the specimens deposited in the Argentinean collections. Sharpshooter species were grouped into biogeographical regions proposed by Morrone (2001, 2006). The Jaccard Index was used to identify the similarities between the biogeographic provinces (Moreno 2001).
Results
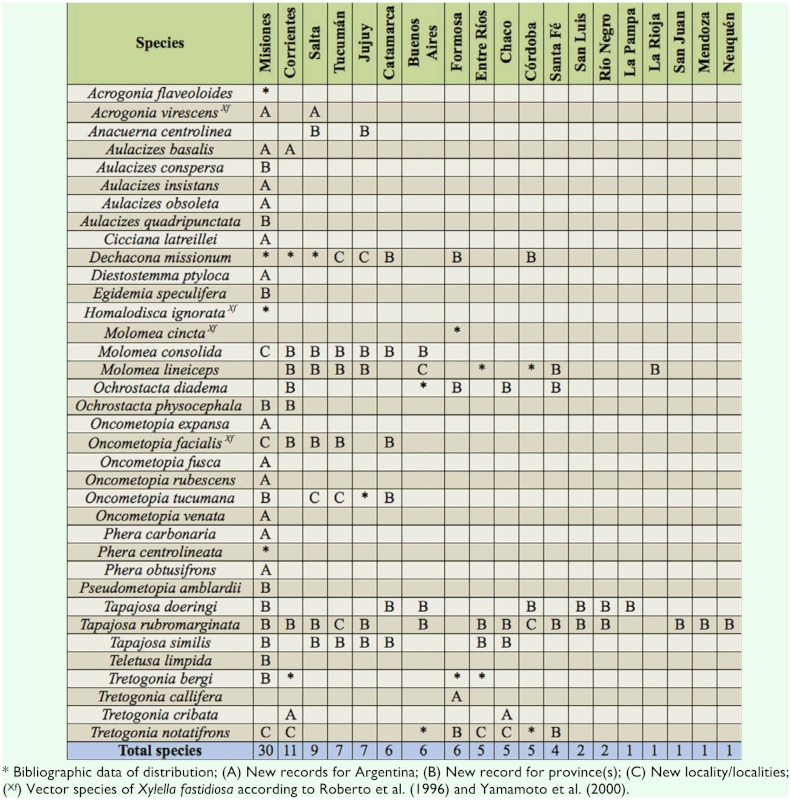
In the Argentinean territory, 40 species of Proconiini were found: 14 of them were reported for first time in Argentina, and 18 species had extended in geographic distribution. Also, new associations with host plants were found for six species of sharpshooters, and new records of parasitoid wasps for four species.
Below, 14 species of Proconiini recorded for the first time in Argentina are listed (Table 1, symbolized with an “A”):
Table 1.
Geographic distribution of the Proconiini in Argentina by provinces, according to political divisions. The species Diestostemma bituberculata, Molomea vermiculata, M. xanthocephala, and Stictoscarta sulcicollis are not listed due to the lack of information about the collection site.
Acrogonia virescens (Metcalf). Salta: Abra Grande, Orán, 2♂♂ 1♀, III/67; 3♂♂ 1♀, 10/I28/II/67, Golbach Leg. (IMLA). Misiones: Eldorado, 1♂ 2♀♀, 31/X/2008, Logarzo and Palottini Legs. (MLP).
Aulacizes basalts Walker. Misiones: San Antonio, 1♀, 7/XII/51, Willink and Monrós Legs.; Bernardo de Irigoyen, 1♀, 5/XII/51, Willink and Monrós Legs.; 2 de Mayo, 2♀♀, 30/XI/51, Willink and Monrós Legs.; Aristóbulo del Valle, 2♀♀, XI/51, Willink and Monrós Legs. (IMLA). Corrientes: Mburucuyá, 1♂, XI/57, BirabenLeg. (MLP).
Aulacizes insistans (Walker). Misiones: Iguazú, 1♀, XII/57, Biraben Leg. (MLP); Misiones: 2♀♀, without other data (MACN).
Aulacizes obsoleta Melichar. Misiones: Puerto Iguazú, 1♀, II/54, Willink and Golbach Legs. (IMLA); Iguazú, 2♀♀, XII/57, Biraben Leg.; Caraguatay, 1♂, I/60, Ronderos and Trotta Legs.; Eldorado (26° 25′ 40″ S, 54° 09′ 38.02″ W), 1♀, 30/X/2008, Logarzo and Palottini Legs. (MLP); 2♀♀, P. Aguirre Leg. (MACN).
Cicciana latreittei (Distant). Misiones: Puerto Iguazú, 4♂♂, 20/XII/2001, Logarzo and Manrique Legs. (MLP).
Diestostemma ptyloca Distant. Misiones: Iguazú, 3♂♂ 1♀, X/27; Iguazú, 1♂, X/77, Pepe Leg. (MACN).
Oncometopia expansa Melichar. Misiones: 4♂♂ 1♀, III/1897, Venturi Leg.; Posadas, 1♂ (MACN); Eldorado, 2♂♂, XI/2008, Logarzo and Palottini Legs. (MLP).
Oncometopia fusca Melichar. Misiones: Rep. Guaraní El Soberbio, 1♂, X/47, Viana Leg. (MACN); Loreto, 1♂, 21/IX/2003, Logarzo and Varone Legs. (MLP).
Oncometopia rubescens Fowler. Misiones: Panambi, 2♂♂, X/51, Monrós and Willink Legs. (PMLA).
Oncometopia venata Schröder. Misiones: Panambi, 1♂, 24/XI/51, Willink and Monrós Legs. (IMLA)
Phera carbonaria (Melichar). Misiones: Iguazú, 3♂♂ 1♀, 10/XI/73, Tonsic and Willink Legs.; Misiones: 1♂, 4/IV/10, Jörgensen Leg.; 1♂, 31/VIII/10, Jörgensen Leg.; Parque Provincial Urugua-i, 1♂, 13/XII/57; San Javier, 1♂, 16/XII/57, Biraben Leg.; Iguazú, 1♂, XI/44, Biraben Leg. (MLP); Misiones: 3♂♂; Dep. Concepción-Sta. Maria, 1♂, X/46, Viana Leg. (MACN).
Phera obtusifrons Fowler. Misiones: 2 de Mayo, 1♂, XI/73, Escobar and Claps Legs. (IMLA).
Tretogonia callifera Melichar. Formosa: Clorinda, 7 specimens, XI/47; Mojón de Fierro, 2♂♂, XII/48, Golbach Leg. (IMLA).
Tretogonia cribata Melichar. Corrientes: 9♂♂ 14♀♀, 2 without abdomen, II/59, Biraben Leg.; Chaco: 1♂ 2♀♀, III/59, Parko Leg. (MLP).
The geographic distributions of 18 species of Proconiini sharpshooters are extended as follows (Table 1, symbolized with “B” and “C”):
Anacuerna centrolinea (Melichar). Jujuy: Morro de la Providencia, Quebrada de Humahuaca, Abra Pampa, Iturbe (IMLA). Salta: Cachipampa (SMNS).
Aulacizes conspersa Walker. Misiones: Puerto Iguazú (IMLA), Caraguatay (MLP).
Aulacizes quadripunctata (Germar). Misiones: San Pedro, Salto Encantado, San Antonio, Tobunas, Campo Grande, Caingua, Aristóbulo del Valle (IMLA); San Javier, 25 de Mayo (MACN); San Ignacio, 2 de Mayo, Eldorado (MLP).
Dechacona missionum (Berg). Tucumán: Horco Molle, Monteras (MACN); La Higuera, Trancas. Salta: Pocitos, Urundel. Catamarca: Arroyo de Infanzón. Córdoba: Dique Los Molinos. Formosa: Estera La Florence, Clorinda (PMLA). Jujuy: Yuto, Gral. San Martín, Dique La Ciénaga. Salta: Bazán. Tucumán: Gonzalo. Misiones: Montecarlo. Corrientes: Empedrado (MLP).
Egidemia speculifera (Walker). Misiones: Puerto Bemberg, San Pedro, 2 de Mayo (PMLA); Guaraní (MACN).
Molomea consolida Schröder. Jujuy: Yuto, Aguas Calientes. Misiones: Montecarlo, Loreto, Garuhapé, Eldorado (MLP). Jujuy: Laguna de Yala, Aguas Calientes. Salta: Embarcación. Misiones: Puerto Bemberg, Iguazú, Oro Verde, San Javier, Panambi, 2 de Mayo. Tucumán. Catamarca: San Antonio. Salta: Tartagal, Aguaray (IMLA). Misiones: Obára, Posadas, Concepción, Santa María. Corrientes: Santo Tomé. Buenos Aires. Salta: Orán (MACN).
Molomea lineiceps Young. Corrientes: Las Marías-Virasoro. Jujuy: Caimancito. Salta: Abra Grande. Tucumán: Las Talitas, El Bachi (PMLA). Buenos Aires: Isla Martin Garcia, Tigre. Corrientes: Monte Caseras, Santo Tomé. La Rioja. Santa Fé: Rosario (MACN). Tucumán: Horco Molle (MLP).
Ochrostacta diadema (Burmeister). Corrientes: Manantiales, Sauce. Formosa: Misión Laishi, Mojón de Fierro (IMLA). Chaco: between Vedia and Pres. Roca, Bermejo River (MACN). Santa Fé: Guadalupe (MLP).
Ochrostacta physocephala (Signoret). Misiones: San Ignacio, Pindapoy. Corrientes: Santo Tomé (MLP).
Oncometopia facialis (Signoret). Misiones: San Javier, Iguazú, Arroyo Urugua-I, Santa Ana, San Antonio, Montecarlo, Aristóbulo del Valle, Panambi. Corrientes: Isla Iyupe Grande. Salta: El Morenillo, San Lorenzo. Tucumán: Cerro San Javier, Lules, Horco Molle, Chilcas, La Ramada. Catamarca: Aconquija, Concepción, Belén, El Rodeo (PMLA). Misiones: Concepción, Santa María. Jujuy: Quebrada Río Blanco (MACN). Misiones: Eldorado, Loreto (MLP).
Oncometopia tucumana Schröder. Salta: Abra Grande, Aguaray, Tartagal, San Lorenzo. Catamarca: El Rodeo, Concepción, Belén. Misiones: Iguazú. Tucumán: San Javier, Cerro San Javier, Burruyacu, Chilcas (IMLA); Tucumán: Las Tipas (MLP).
Pseudometopia amblardii (Signoret). Misiones: Iguazú (IMLA); Loreto (MLP).
Tapajosa doeringi (Berg). Catamarca: El Suncho, Belén, El Alamito, El Rodeo. San Luis: San Francisco, San Martín, Merlo, Villa de Praga, Las Chacras, Cortaderas. Córdoba: Yacanto, Agua de Oro, La Cumbre, Punilla. Río Negro: Choele Choel (PMLA). Córdoba: Calamuchita, El Sauce, Argüello, San Javier. Buenos Aires: San Blas, Bahía Bianca. La Pampa: Conelho. Misiones. Formosa (MACN). Buenos Aires: Sierra de la Ventana, Monte Hermoso. Catamarca: Chumbicha (MLP).
Tapajosa rubromarginata (Signoret). Jujuy: San Salvador, Gral. San Martín. Salta: Orán, Chalicán. Córdoba: Los Molinos, Huerta Grande. Entre Ríos: Concepción del Uruguay. Buenos Aires: Magdalena. Mendoza: Tunuyán (MLP). Jujuy: Calilegua. Salta. Chaco: Resistencia. Córdoba: Calamuchita, El Jag¨el, El Sauce, Arg¨ello, La Paz, La Falda, Alta Gracia. Santa Fé: Garay. Buenos Aires: Rosas FC Sud, Tandil. Corrientes: Monte Caseras. Mendoza: Cacheuta. Neuquén: Loncopué. Río Negro: Río Valcheta (MACN). Salta: Cafayate. Catamarca: Aconquija, El Rodeo. Tucumán: Monteras, Acheral, Aguadita, El Siambon, Monte Bello. San Juan: San Martín. San Luis: Cortaderas. Formosa: Misión Laishi, Clorinda. Misiones: Timbó, San Vicente, Puerto Bemberg. Corrientes: Paso de los Libres, Manantiales. Córdoba: Cabania, Agua de Oro, Dique Los Molinos. Santa Fé: La Gallareta, Villa Ana (IMLA).
Tapajosa similis (Melichar). Jujuy: La Isla. Salta: Cafayate, Campo Quijano, Coronel Moldes. Catamarca: El Rodeo, Arroyo de Infanzón, El Alto. Tucumán: La Mezada, Horco Molle, Trancas, San Pedro de Colalao, Montebello, Río Chico, Tafí Viejo. Entre Ríos: Gualeguaychú (PMLA). Misiones. Salta. Chaco (MACN). Tucumán: Las Tipas (MLP).
Teletusa limpida (Signoret). Misiones: Puerto Bemberg, Puerto Iguazú (IMLA).
Tretogonia bergi Young. Misiones (MACN).
Tretogonia notatifrons Melichar. Formosa: Clorinda, Misión Laishi, Mojón de Fierro. Chaco: Colonia Benítez. Misiones: Apóstoles, San José. Corrientes: Manantiales (IMLA). Chaco: Sáenz Peña, Resistencia, Barranqueras, Zapallar. Formosa: Las Ocas, El Refugio. Misiones: Iguazú, San Ignacio. Entre Ríos: La Paz. (MLP). Misiones: Posadas. Corrientes: Ita Ibaté, Paso de la Patria, San Cosme. Santa Fé: Garay (MACN).
Discussion
The literature provided information on other species of Proconiini found in Argentina such as: Acrogonia flaveoloides Young, Homalodisca ignorata Melichar, Molomea cincta (Signoret), and Phera centrolineata (Signoret) (Gravena et al. 1998; Remes Lenicov et al. 1999; Dellapé and Paradell 2011).
The species Diestostemma bituberculata (Signoret), Molomea vermiculata (Signoret), Molomea xanthocephala (Germar), and Stictoscarta sulcicollis (Germar) were cited for Argentina by Young (1968) and Metcalf (1965), but none of them describe the province or locality where the specimens were collected.
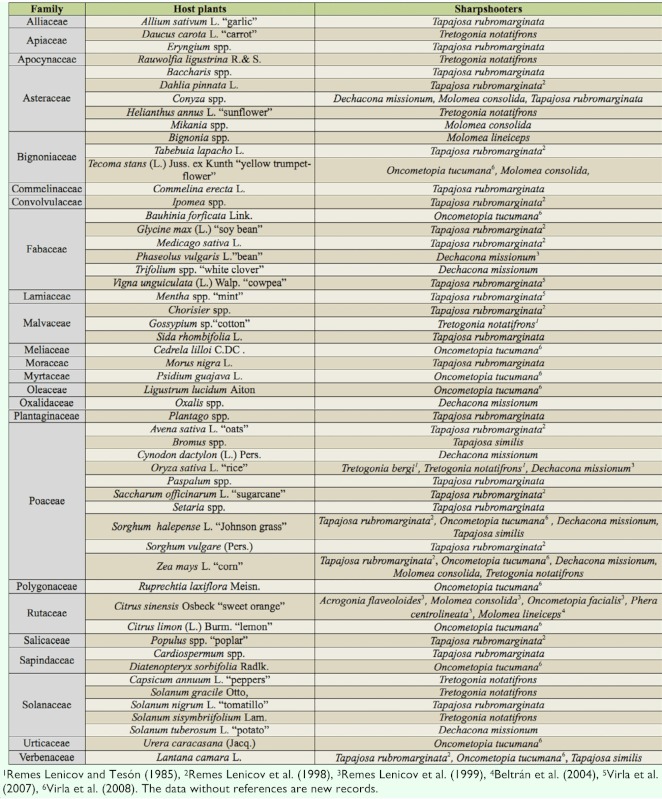
The Proconiini, as other xylem feeding leafhoppers, are considered polyphagous and have evolved with many unusual adaptations, such as host switching, to maximize nutrient uptake (Mizell and Andersen 2001). New associations with host plants were found for 11 Argentinean sharpshooters (27.5%); the cited host plants belong to 24 families (Alliaceae, Apiaceae, Apocynaceae, Asteraceae, Bignoniaceae, Commelinaceae, Convolvulaceae, Fabaceae, Lamiaceae, Malvaceae, Meliaceae, Moraceae, Myrtaceae, Oleaceae, Oxalidaceae, Plantaginaceae, Polygonaceae, Poaceae, Rutaceae, Salicaceae, Sapindaceae, Solanaceae, Urticaceae, and Verbenaceae). Both known and new data of host pi ants-sharpshooter associations are summarized in Table 2.
Table 2.
Host plants records of the sharpshooters occurring in Argentina.
The knowledge about natural enemies of Proconiini in Argentina is insufficient. Sharpshooter species are attacked by egg predators (Dermaptera), entomopathogenic fungus (Ascomycota) (Mariani et al. 1997; Toledo et al. 2006), and several egg parasitoids belonging Trichogrammatidae and Mymaridae families (Hymenoptera). In recent times, investigations conducted to survey the egg parasitoids of the Proconiini sharpshooters resulted in a greater and more comprehensive understanding of egg parasitoid wasps; the majority of the representatives of this guild belong to Gonatocerus Nees (Mymaridae), a wellknown genus showing a certain degree of specificity at level-tribe, because most of its species attacks Cicadellini and Proconiini sharpshooters (Triapitsyn et al. 2010). New records of parasitoids were found for 10 species (25%). Information of known natural enemies and new data are summarized in Table 3.
Table 3.
Summarized records of natural enemies of the Argentinean Proconiini sharpshooters (*).
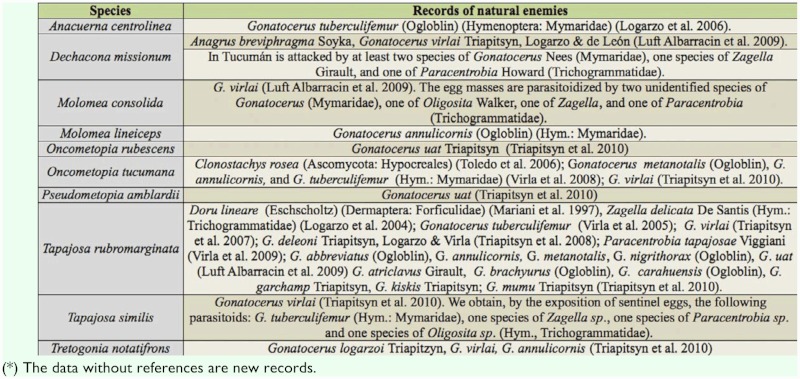
In Argentina, all the species of sharpshooters were found in two zones to north of latitude 40° S: one strip that connects the northeast with the mid-east of the country, and another from the northwestern to the mid-west (Figure 1). The most diverse genera (e.g., Aulacizes and Oncometopia) were found in both places. The eastern fringe includes the Paraná forest and was the most diverse; this is deeply linked to biogeographic systems of the Brazilian territory, which has the greatest diversity of Proconiini (Dellapé et al. 2011). All the studied sites where sharpshooters were found were grouped into the corresponding biogeographic provinces (sensu Morrone 2001, 2006) (Table 4).
Table 4.
Distribution of the Argentinean Proconiini sharpshooters into the biogeographic provinces (according to Morrone 2001, 2006). The range of elevation of the localities in which each species occurs is given.
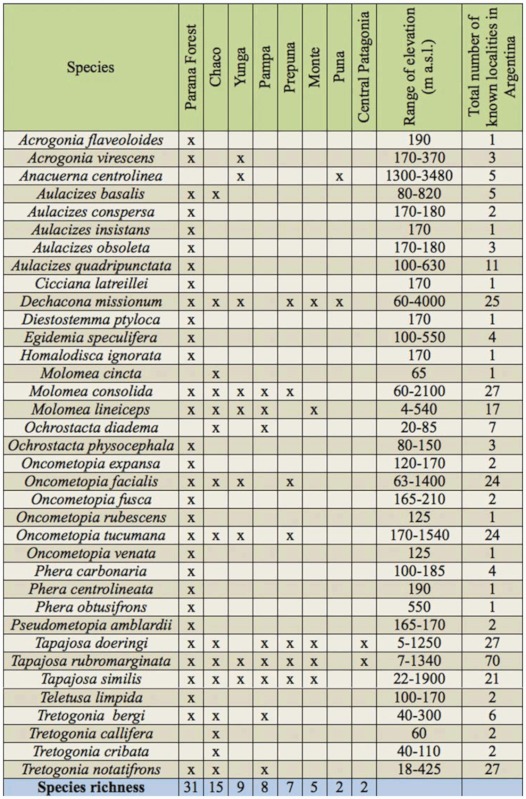
Tapajosa Melichar, the most widely distributed genus, was found in all the biogeographic provinces (except in the Puna); both T. rubromarginata and T. doeringi were the species with southernmost distributional range (Figure 2). Tapajosa rubromarginata was the most frequent and ubiquitous species, which was found in 70 localities of the Argentinean territory.
Figure 2.
Distribution of the species of genus Tapajosa Melichar: T. doeringi ( ), T. rubromarginata (
), T. rubromarginata ( ) and T. similis (
) and T. similis ( ). High quality figures are available online.
). High quality figures are available online.
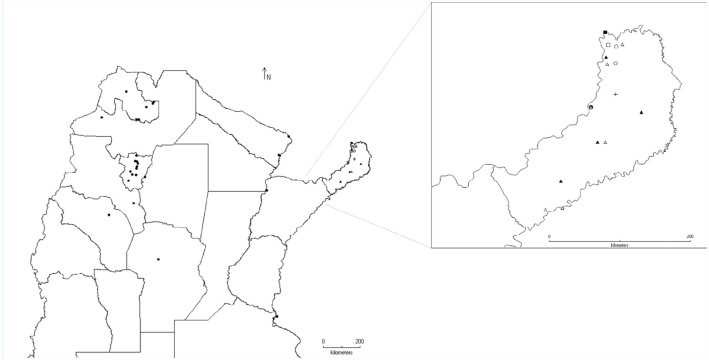
Six genera (Cicciana Metcalf, Diestostemma Amyot and Serville, Egidemia China, Homalodisca Stål, Phera Stål, and Teletusa Distant) were restricted to Paraná Forest—an evergreen forest with altitudes between 500 and 1800 m a.s.l., characterized by abundant trees over 30 m, Bambuceae, and arbustive ferns (Cabrera and Willink 1973) (Figure 3). The monotypic genus Dechacona Young was widely distributed in the northern part of the country, with a broad altitudinal range (from 60 to 4000 m a.s.l.) (Figure 3).
Figure 3.
Distribution of the genera Cicciana Metcalf ( ), Dechacona Young (
), Dechacona Young ( ), Diestostemma Amyot and Serville (
), Diestostemma Amyot and Serville ( ), Egidemia China (
), Egidemia China ( ), Homalodisca Stål (+), Phera Stål (
), Homalodisca Stål (+), Phera Stål ( ), and Teletusa Distant (
), and Teletusa Distant ( ). High quality figures are available online.
). High quality figures are available online.
Three other genera were found in two biogeographic provinces: Acrogonia Stål (associated with jungle environments, both in Paraná and Yunga forest), Anacuerna Young (distributed in high elevations of Yunga and Puna), and Aulacizes Amyot and Serville (linked to forest environments and very humid localities of Chacoan subregion on the shore of the “Esteros de Iberá”) (Figure 4).
Figure 4.
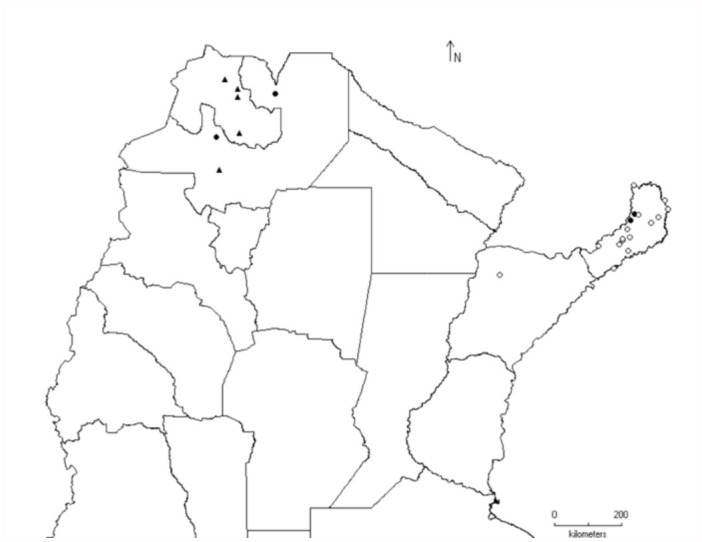
Distribution of the genera Acrogonia Stål ( ), Anacuerna Young (
), Anacuerna Young ( ), and Aulacizes Amyot and Serville (
), and Aulacizes Amyot and Serville ( ). High quality figures are available online.
). High quality figures are available online.
The genus Tretogonia Melichar (Figure 5) was found in sites of the Chaco province, with T. notatifrons being its most widely distributed species. Oncometopia Young is the genus with more species and was mostly linked to forest sites (Figure 5), but the species O. facialis and O. tucumana seemed to have more plasticity, occurring in four biogeographic provinces and a variable range of altitudes. Species of Molomea China were found in six different biogeographic provinces, with M. consolida having the widest range, as it was found to occur in 27 localities, from 60 to 2100 m a.s.l. (Figure 6).
Figure 5.
Distribution of the genera Oncometopia Stål ( ) and Tretogonia Melichar (
) and Tretogonia Melichar ( ). High quality figures are available online.
). High quality figures are available online.
Figure 6.
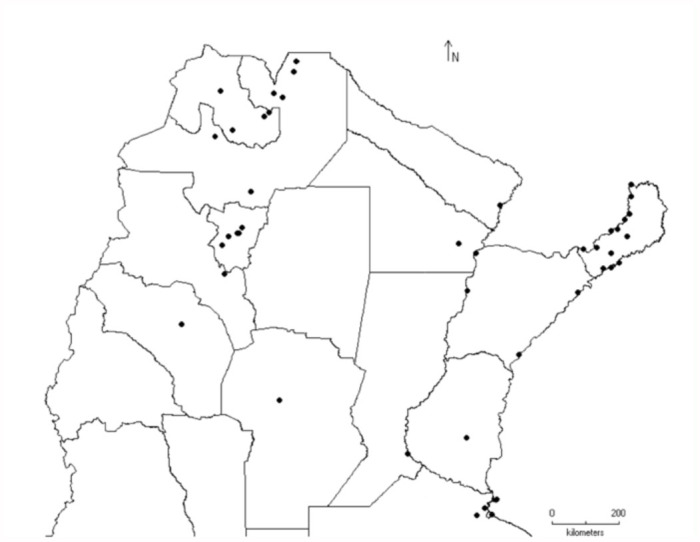
Distribution of the genus Molomea China ( ). High quality figures are available online.
). High quality figures are available online.
Considering the 40 species of sharpshooters inhabiting the Argentinean territory, 19 of them (47.5%) were found only in the Paraná forest, and three species (7.5%) occurred only in the driest region of Chaco. The high elevation of Puna hosted only two species as well as Central Patagonia, where the specimens were collected in oasis located along river valleys.
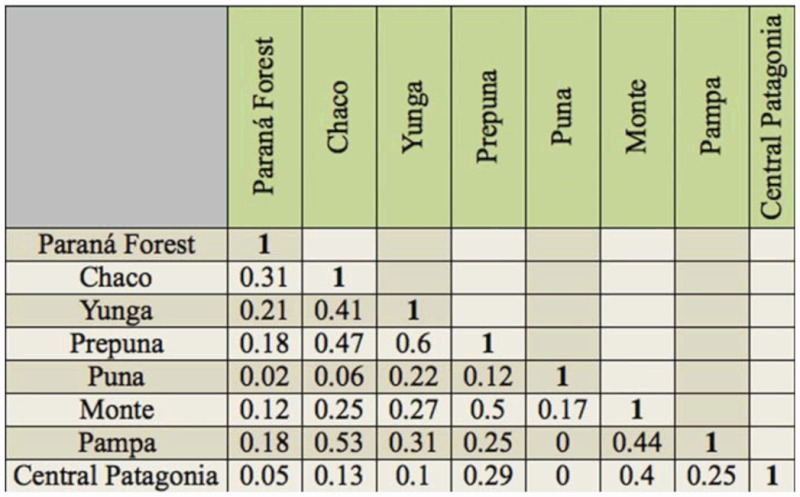
The number of shared species between biogeographic provinces was low. The range of values of the Jaccard index varied between 0–1, representing complete dissimilarity between sampling for any taxon to a perfect match between sampling, respectively. The highest Jaccard index was obtained for adjacent provinces like “Prepuna-Yunga” (0.6), “Chaco-Pampa” (0.53), and “Prepuna-Monte” (0.5), while there were no shared species between “Puna-Pampa” and “PunaCentral Patagonia” (0), located very far from each other (Table 5).
Table 5.
Matrix of Jaccard Similarity Coefficient between Argentinean biogeographic provinces hosting Proconiini sharpshooter species.
This is the most comprehensive compilation of information related to species of sharpshooters in Argentina. The need for knowledge of interrelationships of insect pests and their environment has been emphasized by several authors in order to develop effective management tactics. In this context, the information given in this study could be useful for those involved in vector-control related programs.
Acknowledgements
We thank the curators of IMLA, MLP, and MACN entomological collections, Dr. Pedro Lozada who provided data on specimens deposited in SMNS, the reviewers for helpful comments, and Lic. DA. Barrasso for critically reading the manuscript. Gimena Dellapé is a CONICET fellowship holder.
Editor's note
Paper copies of this article will be deposited in the following libraries. Universitaetsbibliothek Johann Christian Senckenberg, Frankfurt Germany; National Museum of Natural History, Paris, France; Field Museum of Natural History, Chicago, Illinois, U.S.A.; University of Wisconsin, Madison, Wisconsin, U.S.A.; University of Arizona, Tucson, Arizona, U.S.A.; Smithsonian Institution Libraries, Washington D.C, U.S.A.; The Linnean Society, London, England. The date of publication is given in ‘About the Journal’ on the JIS website.
References
- Beltrán VM, Cáceres S, Zubrzycki H, Ploper D, Willink E, Jaldo H. CVC associated vectors in Valencia Orange of Corrientes, Argentina. Proceedings of the International Society of Citriculture, 10th International Citrus Congress. 2004:75–83. [Google Scholar]
- Cabrera AL, Willink A. Biogeografía de América Latina. Serie de Biología. Monografía N° 13. OEA Press; 1973. [Google Scholar]
- Costa N, Plata MI, Garrán SM, Mika R. Detección de Clorosis Variegada de los Cítricos (CVC) en el Departamento de Concordia, Provincia de Entre Ríos. XIII Jornadas Fitosanitarias Argentinas E021. 2009.
- Costilla M, Basco H, Osores V. Primera cita para Tucumán del bicho llovedor de la caña Tapajosa rubromarginata (Signoret) (Homoptera: Cicadellidae), en cultivos de caña de azúcar. IDIA. 1972;28:126–129. [Google Scholar]
- De Coll OR, Remes Lenicov AMM, Agostini J, Paradell S. Detection of Xylella fastidiosa in weeds and sharpshooters in orange groves affected with Citrus Variegated Chlorosis in Misiones, Argentina. Proceeding of the 14th Conference of the International Organization of Citrus Virologists, Insect-Transmitted Procaryotes. 2000. pp. 216–222.
- Dellapé G, Paradell S. First record of the genus Homalodisca (Hemiptera: Cicadellidae) from Argentina and redescription of the female of H. ignorata. Revista de la Sociedad Entomológica Argentina. 2011;70(3–4):363–367. [Google Scholar]
- Dellapé G, Virla EG, Logarzo GA, Paradell S. New records on the geographical distribution of South American sharpshooters (Cicadellidae: Cicadellinae: Proconiini) and their potential as vectors of Xylella fastidiosa. The Florida Entomologist. 2011;94(2):364–366. [Google Scholar]
- Emmrich R. Zur Kenntnis der Gattung Oncometopia Stål, 1869 (Homoptera, Cicadellidae, Cicadellinae). Entomologische Abhandlungen des Staatlichen Museums für Tierkunde Dresden. 1975;40:277–303. [Google Scholar]
- Emmrich R. Weiteres zur Kenntnis der Gattung Oncometopia Stål (s. str.) (Homoptera, Auchenorrhyncha, Cicadellidae, Cicadellinae). Reichenbachia. 1984;22:113–124. [Google Scholar]
- Gravena S, Lopes JRS, Paiva PEB, Yamamoto PT, Roberto SR. The Xylella fastidiosa vetors. In: Donadio LC, Moreira CS, editors. Citrus Variegated Chlorosis. Estação Experimental de Citricultura; 1998. pp. 36–53. [Google Scholar]
- Haelterman RM, Nome CF, Docampo DM, Nome SF. Hospedantes de Xyllela fastidiosa, bacteria causal de la escaldadura del borde de la hoja del almendro (Prunus amygdalus). Revista de Investigaciones Agropecuarias INTA. 1996;26(2):65–72. [Google Scholar]
- Hopkins DL. Xylella fastidiosa: Xylem-limited bacterial pathogen of plants. Annual Review of Phytopathology. 1989;27:271–290. doi: 10.1146/annurev.phyto.34.1.131. [DOI] [PubMed] [Google Scholar]
- Logarzo GA, Virla EG, Triapitsyn SV, Jones W. Biology of Zagella delicata (Hymenoptera: Trichogrammatidae) an egg parasitoid of the sharpshooter Tapajosa rubromarginata (Hemiptera: Clypeorrhyncha: Cicadellidae) in Argentina. The Florida Entomologist. 2004;87(4):511–516. [Google Scholar]
- Logarzo GA, De León JH, Triapitsyn SV, Gonzalez RH, Virla EG. First report of a Proconiine Sharpshooter, Anacuerna centrolinea (Hemiptera: Cicadellidae), in Chile, with notes on its biology, host plants, and egg parasitoids. Annals of the Entomological Society of America. 2006;99(5):879–883. [Google Scholar]
- Luft Albarracin E, Triapitsyn SV, Virla EG. An annotated key to the genera of Mymaridae (Hymenoptera: Chalcidoidea) in Argentina. Zootaxa. 2009;2129:1–28. [Google Scholar]
- Mariani R, Vera L, Virla EG. Aportes al conocimiento de Doru lineare (Dermaptera, Forficulidae), un insecto de importancia agronómica en el noroeste argentino. CIRPON Revista de Investigación. 1997;X(1–4):13–18. [Google Scholar]
- Marucci RC, Cavichioli RR, Zucchi RA. Espécies de cigarrinhas (Hemiptera, Cicadellidae, Cicadellinae) em pomares de citros da região de Bebedouro, SP, com descrição de uma espécie nova de Acrogonia Stål. Revista Brasileira de Entomologia. 2002;46:149–164. [Google Scholar]
- Marucci RC, Lopes JRS, Cavichioli RR. Transmission Efficiency of Xylella fastidiosa by Sharpshooters (Hemiptera: Cicadellidae) in Coffee and Citrus. Journal of Economic Entomology. 2008;101(4):1114–1121. doi: 10.1603/0022-0493(2008)101[1114:TEOXFB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McKamey SH. Taxonomic catalogue of the leafhoppers (Membracoidea). Part 1. Cicadellinae. Memoirs of the American Entomological Institute. 2007;78:1–394. [Google Scholar]
- Metcalf ZP. General Catalogue of the Homoptera. Fascicle IV, Part 1 Tettigellidae. Agricultural Research Service; United States Department of Agriculture: 1965. [Google Scholar]
- Mizell RF, III, Andersen P. Keys to management of glassy-winged sharpshooter: interactions between host plants, malnutrition and natural enemies. Proceedings of the Pierce's Disease Research Symposium. 2001:81–84. [Google Scholar]
- Moreno CE. Métodospara medir la biodiversidad. MandT—Manuales y Tesis SEA, Volumen 1; Zaragoza: 2001. [Google Scholar]
- Morrone JJ. Biogeografía de América Latinay el Caribe. MandT—Manuales y Tesis SEA, Volumen 3; Zaragoza: 2001. [Google Scholar]
- Morrone JJ. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic. Analyses of the entomofauna. Annual Review of Entomology. 2006;51:467–94. doi: 10.1146/annurev.ento.50.071803.130447. [DOI] [PubMed] [Google Scholar]
- Nome SF, Haelterman RM, Docampo DM, Prataviera AG, Di Feo L, Del V. Escaldadura de las hojas del almendro en Argentina. Fitopatologia Brasileira. 1992;17(1):57–60. [Google Scholar]
- Paradell S. Especies argentinas de homópteros Cicadélidos asociados al cultivo de maíz Zea mays L. Revista de la Facultad de Agronomóa. 1995;71(2):213–234. [Google Scholar]
- Pilkington LJ, Irvin NA, Boyd EA, Hoddle MS, Triapitsyn SV, Carey BG, Jones WA, Morgan DJW. Introduced parasitic wasps could control glassy-winged sharpshooter. California Agriculture. 2005;59:223–228. [Google Scholar]
- Rakitov R, Dietrich C. Evolution and historical ecology of the Proconiini sharp shooters. Proceedings of the Pierce's Disease Research Symposium. 2001:139–140. [Google Scholar]
- Redak R, Purcell A, Lopes JRS, Blua M, Mizell RF, III, Andersen P. The biology of Xylem Fluid-Feeding Insect Vectors of Xylella fastidiosa and their relation to disease epidemiology. Annual Review of Entomology. 2004;49:243–270. doi: 10.1146/annurev.ento.49.061802.123403. [DOI] [PubMed] [Google Scholar]
- Remes Lenicov AMM, Tesón A. Cicadélidos que habitan los cultivos de arroz (Homoptera, Cicadellidae). Revista de Investigaciones Agropecuarias INTA. 1985;20(1):131–141. [Google Scholar]
- Remes Lenicov AMM, Paradell S, Virla EG, Mariani R, Costamagna A, Varela G. Cicadélidos y Delfácidos perjudiciales al cultivo de maíz en la República Argentina (Insecta - Homoptera). VI Congreso de Maíz. 1997;I:58–74. [Google Scholar]
- Remes Lenicov AMM, Virla EG, Manca ME. Difusión de Tapajosa rubromarginata (Homoptera: Cicadellidae) sobre cultivos cerealeros de la Argentina. Revista de la Sociedad Entomologica Argentina. 1998;57(1–4):18. [Google Scholar]
- Remes Lenicov AMM, Paradell S, De Coll OR, Agostini J. Cicadelinos asociados a citrus afectados por Clorosis Variegada (CVC) en la República Argentina (Insecta: Homoptera: Cicadellidae). Revista de la Sociedad Entomológica Argentina. 1999;58(3–4):211–225. [Google Scholar]
- Remes Lenicov AMM, Paradell S, Virla EG. Homoptera: Fulgoromorpha y Cicadomorpha. In: Cordo HA, Logarzo GA, Braun K, Di Iorio OR, editors. Catálogo de Insectos fitófagos de la Argentina. Sociedad Entomológica Argentina Press; 2004. pp. 330–342. [Google Scholar]
- Roberto SR, Coutinho A, Lima JEO, Miranda VS, Carlos EF. Transmissão de Xylella fastidiosa pelas cigarrinhas Dilobopterus costalimai, Acrogonia terminalis e Oncometopia facialis em citros. Fitopatologia Brasileira. 1996;21(4):517–518. [Google Scholar]
- Schröeder H. Taxionomische und tiergeographische Studien an neotropischen Zikaden (Cicadellidae, Tettigellinae). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft. 1959;499:1–93. [Google Scholar]
- Silva MRL, Meneguim AM, Paião FG, Meneguim L, Canteri MG, Leite RP., Jr Infectividade Natural por Xylella fastidiosa Wells et al. de Cicadelineos (Hemiptera: Cicadellidae) de Lavouras Cafeeiras do Paraná. Neotropical Entomology. 2007;36(2):274–281. doi: 10.1590/s1519-566x2007000200015. [DOI] [PubMed] [Google Scholar]
- Toledo AV, Virla EG, Humber RA, Paradell S, Lopez Lastra CC. First record of Clonostachys rosea (Ascomycota: Hypocreales) as an entomopathogenic fungus of Oncometopia tucumana and Sonesimia grossa (Hemiptera: Cicadellidae) in Argentina. Journal of Invertebrate Pathology. 2006;92(1):7–10. doi: 10.1016/j.jip.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Triapitsyn SV, Logarzo GA, Virla EG, De León JH. A new species of Gonatocerus (Hymenoptera: Mymaridae) from Argentina, an egg parasitoid of Tapajosa rubromarginata (Hemiptera: Cicadellidae). Zootaxa. 2007;1619:61–68. [Google Scholar]
- Triapitsyn SV, Logarzo GA, De León JH, Virla EG. A new Gonatocerus (Hymenoptera: Mymaridae) from Argentina, with taxonomic notes and molecular data on the G. tuberculifemur species complex. Zootaxa. 2008;1949:1–29. [Google Scholar]
- Triapitsyn SV, Huber JT, Logarzo GA, Berezovskiy VV, Aquino DA. Review of Gonatocerus (Hymenoptera: Mymaridae) in the Neotropical region, with description of eleven new species of Gonatocerus. Zootaxa. 2010;2456:1–243. [Google Scholar]
- Virla EG, Logarzo GA, Jones W, Triapitsyn SV. Biology of Gonatocerus tuberculifemur (Ogloblin) (Hymenoptera: Mymaridae), an egg parasitoid of the sharpshooter, Tapajosa rubromarginata (Hemiptera: Cicadellidae). The Florida Entomologist. 2005;88(1):67–71. [Google Scholar]
- Virla EG, Cangemi L, Logarzo GA. Suitability of different host plants for nymphs of the Sharpshooter Tapajosa rubromarginata (Hemiptera: Cicadellidae: Proconinii). The Florida Entomologist. 2007;90(4):766–769. [Google Scholar]
- Virla EG, Logarzo GA, Paradell S, Triapitsyn SV. Bionomics of Oncometopia tucumana (Hemiptera: Cicadellidae), a sharpshooter from Argentina, with notes on its distribution, host plants, and egg parasitoids. The Florida Entomologist. 2008;91(1):55–62. [Google Scholar]
- Virla EG, Luft Albarracin E, Triapitzyn SV, Viggiani G, Logarzo GA. Description and biological traits of a new species of Paracentrobia (Hymenoptera: Trichogrammatidae), an egg parasitoid of the sharp shooter Tapajosa rubromarginata (Hemiptera: Cicadellidae) in Argentina. Studies on Neotropical Fauna and Environment. 2009;44(1):47–53. [Google Scholar]
- Wilson MR, Turner JA, Mckamey SH. Sharpshooter Leafhoppers of the World (Hemiptera: Cicadellidae subfamily Cicadellinae). Amgueddfa Cymru - National Museum Wales. 2009. Available online, http://naturalhistory.museumwales.ac.uk/Shar pshooters.
- Yamamoto PT, Roberto SR, Praia-Júnior WD, Felippe MR, Miranda VS, Teixeira DC, Lopes JRS. Transmissao de Xylella fastidiosa pelas cigarrinhas Homalodisca ignorata, Acrogonia virescens e Molomea cincta (Hemiptera: Cicadellidae) em plantas cítricas. Summa Phytopathology. 2000;26(1):128. [Google Scholar]
- Young DA. Taxonomy study of the Cicadellinae (Homoptera: Cicadelllidae). Part 1 Proconiini. United States National Museum Bulletin 261. Smithsonian Institution Press; 1968. [Google Scholar]