Lead roles for supporting actors: critical functions of inner ear supporting cells (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 1.
Abstract
Many studies that aim to investigate the underlying mechanisms of hearing loss or balance disorders focus on the hair cells and spiral ganglion neurons of the inner ear. Fewer studies have examined the supporting cells that contact both of these cell types in the cochlea and vestibular end organs. While the roles of supporting cells are still being elucidated, emerging evidence indicates that they serve many functions vital to maintaining healthy populations of hair cells and spiral ganglion neurons. Here we review recent studies that highlight the critical roles supporting cells play in the development, function, survival, death, phagocytosis, and regeneration of other cell types within the inner ear. Many of these roles have also been described for glial cells in other parts of the nervous system, and lessons from these other systems continue to inform our understanding of supporting cell functions.
Keywords: hair cell, supporting cell, spiral ganglion neuron, hearing loss, cochlea, regeneration
Introduction
Hearing loss affects nearly 4 million children and 36 million American adults (NIDCD, 2010, NIDCD, 2006). Aging, noise trauma, ototoxic drugs, and hereditary mutations are all causes of hearing loss (Li-Korotky, 2012, Seixas et al., 2012, Cheng et al., 2009, Friedman and Griffith, 2003), a condition that has limited treatments and no known cure. In addition, in the United States, balance disorders affect over 600,000 individuals and similarly have few treatment options (NIDCD, 2010). Many studies aimed at understanding the mechanisms underlying hearing loss and balance disorders have focused on mechanosensory hair cells, the sensory receptor cells of the auditory and vestibular systems (Phillips et al., 2008). Fewer studies have examined the biology and functions of the supporting cells that surround hair cells. This review will discuss the emerging evidence indicating that auditory and vestibular supporting cells serve many critical functions, some of which are similar to functions carried out by glial cells (astrocytes, microglia, Schwann cells and oligodendrocytes), suggesting that supporting cells may represent a type of specialized glia.
The mammalian cochlea contains several types of supporting cells, each with a distinct morphology and a specific anatomical location within the organ of Corti (reviewed in Raphael and Altschuler, 2003). Deiters’ cells provide structural support for the outer hair cells, which are positioned atop and in direct contact with the Deiters’ cell layer (reviewed in Raphael and Altschuler, 2003). Pillar cells form the tunnel of Corti, which lies between the inner and outer hair cells. Hensen’s and Claudius cells both lie lateral to the outer hair cells in the outer sulcus. Supporting cells are less well-characterized than hair cells and in striving for better characterization, analogies have been drawn between supporting cells of the inner ear and those of other sensory systems, including the olfactory sustentacular cells and the retinal Müller glia (Rubel et al., 1991). Some similarities and significant differences between auditory supporting cells and these other sensory supporting cell types will be discussed in this review.
Emerging evidence suggests that auditory and vestibular supporting cells serve important functions as mediators of hair cell development, function, death and phagocytosis (Tritsch et al., 2007, Jagger and Forge, 2006, Bird et al., 2010, Lahne and Gale, 2008). Recent reports also indicate that supporting cells may mediate the survival and function of SGNs (Zilberstein et al., 2012). Many of these supporting cell functions are paralleled by glia in their relationship with neurons. Glial cells support neuronal function and survival in many ways. For example, both astrocytes and oligodendrocytes provide trophic support for neurons (Wilkins et al., 2003, Banker, 1980). Astrocytes support neuronal function and survival by clearing glutamate from neuronal synapses (Rothstein et al., 1996) and buffering potassium through a system of gap junctions (reviewed in Leis et al., 2005). Microglia play critical roles in the response to neuronal injury, engulfing apoptotic neurons in the central nervous system (reviewed in Napoli and Neumann, 2009). Following neuronal death in the retina, Müller glia serve as neural precursors for regenerated retinal neurons (Fischer and Reh, 2001). Many of these functions of glial cells are similar to those that have been described for auditory and vestibular supporting cells.
Development and survival of hair cells and spiral ganglion neurons
In the developing mammalian cochlea, the onset of neuronal activity results from coordinated signaling from hair cells, supporting cells, and SGNs. Cochlear hair cells are depolarized upon deflection of their stereocilia (Flock, 1965, Russell et al., 1986), which triggers the release of glutamate from inner hair cells (IHCs) (Kataoka and Ohmori, 1994). Glutamate binds to synaptic receptors on adjacent SGNs, resulting in the generation of action potentials and transmission of the afferent signal to the auditory brainstem (Ruel et al., 1999, reviewed in Cunningham and Tan, 2011). While sound energy is the stimulus that ultimately results in the generation of action potentials after the developmental onset of hearing, hair cells depolarize and release glutamate, resulting in spontaneous action potentials prior to hearing onset (Lippe, 1994, Jones et al., 2007, Tritsch et al., 2007). This spontaneous activity may contribute to the guidance and refinement of synaptic connections, including the formation of the tonotopic map of the inner ear (reviewed in Walmsley et al., 2006).
Recent evidence indicates that cochlear supporting cells may mediate the initiation of spontaneous activity during cochlear development. Prior to the onset of hearing in rats, ‘inner’ supporting cells (ISCs), which are the columnar epithelial cells specific to Kolliker’s organ, spontaneously release ATP (Tritsch et al., 2007, Tritsch and Bergles, 2010). As early as the day after birth (P1) this extracellular ATP release results in inward currents and depolarization of IHCs (Tritsch et al., 2007, Tritsch and Bergles, 2010). After P4, IHCs consistently exhibit spontaneous inward currents in response to ATP released by supporting cells. These events occur with increasing frequency and amplitude until the onset of hearing at P10-12 (Tritsch and Bergles, 2010). After hearing onset, the frequency and amplitude of the inward currents rapidly decline. Inward currents tend to occur simultaneously among neighboring IHCs (<100 μm from each other), suggesting that ATP released from supporting cells synchronizes patterns of IHC activity before hearing onset (Tritsch et al., 2007, Tritsch and Bergles, 2010). While not every IHC depolarization generates an action potential, SGN bursting is observed after P4 and appears to rely exclusively upon the ATP release from ISCs (Tritsch and Bergles, 2010). Taken together, these data indicate that supporting cells are important mediators of spontaneous neural activity in the developing organ of Corti. Additional recent data suggests that the resting mechanotransducer current of inner hair cells may also be an important factor in driving this spontaneous activity, although the interplay between the supporting cells and the mechanotransducer current during inner hair cell development remains unclear (Johnson et al., 2012).
Supporting cells are also critical to SGN survival in the mature cochlea. Multiple groups have demonstrated degeneration of SGNs following aminoglycoside-induced hair cell death (Dupont et al., 1993, McFadden et al., 2004). One interpretation of these data is that the survival of SGNs is dependent upon being coupled to viable hair cells (Koitchev et al., 1982), which provide trophic support necessary for SGN survival (reviewed in Gillespie and Shepherd, 2005). However, recent evidence suggests that, at least in the short- to mid-term, SGNs may instead be dependent upon supporting cells for their survival. Using a mutant mouse model with a targeted deletion of a high-affinity thiamine transporter (Slc19a2), in which extensive IHC death can be induced without loss of supporting cells, Zilberstein et al. (2012) showed that SGNs survive for at least three months after the death of adjacent IHCs. These data indicate that viable IHCs may not be necessary for SGN survival, and they are in agreement with other data indicating that Brn3c (now called Pou4f3) null mutant mice, in which hair cells degenerate in the neonatal period, continue to exhibit some surviving SGNs, even in 6-month-old mutant mice (Xiang et al., 2003). Given the above, it is possible that the SGN degeneration that follows aminoglycoside treatment is at least in part a result of toxic effects of aminoglycosides on either supporting cells or SGNs (Sugawara et al., 2005).
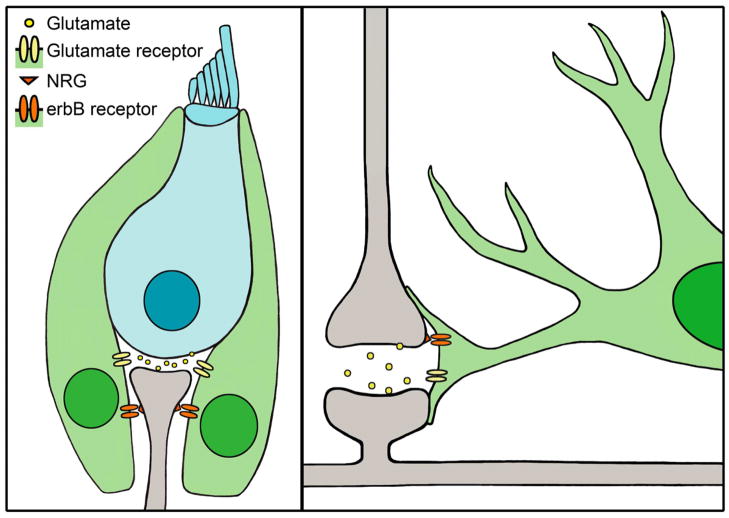
Supporting cells provide trophic factors that promote the survival of SGNs. Brain-derived neurotrophic factor (BDNF), a critical trophic factor in neural development and survival, is expressed in vestibular supporting cells of postnatal mammals (Montcouquiol et al., 1998, Gomez-Casati et al., 2010). BDNF conditional knockout mice (in which the Pax2 promoter drives Cre expression to eliminate BDNF expression in the entire inner ear) exhibit a reduction in IHC synaptic ribbons and afferent SGN fibers (Zuccotti et al., 2012). In addition, neuregulins (NRGs) are critical trophic factors for SGNs and are expressed by SGNs (Figure 1). NRGs bind complementary erbB receptors expressed by multiple cochlear supporting cell types, including inner border cells, inner phalangeal cells, Deiters’ cells, pillar cells, Boettcher cells and inner sulcus cells. When erbB-NRG signaling between supporting cells and SGNs is disrupted in transgenic mice expressing a dominant-negative erbB4 receptor, type I SGNs degenerate (Stankovic et al., 2004). The likely cause of the SGN degeneration in these mice is a reduction in neurotrophin-3 (NT3) expression (Stankovic et al., 2004), which is critical for SGN survival during development (Fritzsch et al., 1999) and is strongly expressed by both auditory and vestibular supporting cells at birth (Sugawara et al., 2007). Gradients of NT3 expression in cochlear and vestibular supporting cells suggest a role for NT3 that extends until at least P15 in mice (Sugawara et al., 2007). Similar trophic interactions are observed between glia and neurons (Figure 1). In the same mouse model of disrupted erbB-NRG signaling, there are significant defects in neuron-glia interactions, including disruption of radial astroglia and severely impaired neuronal migration in vitro (Rio et al., 1997). Additionally, culturing neurons in medium conditioned by astrocytes causes neurons to develop more processes and exhibit less degeneration than those in control medium, suggesting that some trophic factors provided by the astrocytes are soluble (Banker, 1980). Similar improvements in neuronal survival and process length are observed with medium conditioned predominantly by oligodendrocytes (Wilkins et al., 2003). Together, these studies suggest that both glia and supporting cells provide trophic factors that are critical for neuronal survival.
Figure 1.
Supporting cells and glia provide trophic factors to neurons and clear glutamate from the synapse. Left panel, Hair cells (blue) synapse with spiral ganglion neurons (gray), and are surrounded by supporting cells (green). Hair cells release glutamate, which is cleared from the synapse by glutamate receptors expressed by supporting cells. Spiral ganglion neurons express NRG, which binds to erbB receptors located on the supporting cells, thereby promoting SGN survival. Right panel, illustration of a tripartite synapse between two neurons (gray) and an astrocyte (green). The presynaptic neuron (top) releases glutamate into the synapse, which is cleared from the synapse by glutamate receptors on the astrocyte. Neurons also express NRG, which binds to erbB receptors located on the astrocyte, an interaction necessary for normal astroglial morphology and neuronal migration.
In addition to their roles in SGN development and survival, supporting cells also mediate glutamate clearance at synapses. Glutamate, an excitatory neurotransmitter, must be cleared from the synapse to maintain synaptic function and prevent excitotoxicity (reviewed in Pujol and Puel, 1999, and in Gale and Jagger, 2010). In the organ of Corti, supporting cells that surround IHCs express the glutamate aspartate transporter (GLAST, Figure 1) (Furness and Lawton, 2003, Furness and Lehre, 1997). GLAST specifically mediates glutamate transporter currents recorded from the inner phalangeal cells of the rodent cochlea, while transporter currents are not observed in IHCs or afferent dendrites (Glowatzki et al., 2006). The inward currents generated in response to glutamate versus aspartate application are consistent with GLAST transporters, and these inward currents cannot be induced in GLAST knockout mice, indicating that GLAST is the specific mediator of glutamate transporter currents (Glowatzki et al., 2006). Thus, supporting cells mediate the removal of glutamate from excitatory synapses in the cochlea. Similarly, knockdown studies in rats indicate that glia are responsible for glutamate removal in the CNS in vivo (Figure 1, right). The loss of glutamate transporters in astroglia, but not neurons, results in an elevation of extracellular glutamate, leading to excitotoxicity (Rothstein et al., 1996).
Supporting cells are also thought to be critical to the regulation of potassium recycling that is required for hearing. The apical surfaces of hair cells are bathed in potassium-rich endolymph. The process of generating this specialized extracellular environment also results in the generation of the endocochlear potential, a transmembrane potential difference of approximately +80 to 90 mV, relative to the perilymph-filled compartments. This potential difference contributes to the driving force underlying hair cell depolarization and is thereby critical for sound transduction. Potassium is the major charge carrier in the endolymph that depolarizes hair cells upon entry, and defects in potassium transport result in deafness (Knipper et al., 2006, Kubisch et al., 1999, reviewed in Zdebik et al., 2009). Under normal conditions, the potassium concentrations are kept constant at ~150 mM in endolymph, ~6 mM in perilymph, and ~2 mM in the intrastrial fluid of the stria vascularis (reviewed in Wangemann, 2006). The generation and maintenance of this steep ionic gradient requires a significant expenditure of cellular energy, a burden that is placed primarily on the cells of the stria vascularis and then on the cochlear supporting cells. While the exact route of potassium recycling through the organ of Corti is still being elucidated, several models have been proposed that are not mutually exclusive (reviewed in Zdebik et al., 2009). One model of potassium recycling involves potassium returning to the stria directly through the perilymph without entering supporting cells, while two other models rely heavily on supporting cells to buffer potassium from both inner and outer hair cells before its return to the stria vascularis (reviewed in Zdebik et al., 2009, and in Mistrik and Ashmore, 2009). In both of the models that implicate supporting cells, potassium exiting the hair cells after depolarization is taken up by neighboring inner phalangeal cells or Deiters’ cells before either (1) being transported into perilymph and returned to the stria via fibrocytes, or (2) moving laterally through the supporting cell population back to the stria vascularis (reviewed in Zdebik et al., 2009). In all three models, the marginal cells of the stria then pump potassium back into endolymph (reviewed in Zdebik et al., 2009). Supporting evidence for the essential role of potassium buffering by supporting cells comes from studies demonstrating progressive deafness in mice lacking potassium transporters normally expressed in Deiters’ cells and inner phalangeal cells (Boettger et al., 2002, Boettger et al., 2003). In addition, detailed dye transfer studies confirm that connexin channels expressed in supporting cells allow for lateral flow among compartmentalized subsets of the supporting cell population in the postnatal rat organ of Corti, indicating that this might be a viable route for potassium to return to the stria vascularis (Jagger and Forge, 2006). Uncoupling of gap junctions in supporting cells results in impaired compound action potentials and distortion-product otoacoustic emissions, as well as an impaired endocochlear potential, suggesting that ion flow among supporting cells is necessary for cochlear homeostasis and thus normal function (Spiess et al., 2002). These data implicate supporting cells as important mediators of ion transport within the cochlea, a role that has also been described for glia. Using both passive diffusion and an extensive system of gap junctions, astrocytes spatially buffer extracellular potassium and redistribute local potassium elevations (reviewed in Leis et al., 2005).
Hair cell death and survival
Auditory hair cells are both very sensitive in terms of detecting sound and also highly susceptible to damage. The mechanisms that determine whether a hair cell under stress ultimately lives or dies are only beginning to be understood. Recent studies have revealed some of the molecular signals that are activated in supporting cells in response to hair cell damage, and these findings suggest that supporting cells may act as critical mediators of hair cell death (reviewed in Gale and Jagger, 2010) and survival. Laser ablation or mechanical damage to hair cells results in an intercellular calcium wave that propagates in supporting cells and moves away from the site of damage, suggesting a supporting cell-specific response to hair cell stress (Lahne and Gale, 2010, Gale et al., 2004). Recent work indicates that there are two distinct calcium waves - a faster wave that utilizes extracellular calcium and travels through outer hair cells, and a slower wave that relies upon intracellular calcium stores and propagates in Deiters’ and other supporting cells. Both of these calcium waves rely upon extracellular ATP acting on purinergic P2 receptors for propagation (Lahne and Gale, 2010). Evidence for intercellular signaling between stressed hair cells and surrounding supporting cells comes from studies examining the roles of extracellularly regulated kinases 1 and 2 (ERK 1/2), which are activated exclusively in supporting cells in postnatal rat cochlea following either mechanical damage or aminoglycoside treatment in vitro (Lahne and Gale, 2008). The activation of ERK1/2 is immediately preceded by a calcium wave in the hair cell region and is reduced in a calcium-chelated environment (Lahne and Gale, 2008). Pharmacological inhibition of ERK signaling in supporting cells inhibits hair cell death (Lahne and Gale, 2008), suggesting that supporting cells act as mediators of hair cell death. Similar results have also been reported for glia in an in vitro rat model of Parkinson’s disease, in which glutathione depletion leads to neuronal death, as well as an increase in ERK1/2 activation in glial cells (de Bernardo et al., 2004). Inhibition of ERK signaling reduces neuronal death, but only in mixed neuron/glia co-cultures and not in enriched neuronal cultures, implicating glial cells as mediators of neuronal death.
Supporting cells can also act as mediators of hair cell survival. In response to heat stress, supporting cells of the mouse utricle rapidly upregulate expression of heat shock protein 70 (HSP70), which is not upregulated in hair cells. Viral-mediated expression of HSP70 in supporting cells protects hair cells against aminoglycoside-induced death (Lindsey May, Carlene Brandon, and Lisa Cunningham, unpublished observations), suggesting that supporting cells can promote hair cell survival. Taken together with the studies above indicating that supporting cells can mediate hair cell death, these data suggest that supporting cells may be critical determinants of whether hair cells under stress ultimately live or die.
Supporting cells also play important roles in the clearance of dead or damaged hair cells. When a hair cell dies, its corpse is removed from the epithelium with remarkable precision and minimal disruption of the surrounding tissue architecture and function. Indirect evidence indicates that the process of hair cell removal is a coordinated series of events, which may include extrusion of the hair cell and/or sub-luminal phagocytosis (Li et al., 1995, Forge, 1985, Seoane and Llorens, 2005, Hirose et al., 1999). A transmission electron microscopy (TEM) study in guinea pig cochlea after systemic gentamicin treatment provides evidence for both modes of hair cell corpse removal in the same lesion: some hair cells appear to undergo extrusion from the epithelium, while other hair cells are phagocytosed within the epithelium by surrounding supporting cells (Li et al., 1995). During sub-luminal phagocytosis, the majority of the hair cell, with the exception of the stereocilia bundle and cuticular plate, is destroyed beneath the epithelial surface (Figure 2). Processes from adjacent supporting cells invade the hair cell just below the cuticular plate, separating the stereocilia bundle from the rest of the hair cell body (Li et al., 1995, Gale et al., 2002). A definitive study of this process utilizing time-lapse confocal imaging in the living chick utricle following streptomycin treatment shows that supporting cells form a thick actin cable below the level of the reticular lamina. This actin cable forms a ring beneath the cuticular plate of a damaged hair cell. As the actin ring constricts, the cuticular plate and stereocilia bundle are pinched off from the rest of the hair cell body (Bird et al., 2010). Within 2–3 hours of bundle removal, the supporting cells form phagocytic structures composed of multiple cells, which the authors termed “phagosomes”. These phagosomes engulf the remaining bundleless hair cell beneath the luminal surface, thereby completing the process of hair cell elimination. Both sub-luminal phagocytosis and hair cell extrusion may allow for the removal of hair cells from the epithelium while maintaining the integrity of the reticular lamina. While hair cell extrusion was not observed in the extrastriolar regions of the utricles in the live imaging study, extrusion did occur in the striola, as well as in the basilar papilla (J. Bird and J. Gale, unpublished observations). Hair cell extrusion is observed via electron and fluorescent microscopy in the basilar papilla following either acoustic overstimulation (Cotanche et al., 1987, Cotanche and Dopyera, 1990, Marsh et al., 1990, Nakagawa et al., 1997, Stone and Cotanche, 1992) or high-dose aminoglycoside treatment (Hirose et al., 2004, Mangiardi et al., 2004). Extrusion can occur rapidly, with initial signs observed as early as 4 hours after acoustic trauma (Stone and Cotanche, 1992) or 8–12 hours after a single injection of high-dose gentamicin (Hirose et al., 2004, Mangiardi et al., 2004). The span of time required for ejection from the epithelium is approximately 1–2 days after gentamicin injection, with the extrusion process complete by 54 hours post-injection (Hirose et al., 2004, Mangiardi et al., 2004). During extrusion, hair cells swell and are typically observed emerging from the reticular lamina whole, although some cells are fragmented (Hirose et al., 2004, Nakagawa et al., 1997). In fact, hair cells often do not lose membrane integrity until after they have been completely extruded from the epithelium, with a portion of ejected hair cells continuing to exhibit esterase activity, a sign of cellular viability (Mangiardi et al., 2004). However, hair cells exhibit some signs of apoptosis directly before and during extrusion, including translocation of T-cell restricted intracellular antigen-related protein (TIAR), release of cytochrome c from mitochondria, and activation of caspases-3 and -9 (Mangiardi et al., 2004, Kaiser et al., 2008). The intercellular junctions between the hair cell and surrounding supporting cells remain uncompromised throughout the extrusion process, with points of contact at tight junctions gradually reducing contact until the hair cell is extruded into endolymph, suggesting that supporting cells are actively extruding the hair cell and maintaining the sensory epithelium (Hirose et al., 2004). Other evidence indicating concerted supporting cell activity during extrusion comes from studies of acoustic overexposure, in which the apical surface area of affected hair cells shrinks and supporting cells expand across the epithelium surface (Cotanche et al., 1987, Marsh et al., 1990, Stone and Cotanche, 1992).
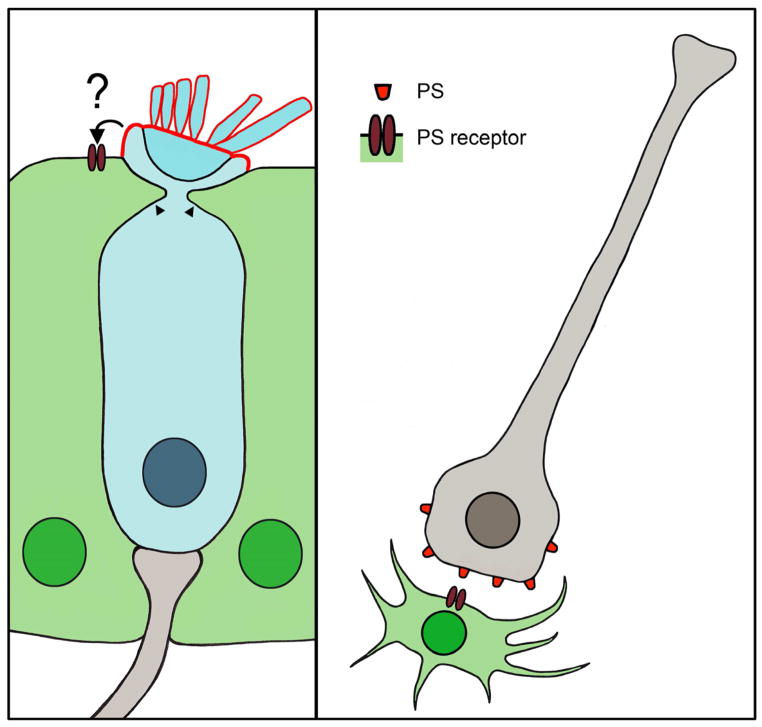
Figure 2.
Supporting cells and glia eliminate dead cells. Left panel, illustration of supporting cell processes (arrowheads) invading a neighboring hair cell (blue) during the process of hair bundle excision. Phosphatidylserine (PS, red outline) exposure is restricted the apical membrane of the hair cell, but its interaction with a PS receptor on neighboring supporting cells is unclear. Right panel, illustration of a neuron (gray) as a microglia (green) approaches for phagocytosis. The damaged neuron exposes PS, which binds a PS receptor expressed by the microglia.
While cell death and corpse removal are generally thought to be sequential events, emerging data from other systems indicate that phagocytosis can be a primary cause of cell death in some cases (Neher et al., 2011, Zoller et al., 2011, Neniskyte et al., 2011). This process of cellular execution, termed “phagoptosis”, is thought to be a combination of phagocytosis and apoptosis (Brown and Neher, 2012). Microglia can eliminate neurons in this manner (Neher et al., 2011, Neniskyte et al., 2011, Fricker et al., 2012), which has also been observed between macrophages and blood cells (Zoller et al., 2011). Exposure of phosphatidylserine (PS) on the outer leaflet of the cell membrane is the best-characterized cellular signal that marks cells for engulfment by professional phagocytic cells, such as macrophages or microglia (Figure 2) (Neher et al., 2011, reviewed recently in Ravichandran, 2011). This so-called “eat295 me signal” is an indication of cellular distress, allowing for the initiation of phagocytosis (Ravichandran, 2010). Cells exposing PS were thought to have initiated an irreversible apoptotic program and thus be beyond repair. However, preventing microglial phagocytosis of neurons by masking PS or by blocking PS signaling prevents neuronal death in the central nervous system (Neher et al., 2011, Neniskyte et al., 2011). These data have two important implications: the first is that exposure of an “eat-me” signal does not necessarily signal irreversible death, but rather may indicate a temporary distressed condition from which the cell is able to recover. The second is that glial cells can destroy viable cells by initiating phagocytosis of living neurons.
An intriguing possibility is that supporting cells may be able to remove “undead” hair cells. As mentioned above, supporting cells remove hair cells from the sensory epithelium (Raphael and Altschuler, 1991, Bird et al., 2010, Li et al., 1995), but the assumption has been that those hair cells were dead (or at least irreversibly dying) before they were engulfed by supporting cells. However, recent evidence indicates that hair cells can reversibly expose PS, suggesting that supporting cells may have an opportunity to eliminate living hair cells via phagoptosis (Goodyear et al., 2008). Following short-term aminoglycoside exposure, PS translocation to the outer membrane is reversible in hair cells, suggesting a temporary stressed state (Goodyear et al., 2008). Since the PS externalization is restricted to the apical membrane, which is an area that is not in contact with any surrounding cells, it is unclear whether this signal is an important signal for phagocytosis. It is also unclear whether apoptosis always precedes phagocytosis. One of the inherent challenges in these studies is the lack of a consistent, rigorous definition of cell death in general (Kroemer et al., 2005). PS externalization that is restricted to the apical membrane has also been reported in hair cells seemingly unassociated with either apoptosis or necrosis, and in those cells that do show obvious signs of degeneration, PS exposure was more prominently expressed over the entire cell membrane (Shi et al., 2007). Additionally, macrophages have been reported to phagocytose ostensibly healthy supporting cells and supporting cell progeny (some of which appear to be differentiating hair cells) following selective hair cell laser ablation in the axolotl lateral line (Jones and Corwin, 1996).
Additional evidence that supporting cells may eliminate viable hair cells comes from studies in the bullfrog saccule showing that hair cells can survive even after removal of the bundle from the cell body. Hair cells underwent bundle excision following low-dose gentamicin treatment, yet the bundleless hair cells continued to stain positively for HCS-1 and Calcein-AM and negatively for ethidium homodimer, suggesting that the hair cells may have remained viable after bundle excision (Gale et al., 2002). The neighboring supporting cells in the bullfrog saccule do not appear to phagocytose the bundleless hair cells, yet the mechanism of bundle removal appears similar to that observed in the chick. Following bundle removal in the chick utricle, until the point of phagosome engulfment, the hair cell membrane remained intact, with the engulfment and loss of membrane integrity so closely correlated that they were temporally indistinguishable (Bird et al., 2010). Taken together with the reversibility of PS expression, these data suggest that supporting cells may eliminate hair cells that could otherwise survive. If this is the case, inhibition of supporting cell phagocytosis may represent an opportunity for development of clinical therapies aimed at preventing hair cell death. To more accurately gauge whether phagocytosis is a primary cause of hair cell death, the phagocytic activity must be inhibited under stress and hair cell death re-evaluated. This is technically challenging, since the most effective method of blocking PS signaling in hair cells requires calcium chelation, a treatment that disrupts tip links, thereby interfering with hair cell function (Goodyear et al., 2008). Additional methods of inhibiting supporting cell phagocytic activity will likely further illuminate the potential roles of the supporting cells as hair cell executioners.
Supporting cells rapidly expand to fill the empty spaces and form scars in the areas previously occupied by hair cells (Raphael and Altschuler, 1991). The process of phalangeal scar formation has been documented in the guinea pig cochlear and vestibular systems (Raphael and Altschuler, 1991, Meiteles and Raphael, 1994). As described above during the process of hair cell elimination, supporting cells form a thick actin ring around the injured hair cell, and disruption of actin polymerization blocks scar formation in the bullfrog saccule (Hordichok and Steyger, 2007). The scar is ultimately formed by supporting cells that contact the injured hair cell (Meiteles and Raphael, 1994, Raphael and Altschuler, 1991). The surrounding supporting cells expand to fill the void left by the degenerating hair cell, and they also invade regions not typically occupied by supporting cells, including the spaces of Nuel. The integrity of the reticular lamina appears to be maintained by the supporting cells throughout the process of scar formation (Raphael and Altschuler, 1991, Meiteles and Raphael, 1994, Gale et al., 2002, Bird et al., 2010). Previous work using lanthanum as an electron-dense tracer in the gentamicin-damaged guinea pig organ of Corti supports the concept of a continuously intact reticular lamina during aminoglycoside treatment (McDowell et al., 1989). However, a study using a carbon tracer in the noise-damaged chinchilla organ of Corti found that intense noise exposure may create transient lesions in the reticular lamina, which are repaired by supporting cells as they form phalangeal or epithelial scars (Ahmad et al., 2003).
Hair cell regeneration
While the mature mammalian vestibular system exhibits a limited capacity for hair cell regeneration, spontaneous hair cell regeneration has not been reported in the mature organ of Corti (Li and Forge, 1997, Zheng et al., 1999, Yamasoba and Kondo, 2006, Warchol et al., 1993). However, non-mammalian vertebrates have the capacity for robust regeneration of lost hair cells. The first reports of hair cell regeneration in birds were published in the 1980’s (Cotanche, 1987, Corwin and Cotanche, 1988, Cruz et al., 1987, Ryals and Rubel, 1988), and these reports launched the field of hair cell regeneration, which has since led to a detailed understanding of how lost hair cells are replaced in these species. Since mammals and birds/amphibians evolved from a distant common ancestor (Aboitiz et al., 2002), it seems possible that an evolutionary mechanism that prevents hair cell regeneration is present in mammals but not in birds and amphibians. Circumventing this inhibitory mechanism might therefore result in the restoration of lost hair cells in mammals. The past 25 years have seen discoveries in both the process of hair cell regeneration as well as the factors that control this process.
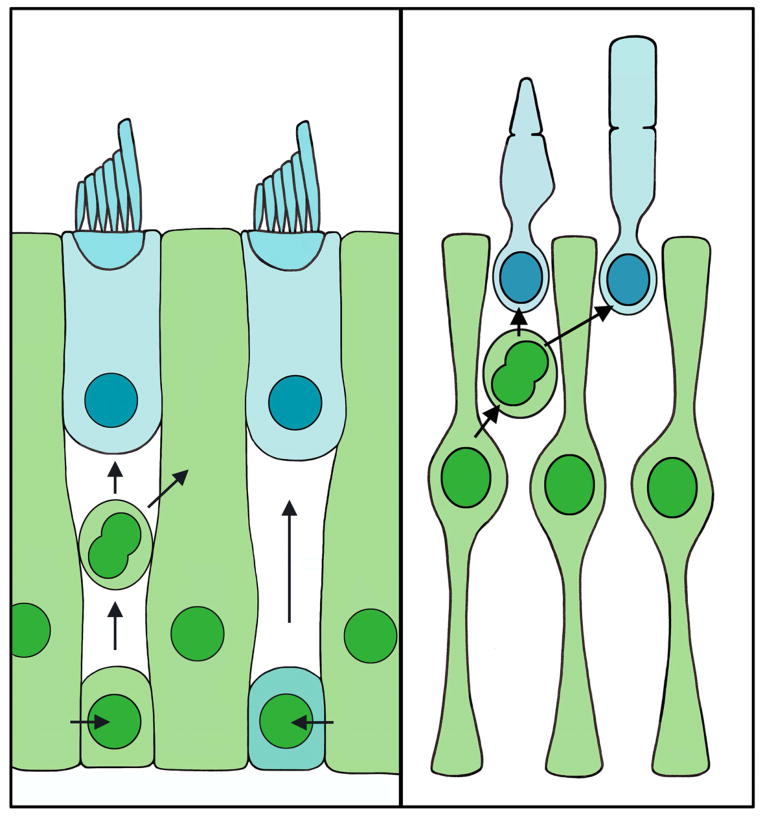
Hair cell regeneration occurs via two primary mechanisms, mitotic replacement and direct transdifferentiation. Supporting cells mediate both of these modes of hair cell regeneration (Figure 3). During mitotic replacement, supporting cells divide to generate new hair cells, while direct transdifferentiation involves a supporting cell undergoing a transformation to become a hair cell without dividing. Numerous studies indicate that supporting cells are the progenitor cells for proliferative (mitotic) hair cell regeneration following damage (Matsui et al., 2000, Warchol and Corwin, 1996, Jones and Corwin, 1996, Raphael, 1992, Balak et al., 1990, Bhave et al., 1998, Stone and Cotanche, 1994). In the mature, undamaged avian auditory system, hair cells exist in a quiescent state, while in the avian vestibular system, hair cells are continuously replaced (Jorgensen and Mathiesen, 1988, Roberson et al., 1992). Despite this difference in basal states, both systems are capable of regenerating hair cells following damage. Following ototoxic drug exposure, laser ablation or acoustic trauma, supporting cells re-enter the cell cycle, with those near the site of damage progressing fully through the cell cycle to produce new hair cells and supporting cells (Matsui et al., 2000, Raphael, 1992, Warchol and Corwin, 1996). Hair cell regeneration via supporting cell proliferation has been demonstrated in multiple species, including amphibians, fish, and birds (Balak et al., 1990, Ma et al., 2008, reviewed in Bermingham-McDonogh and Reh, 2011), but it has been best characterized in the avian inner ear (reviewed in Stone and Rubel, 2000, and in Stone and Cotanche, 2007). Supporting cells in the damaged avian auditory epithelium label positively with the mitotic markers tritiated thymidine or BrdU (Corwin and Cotanche, 1988, Ryals and Rubel, 1988, Lippe et al., 1991, Weisleder et al., 1995, Stone et al., 1999). When hair cell loss is localized using a laser microbeam, proliferation is evoked in supporting cells within ~200 μm of the lesion (Warchol and Corwin, 1996). The mechanisms that regulate cell cycle re-entry in supporting cells are not fully understood, but supporting cells likely de-differentiate at least partially from their mature phenotypes to downregulate expression of several supporting cell-specific molecules (Warchol, 2007). Supporting cells enter S-phase as early as 16 hours after hair cell death caused by laser ablation or noise exposure in chicks (Warchol and Corwin, 1996, Stone and Cotanche, 1994), although the peak of supporting cell mitotic activity occurs 2–5 days following noise exposure or aminoglycoside treatment (Bhave et al., 1995, Bhave et al., 1998, Cafaro et al., 2007, Stone et al., 1999, Roberson et al., 2004, Stone and Cotanche, 1994, Raphael, 1992, Shang et al., 2010). Supporting cells divide either symmetrically (resulting in either two new hair cells or two new supporting cells), or asymmetrically (resulting in one new hair cell and one new supporting cell) (Jones and Corwin, 1996, Stone and Cotanche, 1994, Stone and Rubel, 1999). It is unclear whether supporting cells can undergo multiple rounds of division, which could conceivably generate new hair cells without depleting the supporting cell population (Stone and Cotanche, 1994, Wilkins et al., 1999). Mitotic replacement is also observed in the glial cells of the retina, where Müller glia can serve as retinal progenitor cells, proliferating after damage to replace lost retinal neurons in fish, and to a more limited extent in birds (Figure 3) (reviewed in Bermingham-McDonogh and Reh, 2011). However, unlike supporting cells, there is no evidence that Müller glia can replace retinal neurons by direct transdifferentiation.
Figure 3.
Supporting cells and glia mediate the regeneration of dead cells. Left panel, illustration of supporting cells (green) acting as progenitors for regenerated hair cells (blue). Supporting cells can replace damaged hair cells and supporting cells through mitotic regeneration. Alternatively, supporting cells can replace hair cells by direct transdifferentiation. Right panel, illustration of Müller glia (green) dividing to replace damaged photoreceptors (blue).
Supporting cells undergo direct transdifferentiation in frogs, newts, birds, and to a more limited extent, mammals (Baird et al., 1996, Baird et al., 2000, Taylor and Forge, 2005, Shang et al., 2010, Lin et al., 2011, Li and Forge, 1997). In this alternative mechanism of regeneration, a supporting cell alters its phenotype into that of a hair cell without undergoing mitosis. In the chick, hair cells regenerated by direct transdifferentiation appear before those generated by proliferative regeneration (Cafaro et al., 2007, Roberson et al., 2004). In the gentamicin-treated chick utricle, expression of Atoh1, a transcription factor that is necessary for hair cell differentiation, is activated in those supporting cells undergoing transdifferentiation into hair cells (Cafaro et al., 2007). Recent studies in both amphibians and chicks indicate that transdifferentiation may account for a higher proportion of regenerated hair cells than previously thought (Taylor and Forge, 2005), with one recent study reporting that an average of 87% of hair cells regenerated in the chick BP following streptomycin treatment lacked the mitotic marker BrdU (Taylor and Forge, 2005, Shang et al., 2010). However, BrdU-positive supporting cells were present in higher numbers in the streptomycin-treated organ cultures relative to the control cultures, suggesting that supporting cell division does contribute to hair cell regeneration. This mode of hair cell regeneration may be important to prevent the depletion of the supporting cell population. When proliferation in streptomycin-treated cultures is blocked using aphidicolin, direct transdifferentiation still occurs, but it is accompanied by a 28% decrease in the total epithelial cell population (Shang et al., 2010). This finding highlights the importance of supporting cell division in maintaining the steady-state cell population of the tissue when the predominant mode of hair cell replacement is non-mitotic.
The factors that determine whether supporting cells undergo proliferative regeneration or direct transdifferentiation to replace damaged hair cells are still being investigated. Inhibition of fibroblast growth factor (FGFR) signaling in chick progenitor and supporting cells caused an increase in hair cell number but no increase in proliferation (Jacques et al., 2012), suggesting that FGF signaling inhibits transdifferentiation. Notch signaling is critical to hair cell development, both in prosensory patch formation within the developing cochlea as well as lateral inhibition of surrounding supporting cells from differentiating into hair cells themselves (Hartman et al., 2010, reviewed in Kelley, 2006). In addition to these roles during development, the Notch signaling pathway also regulates hair cell regeneration by supporting cells. Inhibiting Notch signaling augments the total number of regenerated hair cells in the zebrafish lateral line, postnatal mouse utricle, guinea pig cochlea, and avian basilar papilla, indicating that this pathway is a key regulator of the supporting cell-to-hair cell transition (Lin et al., 2011, Ma et al., 2008, Hori et al., 2007, Daudet et al., 2009). However, the mode of differentiation into hair cells appears to differ among species. Inhibition of Notch signaling promotes supporting cell proliferation followed by differentiation in the zebrafish (Lin et al., 2011) and direct transdifferentiation in the mouse utricle (Ma et al., 2008), while both mechanisms are reported in the chick (Daudet et al., 2009).
An important open question is whether every supporting cell has the capacity to replace damaged hair cells, or if regenerative capacity is restricted to a subset of supporting cells. In the aminoglycoside-damaged avian cochlea, most of the supporting cells re-enter the cell cycle (Bhave et al., 1995). However, only those supporting cells located in the region of hair cell loss ultimately progress through cell division following either gentamicin exposure (Bhave et al., 1995) or acoustic trauma (Corwin and Cotanche, 1988, Ryals and Rubel, 1988). These data suggest that multiple signals are necessary for supporting cells to regenerate hair cells - at least one signal initiates cell cycle re-entry, while additional signal(s) that are localized to the damage site are required for supporting cells to fully progress through the cell cycle and produce new hair cells. While the entire supporting cell population demonstrates the capacity for mitotic re-entry, it is unclear if some possess more stem cell-like qualities than others. Murine supporting cells in the auditory and vestibular systems express markers that are associated with (but perhaps not exclusive to) progenitor cells, including Sox2 (Hume et al., 2007), Nestin (Lopez et al., 2004) Notch (Lanford et al., 1999), and Musashi1 (Sakaguchi et al., 2004). The genes that encode these markers are all developmentally-regulated in the mouse inner ear, becoming more spatially restricted in mature animals (Lopez et al., 2004, Lanford et al., 1999, Hume et al., 2007, Sakaguchi et al., 2004). However, expression of these progenitor cell markers persists in at least a subset of mature supporting cells, suggesting that the mammalian inner ear retains some of the signals that mediate hair cell replacement in regenerative systems. Recent work suggests that Lgr5, a stem cell marker that is regulated by Wnt signaling (Haegebarth and Clevers, 2009), may identify hair cell precursors in the early postnatal mouse cochlea (Chai et al., 2012, Shi et al., 2012). In birds, supporting cells express the progenitor markers Islet-1 (Li et al., 2004) and Prox1 (Stone et al., 2004). Prox1 expression may regulate which supporting cells regenerate damaged hair cells in the basilar papilla (Stone et al., 2004). However, in mice, Prox1 is downregulated in supporting cells during the second postnatal week (Bermingham-McDonogh et al., 2006), which may represent a key difference in regenerative capacity between birds and mammals. The expression of progenitor cell markers by supporting cells is consistent with the general concept of ‘stemness’ within the supporting cell population. Currently, no consistent pattern of expression has definitively demarcated certain supporting cells as a dedicated progenitor population.
Beyond progenitor cell markers, there may be physical characteristics indicative of a highly differentiated cellular state that are specific to mammalian supporting cells, thereby limiting their ability to regenerate lost hair cells in adulthood (reviewed in Bermingham-McDonogh et al., 2012). Previous work examining the relationship between a supporting cell’s ability to change shape and its capacity for proliferation demonstrated that cells within mouse utricular maculae that have spread over a substrate in culture are more likely to enter S-phase than their counterparts in culture conditions that do not favor spreading (Collado et al., 2011). That same study found that this ability of supporting cells to spread and change shape decreases as mice age, but is retained into adulthood by chickens, and correlates with effective wound healing. (Collado et al., 2011) This reduced ability to change shape may be related to the circumferential F-actin bands that are present at the apical junctions between supporting cells. These bands thicken significantly with adulthood in mice and humans, but remain thin in the mature chicken utricle, providing another potential explanation for why regeneration is robust in the avian system, but much more limited in mammals (Burns et al., 2008). Major questions regarding hair cell regeneration persist - why do some supporting cells go on to regenerate hair cells following injury while others remain committed to the supporting cell lineage? What mechanisms prevent auditory hair cell regeneration?
Summary
Supporting cells play many important roles in the inner ear, and some of those functions are analogous to those of glia in the central nervous system. During development, supporting cells are thought to mediate spontaneous inward currents in hair cells and SGN firing of rapid action potentials, which may be essential to develop neural connections and refine the tonotopic map of the cochlea. Supporting cells provide critical trophic factors to SGNs and are responsible for clearing neurotransmitters from synapses, two functions that are also served by glial cells. While one model of potassium recycling postulates that potassium returns to the stria directly via perilymph, two other models of potassium recycling rely on supporting cells to remove potassium from the vicinity of depolarized hair cells, a buffering role that astrocytes also perform to maintain balanced ionic conditions for neurons.
Supporting cells eliminate dead hair cells via phagocytosis in a manner that is reminiscent of the removal of neuronal corpses by microglia. Microglia have been implicated in phagoptosis, a process in which viable cells are eliminated by phagocytosis. Whether supporting cells are capable of phagoptosis remains to be seen, although the reversibility of PS externalization in hair cells suggests that such a mechanism may contribute to supporting cell-mediated hair cell death.
In the systems that regenerate hair cells, supporting cells serve as the precursors for new hair cells. Supporting cells can regenerate hair cells through a mitotic mechanism, which is reminiscent of the manner in which Müller glia give rise to new retinal neurons. Alternatively, supporting cells can convert directly into new hair cells via transdifferentiation, which has not been reported for Müller glia.
As was the case for glia ~20 years ago, supporting cells are emerging as central players in the auditory and vestibular systems. Supporting cells perform critical functions in development, survival, phagocytosis, death and regeneration in the inner ear. Many of these functions are analogous to those that are served by glia in other parts of the nervous system. From a clinical/translational perspective, supporting cells are emerging as potential targets in the development of clinical therapies aimed at both preventing and reversing hearing loss.
Highlights.
- Supporting cells are vital to the development and function of hair cells and neurons
- Supporting cells mediate hair cell survival, death, and regeneration
- Supporting cells eliminate damaged or dying hair cells from the sensory epithelium
- Many functions of supporting cells are similar to those reported for glial cells
Acknowledgments
The authors would like to thank Dr. Doris Wu, Dr. Matthew Kelley, Dr. Meghan Drummond, and Dr. Jonathan Bird for their helpful comments and suggestions on the manuscript. The authors are also grateful to Dr. Jonathan Gale for many thoughtful conversations about supporting cells as well as constructive notes on the development of the text and figures of this manuscript. This work was supported by the NIDCD Division of Intramural Research.
Abbreviations
SGN
spiral ganglion neuron
IHC
inner hair cell
ISC
inner supporting cell
BDNF
brain-derived neurotrophic factor
NRG
neuregulin
NT3
neurotrophin-3
GLAST
glutamate aspartate transporter
ERK 1/2
extracellularly regulated kinases 1 and 2
HSP70
heat shock protein 70
TEM
transmission electron microscopy
TIAR
T-cell restricted intracellular antigen-related protein
PS
phosphatidylserine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Montiel J, Morales D, Concha M. Evolutionary divergence of the reptilian and the mammalian brains: considerations on connectivity and development. Brain Res Brain Res Rev. 2002;39:141–153. doi: 10.1016/s0165-0173(02)00180-7. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Bohne BA, Harding GW. An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res. 2003;175:82–100. doi: 10.1016/s0378-5955(02)00713-x. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Lysakowski A, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci U S A. 2000;97:11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann N Y Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Corwin JT, Hauswirth WW, Heller S, Reed R, Reh TA. Regenerative Medicine for the Special Senses: Restoring the Inputs. J Neurosci. 2012;32:14053–14057. doi: 10.1523/JNEUROSCI.3336-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71:389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SA, Oesterle EC, Coltrera MD. Macrophage and microglia-like cells in the avian inner ear. J Comp Neurol. 1998;398:241–256. doi: 10.1002/(sici)1096-9861(19980824)398:2<241::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Bhave SA, Stone JS, Rubel EW, Coltrera MD. Cell cycle progression in gentamicin-damaged avian cochleas. J Neurosci. 1995;15:4618–4628. doi: 10.1523/JNEUROSCI.15-06-04618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JE, Daudet N, Warchol ME, Gale JE. Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear. J Neurosci. 2010;30:12545–12556. doi: 10.1523/JNEUROSCI.3042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–878. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Volkl H, Hubner CA, Jentsch TJ. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J. 2003;22:5422–5434. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci. 2012 doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Burns JC, Christophel JJ, Collado MS, Magnus C, Carfrae M, Corwin JT. Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds. J Comp Neurol. 2008;511:396–414. doi: 10.1002/cne.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, Zuo J, Cheng AG. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Johnston PR, Luz J, Uluer A, Fligor B, Licameli GR, Kenna MA, Jones DT. Sensorineural hearing loss in patients with cystic fibrosis. Otolaryngol Head Neck Surg. 2009;141:86–90. doi: 10.1016/j.otohns.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Collado MS, Burns JC, Meyers JR, Corwin JT. Variations in shape-sensitive restriction points mirror differences in the regeneration capacities of avian and mammalian ears. PLoS One. 2011;6:e23861. doi: 10.1371/journal.pone.0023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181–195. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Dopyera CE. Hair cell and supporting cell response to acoustic trauma in the chick cochlea. Hear Res. 1990;46:29–40. doi: 10.1016/0378-5955(90)90137-e. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Saunders JC, Tilney LG. Hair cell damage produced by acoustic trauma in the chick cochlea. Hear Res. 1987;25:267–286. doi: 10.1016/0378-5955(87)90098-0. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Tan J. Cell Death in the Inner Ear. In: Reed JC, Green DR, editors. Apoptosis : physiology and pathology. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernardo S, Canals S, Casarejos MJ, Solano RM, Menendez J, Mena MA. Role of extracellular signal-regulated protein kinase in neuronal cell death induced by glutathione depletion in neuron/glia mesencephalic cultures. J Neurochem. 2004;91:667–682. doi: 10.1111/j.1471-4159.2004.02744.x. [DOI] [PubMed] [Google Scholar]
- Dupont J, Guilhaume A, Aran JM. Neuronal degeneration of primary cochlear and vestibular innervations after local injection of sisomicin in the guinea pig. Hear Res. 1993;68:217–228. doi: 10.1016/0378-5955(93)90125-k. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Flock A. Transducing mechanisms in the lateral line canal organ receptors. Cold Spring Harb Symp Quant Biol. 1965;30:133–145. doi: 10.1101/sqb.1965.030.01.016. [DOI] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985;19:171–182. doi: 10.1016/0378-5955(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Fricker M, Neher JJ, Zhao JW, Thery C, Tolkovsky AM, Brown GC. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci. 2012;32:2657–2666. doi: 10.1523/JNEUROSCI.4837-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TB, Griffith AJ. Human nonsyndromic sensorineural deafness. Annu Rev Genomics Hum Genet. 2003;4:341–402. doi: 10.1146/annurev.genom.4.070802.110347. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci. 2003;23:11296–11304. doi: 10.1523/JNEUROSCI.23-36-11296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lehre KP. Immunocytochemical localization of a high-affinity glutamate aspartate transporter, GLAST, in the rat and guinea-pig cochlea. Eur J Neurosci. 1997;9:1961–1969. doi: 10.1111/j.1460-9568.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Gale JE, Jagger DJ. Cochlear supporting cells. In: Fuchs PA, editor. The Oxford Handbook of Auditory Science: The Ear. Oxford; New York: Oxford University Press; 2010. [Google Scholar]
- Gale JE, Meyers JR, Periasamy A, Corwin JT. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog’s saccule. J Neurobiol. 2002;50:81–92. doi: 10.1002/neu.10002. [DOI] [PubMed] [Google Scholar]
- Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr Biol. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Cheng N, Hiel H, Yi E, Tanaka K, Ellis-Davies GC, Rothstein JD, Bergles DE. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J Neurosci. 2006;26:7659–7664. doi: 10.1523/JNEUROSCI.1545-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Casati ME, Murtie JC, Rio C, Stankovic K, Liberman MC, Corfas G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci U S A. 2010;107:17005–17010. doi: 10.1073/pnas.1008938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Gale JE, Ranatunga KM, Kros CJ, Richardson GP. Aminoglycoside-induced phosphatidylserine externalization in sensory hair cells is regionally restricted, rapid, and reversible. J Neurosci. 2008;28:9939–9952. doi: 10.1523/JNEUROSCI.1124-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Westrum LE, Cunningham DE, Rubel EW. Electron microscopy of degenerative changes in the chick basilar papilla after gentamicin exposure. J Comp Neurol. 2004;470:164–180. doi: 10.1002/cne.11046. [DOI] [PubMed] [Google Scholar]
- Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann N Y Acad Sci. 1999;884:389–409. doi: 10.1111/j.1749-6632.1999.tb08657.x. [DOI] [PubMed] [Google Scholar]
- Hordichok AJ, Steyger PS. Closure of supporting cell scar formations requires dynamic actin mechanisms. Hear Res. 2007;232:1–19. doi: 10.1016/j.heares.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–1914. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Dabdoub A, Kelley MW. Fgf signaling regulates development and transdifferentiation of hair cells and supporting cells in the basilar papilla. Hear Res. 2012;289:27–39. doi: 10.1016/j.heares.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger DJ, Forge A. Compartmentalized and signal-selective gap junctional coupling in the hearing cochlea. J Neurosci. 2006;26:1260–1268. doi: 10.1523/JNEUROSCI.4278-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kennedy HJ, Holley MC, Fettiplace R, Marcotti W. The resting transducer current drives spontaneous activity in prehearing mammalian cochlear inner hair cells. J Neurosci. 2012;32:10479–10483. doi: 10.1523/JNEUROSCI.0803-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. J Neurosci. 1996;16:649–662. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B. Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. J Neurophysiol. 2007;98:1898–1908. doi: 10.1152/jn.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM, Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- Kaiser CL, Chapman BJ, Guidi JL, Terry CE, Mangiardi DA, Cotanche DA. Comparison of activated caspase detection methods in the gentamicin-treated chick cochlea. Hear Res. 2008;240:1–11. doi: 10.1016/j.heares.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Ohmori H. Activation of glutamate receptors in response to membrane depolarization of hair cells isolated from chick cochlea. J Physiol. 1994;477 (Pt 3):403–414. doi: 10.1113/jphysiol.1994.sp020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Knipper M, Claussen C, Ruttiger L, Zimmermann U, Lullmann-Rauch R, Eskelinen EL, Schroder J, Schwake M, Saftig P. Deafness in LIMP2-deficient mice due to early loss of the potassium channel KCNQ1/KCNE1 in marginal cells of the stria vascularis. J Physiol. 2006;576:73–86. doi: 10.1113/jphysiol.2006.116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koitchev K, Guilhaume A, Cazals Y, Aran JM. Spiral ganglion changes after massive aminoglycoside treatment in the guinea pig. Counts and ultrastructure. Acta Otolaryngol. 1982;94:431–438. doi: 10.3109/00016488209128931. [DOI] [PubMed] [Google Scholar]
- Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Lahne M, Gale JE. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci. 2008;28:4918–4928. doi: 10.1523/JNEUROSCI.4914-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Gale JE. Damage-induced cell-cell communication in different cochlear cell types via two distinct ATP-dependent Ca waves. Purinergic Signal. 2010;6:189–200. doi: 10.1007/s11302-010-9193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Leis JA, Bekar LK, Walz W. Potassium homeostasis in the ischemic brain. Glia. 2005;50:407–416. doi: 10.1002/glia.20145. [DOI] [PubMed] [Google Scholar]
- Li-Korotky HS. Age-related hearing loss: quality of care for quality of life. Gerontologist. 2012;52:265–271. doi: 10.1093/geront/gnr159. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Sage C, Huang M, Chen ZY, Heller S. Islet-1 expression in the developing chicken inner ear. J Comp Neurol. 2004;477:1–10. doi: 10.1002/cne.20190. [DOI] [PubMed] [Google Scholar]
- Li L, Forge A. Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. Int J Dev Neurosci. 1997;15:433–446. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31:15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR, Westbrook EW, Ryals BM. Hair cell regeneration in the chicken cochlea following aminoglycoside toxicity. Hear Res. 1991;56:203–210. doi: 10.1016/0378-5955(91)90171-5. [DOI] [PubMed] [Google Scholar]
- Lopez IA, Zhao PM, Yamaguchi M, de Vellis J, Espinosa-Jeffrey A. Stem/progenitor cells in the postnatal inner ear of the GFP-nestin transgenic mouse. Int J Dev Neurosci. 2004;22:205–213. doi: 10.1016/j.ijdevneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004;475:1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Marsh RR, Xu LR, Moy JP, Saunders JC. Recovery of the basilar papilla following intense sound exposure in the chick. Hear Res. 1990;46:229–237. doi: 10.1016/0378-5955(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Oesterle EC, Stone JS, Rubel EW. Characterization of damage and regeneration in cultured avian utricles. J Assoc Res Otolaryngol. 2000;1:46–63. doi: 10.1007/s101620010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell B, Davies S, Forge A. The effect of gentamicin-induced hair cell loss on the tight junctions of the reticular lamina. Hear Res. 1989;40:221–232. doi: 10.1016/0378-5955(89)90163-9. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Meiteles LZ, Raphael Y. Scar formation in the vestibular sensory epithelium after aminoglycoside toxicity. Hear Res. 1994;79:26–38. doi: 10.1016/0378-5955(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Mistrik P, Ashmore J. The role of potassium recirculation in cochlear amplification. Curr Opin Otolaryngol Head Neck Surg. 2009;17:394–399. doi: 10.1097/MOO.0b013e328330366f. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Valat J, Travo C, Sans A. A role for BDNF in early postnatal rat vestibular epithelia maturation: implication of supporting cells. Eur J Neurosci. 1998;10:598–606. doi: 10.1046/j.1460-9568.1998.00070.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamane H, Shibata S, Takayama M, Sunami K, Nakai Y. Two modes of auditory hair cell loss following acoustic overstimulation in the avian inner ear. ORL J Otorhinolaryngol Relat Spec. 1997;59:303–310. doi: 10.1159/000276961. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- Neniskyte U, Neher JJ, Brown GC. Neuronal death induced by nanomolar amyloid beta is mediated by primary phagocytosis of neurons by microglia. J Biol Chem. 2011;286:39904–39913. doi: 10.1074/jbc.M111.267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDCD. Statistical Report: Prevalence of Hearing Loss in US Children, 2005. 2006 [Online]. Available: http://www.nidcd.nih.gov/funding/programs/hb/outcomes/Pages/report.aspx.
- NIDCD. Quick statistics. 2010 [Online]. Available: http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx.
- Phillips KR, Biswas A, Cyr JL. How hair cells hear: the molecular basis of hair-cell mechanotransduction. Curr Opin Otolaryngol Head Neck Surg. 2008;16:445–451. doi: 10.1097/MOO.0b013e32830f4ac8. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J Neurocytol. 1992;21:663–671. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear Res. 1991;51:173–183. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Roberson DF, Weisleder P, Bohrer PS, Rubel EW. Ongoing production of sensory cells in the vestibular epithelium of the chick. Hear Res. 1992;57:166–174. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Watt FM, Potten CS, Farbman AI, Lewis J, Calof AL, Margolis FL, Reh JA, Raymond PA, Corwin JT, Presson JC, Fernald RD, Steinberg RH, Cotanche DA, Reasner DA, Oakley B, Ryals BM. Regeneration of Vertebrate Sensory Receptor-Cells- Final General Discussion. Ciba Foundation Symposia. 1991;160:314–329. [Google Scholar]
- Ruel J, Chen C, Pujol R, Bobbin RP, Puel JL. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea-pig. J Physiol. 1999;518 (Pt 3):667–680. doi: 10.1111/j.1469-7793.1999.0667p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Richardson GP, Cody AR. Mechanosensitivity of mammalian auditory hair cells in vitro. Nature. 1986;321:517–519. doi: 10.1038/321517a0. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Yaoi T, Suzuki T, Okano H, Hisa Y, Fushiki S. Spatiotemporal patterns of Musashi1 expression during inner ear development. Neuroreport. 2004;15:997–1001. doi: 10.1097/00001756-200404290-00013. [DOI] [PubMed] [Google Scholar]
- Seixas NS, Neitzel R, Stover B, Sheppard L, Feeney P, Mills D, Kujawa S. 10-Year prospective study of noise exposure and hearing damage among construction workers. Occup Environ Med. 2012 doi: 10.1136/oemed-2011-100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane A, Llorens J. Extruding auditory hair cells in rats exposed to subchronic 3,3′-iminodipropionitrile. Environ Toxicol Pharmacol. 2005;19:571–574. doi: 10.1016/j.etap.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Shang J, Cafaro J, Nehmer R, Stone J. Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro. J Assoc Res Otolaryngol. 2010;11:203–222. doi: 10.1007/s10162-009-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. Wnt-responsive lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Gillespie PG, Nuttall AL. Apical phosphatidylserine externalization in auditory hair cells. Mol Membr Biol. 2007;24:16–27. doi: 10.1080/09687860600926883. [DOI] [PubMed] [Google Scholar]
- Spiess AC, Lang H, Schulte BA, Spicer SS, Schmiedt RA. Effects of gap junction uncoupling in the gerbil cochlea. Laryngoscope. 2002;112:1635–1641. doi: 10.1097/00005537-200209000-00020. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Choi YS, Woolley SM, Yamashita H, Rubel EW. Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol. 1999;28:863–876. doi: 10.1023/a:1007022205821. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Synchronization of hair cell regeneration in the chick cochlea following noise damage. J Cell Sci. 1992;102 (Pt 4):671–680. doi: 10.1242/jcs.102.4.671. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–973. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Cellular studies of auditory hair cell regeneration in birds. Proc Natl Acad Sci U S A. 2000;97:11714–11721. doi: 10.1073/pnas.97.22.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. cProx1 immunoreactivity distinguishes progenitor cells and predicts hair cell fate during avian hair cell regeneration. Dev Dyn. 2004;230:597–614. doi: 10.1002/dvdy.20087. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol. 2007;501:30–37. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. J Comp Neurol. 2005;484:105–120. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Berntson A, Leao RN, Fyffe RE. Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways. J Physiol. 2006;572:313–321. doi: 10.1113/jphysiol.2006.104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME. Characterization of supporting cell phenotype in the avian inner ear: implications for sensory regeneration. Hear Res. 2007;227:11–18. doi: 10.1016/j.heares.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Tsue TT, Rubel EW. Hair cell replacement in avian vestibular epithelium: supporting cell to type I hair cell. Hear Res. 1995;82:125–133. doi: 10.1016/0378-5955(94)00169-q. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins HR, Presson JC, Popper AN. Proliferation of vertebrate inner ear supporting cells. J Neurobiol. 1999;39:527–535. doi: 10.1002/(sici)1097-4695(19990615)39:4<527::aid-neu6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasoba T, Kondo K. Supporting cell proliferation after hair cell injury in mature guinea pig cochlea in vivo. Cell Tissue Res. 2006;325:23–31. doi: 10.1007/s00441-006-0157-9. [DOI] [PubMed] [Google Scholar]
- Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 2009;24:307–316. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J Neurosci. 1999;19:2161–2170. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32:405–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller EE, Lykens JE, Terrell CE, Aliberti J, Filipovich AH, Henson PM, Jordan MB. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208:1203–1214. doi: 10.1084/jem.20102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti A, Kuhn S, Johnson SL, Franz C, Singer W, Hecker D, Geisler HS, Kopschall I, Rohbock K, Gutsche K, Dlugaiczyk J, Schick B, Marcotti W, Ruttiger L, Schimmang T, Knipper M. Lack of brain-derived neurotrophic factor hampers inner hair cell synapse physiology, but protects against noise-induced hearing loss. J Neurosci. 2012;32:8545–8553. doi: 10.1523/JNEUROSCI.1247-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]